Abstract
The stem-cell pool is considered to be maintained by a balance between symmetric and asymmetric division of stem cells. The cell polarity model proposes that the facultative use of symmetric and asymmetric cell division is orchestrated by a polarity complex consisting of partitioning-defective proteins Par3 and Par6, and atypical protein kinase C (aPKCζ and aPKCλ), which regulates planar symmetry of dividing stem cells with respect to the signaling microenvironment. However, the role of the polarity complex is unexplored in mammalian adult stem-cell functions. Here we report that, in contrast to accepted paradigms, polarization and activity of adult hematopoietic stem cell (HSC) do not depend on either aPKCζ or aPKCλ or both in vivo. Mice, having constitutive and hematopoietic-specific (Vav1-Cre) deletion of aPKCζ and aPKCλ, respectively, have normal hematopoiesis, including normal HSC self-renewal, engraftment, differentiation, and interaction with the bone marrow microenvironment. Furthermore, inducible complete deletion of aPKCλ (Mx1-Cre) in aPKCζ−/− HSC does not affect HSC polarization, self-renewal, engraftment, or lineage repopulation. In addition, aPKCζ- and aPKCλ-deficient HSCs elicited a normal pattern of hematopoietic recovery secondary to myeloablative stress. Taken together, the expression of aPKCζ, aPKCλ, or both are dispensable for primitive and adult HSC fate determination in steady-state and stress hematopoiesis, contrary to the hypothesis of a unique, evolutionary conserved aPKCζ/λ-directed cell polarity signaling mechanism in mammalian HSC fate determination.
Stem cells are characterized by self-renewal and multilineage differentiation potential (1). The choice between these two processes remains a critical issue in stem cell and cancer biology (1–4). It has been proposed that the selection of cell fate determination toward either self-renewal or differentiation of stem cells results from a choice between maintaining contact with the bone marrow (BM) microenvironment to regulate self-renewal, and changing the cell planar polarity to establish an orientation axis for asymmetric distribution (5, 6). Whether stem cell activity is truly dependent on asymmetric cell division remains controversial (1).
Distinct polarity proteins have been shown to act as core components of the cell polarization machinery in animals ranging from Caenorhabditis elegans to humans (6–12). Asymmetric cell division requires polar distribution of a set of proteins that accumulate before mitosis. Classical cell-polarity complex proteins consist of partitioning-defective proteins: Par3, Par6, and atypical protein kinase C (aPKCζ and aPKCλ) (7). In the hematopoietic system, aPKC signaling has been implicated in the establishment of T-cell polarity during immunological synapse and in the regulation of pathogen-specific CD8+ T-cell activity (12, 13). aPKCs have been shown to play a crucial role in Drosophila neuroblast self-renewal and differentiation (4, 8, 14, 15). Studies based on Drosophila and C. elegans have indicated that spatiotemporal gradients of several polarity aPKC phosphorylated determinants, such as Lgl or Numb, can determine asymmetric stem cell division through microtubule polarization to orient the mitotic spindle (4, 16). However, while the molecules implicated in stem cell polarity are highly conserved in vertebrates, their mechanism of action could be tissue specific, and in some cases differ substantially from that in the invertebrates (1, 5).
Similarly to embryonic and other tissue-specific stem cells, mammalian hematopoietic stem-cell (HSC) fate could be regulated by an active cell-polarity complex (17–21). Using a Notch-reporter system, it has been shown that the differential cell fate of paired daughter cells in culture is related to asymmetric acquisition of Numb in vitro (18). A recent RNA-interference (RNAi)-based screening has suggested that aPKCζ and Par6 could positively regulate mammalian HSC activity (19). In a separate study, it has also been shown that the pharmacological attenuation of aPKCζ signaling can induce mobilization of murine hematopoietic progenitors (20). However, proper genetic evaluation of the function of aPKCζ and aPKCλ in mammalian HSC fate mapping in vivo has been unexplored because of the unavailability of tissue-specific conditional knock-out animal models.
To elucidate the individual and combined role of aPKCζ and aPKCλ signaling in mammalian HSC activity and hematopoiesis, we generated triple-transgenic, hematopoietic-specific or inducible knock-out mice. We have found that the polarization and activity of mammalian HSC do not depend on aPKCζ and aPKCλ in vivo. Therefore, an alternative tissue-specific and context-dependent signaling complex may be involved in controlling stem-cell fate.
Results
aPKCζ−/− Mice Have Normal Steady-State Hematopoiesis.
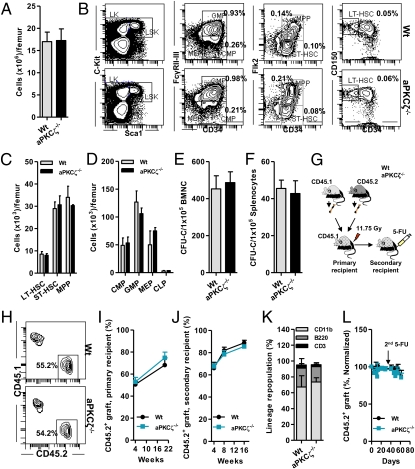
Because aPKCζ has been implicated in determining HSC activity (19, 20), to study the effects of genetic deletion of aPKCζ in mammalian hematopoietic systems in vivo, we analyzed aPKCζ−/− mice (22). aPKCζ−/− mice are viable and are born following a Mendelian ratio of inheritance (22). We hypothesized that if aPKCζ-dependent polarity signals regulate HSC and progenitor (HSC/P) activity, we could expect an alteration in the steady-state hematopoiesis of aPKCζ-deficient mice. However, aPKCζ-deficient mice had normal bone marrow (BM) cellularity, similar to their littermates (Fig. 1A). There was no effect of loss of aPKCζ on BM-HSC/P frequency or content (Fig. 1 B–D) or in the frequency of colony-forming progenitors (CFU-C) present in the BM and spleen (Fig. 1 E and F). This finding suggested that aPKCζ does not regulate steady-state hematopoiesis.
Fig. 1.
Deficiency of aPKCζ does not affect HSC activity or steady-state hematopoiesis. (A) Absolute numbers of nuclear cells present in the BM of WT or aPKCζ−/− mice (n = 3–4 mice per group). (B) Representative flow cytometry (FACS) contour diagram showing the frequency of hematopoietic stem cell and progenitors (HSC/P) present in the BM of WT or aPKCζ−/− mice. (C) Absolute numbers of HSCs present in the BM of WT or aPKCζ−/− mice (n = 3–4 mice per group). (D) Absolute numbers of hematopoietic progenitors (HPC) present in the BM of WT or aPKCζ−/− mice (n = 3–4 mice per group). (E) Absolute numbers of colony-forming progenitors (CFU-C) present in the BM of WT or aPKCζ−/− mice (n = 3–4 mice per group). (F) Absolute numbers of colony-forming progenitors (CFU-C) present in the spleen of WT or aPKCζ−/− mice (n = 3–4 mice per group). (A–F) Error bars represent SD. (G) Experimental set up: 2 × 106 BM cells from CD45.2+ WT or aPKCζ−/− mice were mixed with 2 × 106 CD45.1+ B6.SJLPtprca Pep3b/BoyJ WT BM cells and competitively (1:1 ratio) transplanted into lethally irradiated primary, secondary, and tertiary recipient mice and peripheral blood chimera was monitored at different time points. After 16 wk of engraftment, secondary recipient mice were further challenged (intraperitoneally) with two cycles (day 0 and day 40) of 5-FU injections (150 mg/kg body weight) and peripheral blood donor-derived chimera (CD45.2+) was monitored for 8 to 10 wk. (H) Representative FACS contour diagram showing competitive chimera of CD45.2+ WT or aPKCζ−/− cells serially transplanted into lethally irradiated CD45.1+ primary recipient mice from G. (I) Change in donor chimera (CD45.2+) in the peripheral blood of primary recipient mice (n = 6–8 mice per group). (J) Change in donor chimera (CD45.2+) in the peripheral blood of secondary recipient mice (n = 7 mice per group). (K) Frequency of lineage-repopulating myeloid (CD45.2+CD11b+) or B (CD45.2+B220+) or T (CD45.2+CD3+) cells present in the BM of the primary recipient mice (n = 6–8 mice per group). (L) Change in donor chimera (CD45.2+) in the peripheral blood of 5-FU–treated secondary recipient mice (n = 7 mice per group). (I–L) Error bars represent SEM.
aPKCζ−/− HSCs Have Normal Self-Renewal and Lineage Repopulation Activity.
If aPKCζ-dependent polarity signals regulate HSC self-renewal and differentiation, we would expect an alteration in HSC repopulation ability during serial competitive BM transplantation, the gold-standard assay used to quantitate HSC self-renewal and differentiation in vivo (23–25). To test this hypothesis, we serially transplanted BM nucleated cells (BMNC) from CD45.2+ WT or CD45.2+ aPKCζ−/− mice along with CD45.1+ B6.SJLPtprca Pep3b/BoyJ competitor cells into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ primary and secondary recipient mice (Fig. 1G). The donor-derived CD45.2+ chimera level was monitored in the peripheral blood and in the BM in both the primary and secondary recipient mice (Fig. 1H). HSC-repopulation ability did not change in either the primary or secondary recipients because of the deficiency of aPKCζ (Fig. 1 I and J). There was no change in CD45.2+ myeloid (CD11b+ graft) or lymphoid (B220+ graft and CD3+ graft) cell reconstitution in the recipient BM (Fig. 1K), suggesting that aPKCζ does not regulate HSC self-renewal and lineage repopulation in vivo.
Because treatment with 5-fluorouracil (5-FU), a potent myeloablative agent, rapidly kills cycling BM cells and brings quiescent HSC into cell division (26, 27), 5-FU administration is used to assess HSC self-renewal and repopulation in vivo (28). Secondary recipient mice, after 16 wk of engraftment, were further challenged with serial administration of 5-FU (on days 0 and 40), and CD45.2+ chimera was monitored over 2 mo (Fig. 1 G and L). There was no significant change in the level of CD45.2+ chimera (Fig. 1L), suggesting that aPKCζ is dispensable in HSC repopulation activity and stressed hematopoiesis in vivo.
Constitutive and Hematopoietic-Specific (Vav1-Cre) Genetic Deletion of aPKCζ and aPKCλ Does Not Affect Steady-State Hematopoiesis.
aPKCζ shares a homologous domain architecture with aPKCλ (29). We wondered whether the loss of function of aPKCζ is compensated by aPKCλ in vivo. aPKCλ-deficient mice die at very early embryonic stages before hematopoiesis occurs (12). Mice with aPKCλf/f alleles (12) were crossed with Vav1-Cre to generate WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre mice. Expression of Cre recombinase under the promoter of Vav1, a guanine nucleotide exchange factor for Rho-GTPases, induces deletion of aPKCλ alleles, specifically in the fetal and adult hematopoietic system (26, 30).
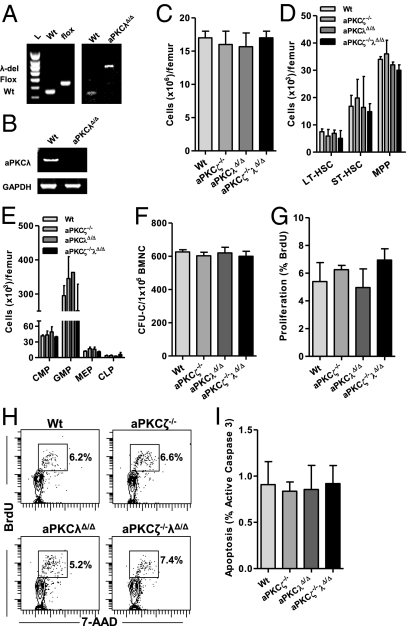
Genomic DNA PCR revealed complete deletion of aPKCλ alleles in purified HSCs (Lineage−Sca1+c-Kit+CD34−Flk2−) from aPKCλΔ/Δ; Vav1-Cre BM (Fig. 2A). We did not detect mRNA expression of aPKCλ in the HSCs isolated from aPKCλΔ/Δ; Vav1-Cre mice by RT-PCR (Fig. 2B). aPKCλΔ/Δ; Vav1-Cre and aPKCζ−/−λΔ/Δ; Vav1-Cre mice were born following Mendelian ratio of inheritance (Fig. S1), suggesting that the expression of aPKCζ and aPKCλ is not crucial during fetal hematopoiesis (31).
Fig. 2.
Constitutive and hematopoietic system-specific loss of aPKCζ/λ does not affect steady-state hematopoiesis. (A) Genomic DNA PCR analysis of WT, floxed, and aPKCλ-deleted alleles in Lineage−Sca1+c-Kit+ (LSK) CD34−Flk2− cells isolated from the BM of WT; Vav1-Cre or aPKCλΔ/Δ;Vav1-Cre mice. (B) mRNA expression (RT-PCR) analysis of aPKCλ in LSKCD34−Flk2− cells isolated from the BM of WT; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre mice. (C) Absolute numbers of nuclear cells present in the BM of WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ;Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ;Vav1-Cre mice (n = 3–4 mice per group). (D) Absolute numbers of HSCs present in the BM of WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ; Vav1-Cre mice (n = 3 mice per group). Error bars represent SD. (E) Absolute numbers of HPC present in the BM of WT; Vav1-Cre or aPKCζ−/−;Vav1-Cre or aPKCλΔ/Δ;Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ; Vav1-Cre mice (n = 3 mice per group). (F) Absolute numbers of colony-forming progenitors (CFU-C) present in the BM of WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ; Vav1-Cre mice (n = 3 mice per group). (G) Apoptosis (active Caspase 3) of BM-LSK cells in WT;Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ; Vav1-Cre mice in vivo (n = 3 mice per group). (H) Representative FACS contour diagram showing BrdU (300 μg i.p. injection, 45-min pulse) incorporation into BM-LSK cells in WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ; Vav1-Cre mice in vivo. (I) Proliferation of BM-LSK cells in WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/− aPKCλΔ/Δ; Vav1-Cre mice in vivo (n = 3 mice per group). (A–I) Error bars represent SD.
Because both fetal and adult hematopoiesis use distinct microenvironments and differ in their cell cycle activation (32), we speculated that aPKCζ and aPKCλ may be required for normal HSC activity in adult BM. However, aPKCζ- and aPKCλ-deficient mice had normal BM cellularity, similar to their littermates (Fig. 2C). Loss of aPKCζ and aPKCλ had no detectable effect on BM-HSC/P content (Fig. 2 D and E) or the frequency of BM colony-forming progenitors (Fig. 2F). aPKCζ- and aPKCλ-deficient HSCs showed normal apoptosis (Fig. 2G) and proliferation in vivo (Fig. 2 H and I), similar to WT cells, which suggests that expression of aPKCζ and aPKCλ does not regulate steady-state hematopoiesis.
Deficiency of aPKCζ and aPKCλ Do Not Affect Hematopoietic Recovery from 5-FU–Induced Myeloablative Stress.
We examined the hematopoietic response to forced cell cycle activation of quiescent HSC in vivo of WT; Vav1-Cre or aPKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre BM. When challenged with 5-FU, aPKCζ- and aPKCλ-deficient mice elicited a normal hematopoietic recovery response similar to littermate control mice (Fig. S2). There were no differences among the groups in the peripheral blood leukocyte, neutrophil, or platelet counts (Fig. S2 A–C), nor in the evolution of reticulocyte frequency in the peripheral blood (Fig. S2 D and E). No differences were seen in the red blood cell counts in WT and aPKCζ- and aPKCλ-deficient mice (Fig. S2F). These data suggest that aPKCζ and aPKCλ are also dispensable during stressed adult hematopoiesis.
Inducible (Mx1-Cre) Genetic Deletion of aPKCζ and aPKCλ Does Not Affect Steady-State Hematopoiesis.
We thought that the constitutive and hematopoietic-specific deletion of aPKCλ, as seen in the Vav1-Cre mice, might provide a selection advantage during development through functional compensation toward normal HSC activity in the aPKCλ-dependent downstream signaling pathway in vivo. We therefore wanted to specifically delete aPKCλ in the adult hematopoietic system using IFN-inducible Mx1-Cre mice (23). Mice with aPKCλf/f alleles (12), were crossed with Mx1-Cre transgenic mice similar to other inducible models of gene deletion (23) and WT; Mx1-Cre or aPKCζ−/−; Mx1-Cre or aPKCλf/f; Mx1-Cre or aPKCζ−/−λf/f; Mx1-Cre mice were generated (Fig. S3A). Administration of polyinosinic:polycytidylic acid (pI-pC) resulted in the loss of expression of aPKCλ in the Lineage−Sca1+c-Kit+CD34−Flk2− cells that were isolated from aPKCλf/f; Mx1-Cre mice, but not those from WT; Mx1-Cre mice (Fig. S3B). Genomic DNA PCR analysis confirmed that there was complete deletion of aPKCλ alleles in the adult hematopoietic system (Fig. S3C).
If aPKCζ/λ-dependent polarity signals regulate HSC activity, we should expect an alteration in the steady-state hematopoiesis in aPKCζ- and aPKCλ-deficient mice. However, there was no detectable effect of the loss of aPKCζ and aPKCλ on the content of BM-HSC/P subpopulations when assessed by immunofluorescence or functional assays (Fig. S3 D–F). Likewise, we did not detect any effect of aPKCζ and aPKCλ deficiency in BM-CD11b+ myeloid cell or B220+ B-cell or CD3+ T-cell (Fig. S3 G–I) contents. In addition, aPKCζ- and aPKCλ-deficient HSCs showed normal apoptosis and proliferation (Fig. S3 J and K) in vivo, similar to the WT cells, suggesting that the inducible genetic inactivation of aPKCζ and aPKCλ in the adult hematopoietic system does not significantly affect steady state HSC activity or hematopoiesis.
Inducible Deletion of aPKCλ and Constitutive Deficiency of aPKCζ Does Not Affect HSC Self-Renewal and Lineage Repopulation Activity.
Despite the normal content, proliferation, and survival of BM-HSC/Ps in the primary mice, it was possible that the self-renewal ability of long-term HSC (LT-HSC) in the adult hematopoietic system is regulated by aPKCζ and aPKCλ. Compensatory mechanisms during development in Vav1-Cre; aPKCζ−/−λf/f mice and the existence of a subtle HSC phenotype in Mx1-Cre; aPKCζ−/−λf/f might possible explanations for our findings. To address whether the deficiency of aPKCζ and aPKCλ resulted in a loss of LT-HSC activity, we first analyzed whether aPKCζ and aPKCζ-deficient HSCs are able to reconstitute the hematopoietic system. To test this, we transplanted BM cells from CD45.2+ WT; Mx1-Cre or aPKCλf/f; Mx1-Cre or aPKCζ−/−λf/f; Mx1-Cre mice into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice. After 4 wk of engraftment, the recipient mice were treated with pI-pC and their peripheral blood CD45.2+ chimera monitored for 16 wk (Fig. S4A). pI-pC treatment resulted in the complete excision of donor-derived aPKCλ alleles in the transplanted mice (Fig. S4B). However, the deficiency of aPKCζ and aPKCλ did not affect the evolution of myeloid cell (CD11b+ cells gated on CD45.2+ graft) and B-cell (B220+ cells gated on CD45.2+ graft) content in the peripheral blood of the recipient mice (Fig. S4 C and D). These results indicate that aPKCζ and aPKCλ are not required for hematopoietic reconstitution.
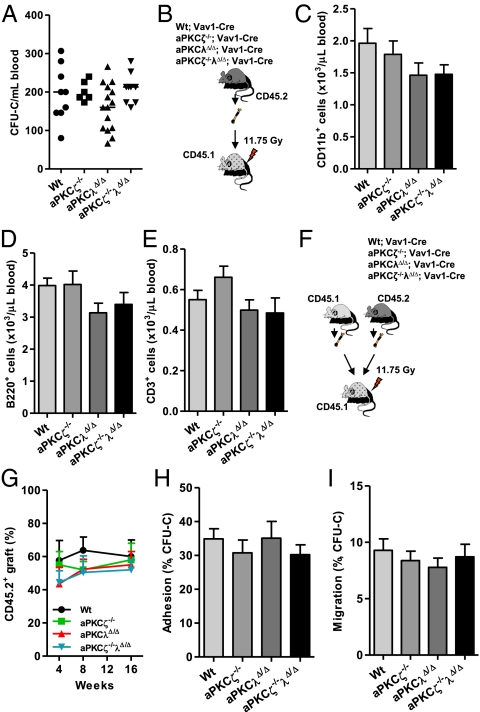
Secondly, to analyze the ability of aPKC-deficient cells to compete with WT cells, we generated mixed chimeric animals by transplanting BMNC from CD45.2+ WT; Mx1-Cre or aPKCλf/f; Mx1-Cre or aPKCζ−/−λf/f; Mx1-Cre mice along with CD45.1+ B6.SJLPtprca Pep3b/BoyJ competitor cells into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice (Fig. 3A). After 4 wk of engraftment, the recipient mice were treated with pI-pC (administered on every other day for 14 d) and peripheral blood CD45.2+ chimera was monitored for another 16 wk (Fig. 3A). BMNC from primary recipient mice were serially transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ secondary and tertiary recipients and peripheral blood CD45.2+ cell chimera was determined (Fig. 3A).
Fig. 3.
aPKCζ and aPKCλ does not regulate HSC self-renewal, engraftment, and multilineage differentiation. (A) Experimental set up. The 2 × 106 BM cells from CD45.2+ WT; Mx1-Cre or aPKCλf/f; Mx1-Cre or aPKCζ−/−λf/f; Mx1-Cre mice were mixed with 2 × 106 CD45.1+ B6.SJLPtprca Pep3b/BoyJ WT BM cells and competitively (1:1 ratio) transplanted into lethally irradiated primary mice. Serial transplantation of 1 × 107 pooled BM cells from primary mice was performed into secondary and tertiary recipient mice. After BM engraftment (4 wk), aPKCλ deletion was induced by administration of pI-pC and CD45.2+ chimera was monitored in different time points. (B) Evolution (normalized to baseline) of CD45.2+ chimera in peripheral blood of primary recipient mice (n = 7–9 mice per group). (C) Evolution (normalized to baseline) of CD45.2+ chimera in peripheral blood of secondary recipient mice (n = 8 mice per group). (D) Representative FACS contour diagram showing CD45.2+CD11b+-myeloid and CD45.2+B220+-B-lymphoid cells during HSC engraftment. (E) Evolution (normalized to baseline) of myeloid-cell chimera (CD45.2+ cells gated on total CD11b+ graft) in the peripheral blood of primary recipient mice (n = 7–9 mice per group). (F) Evolution (normalized to baseline) of myeloid-cell chimera (CD45.2+ cells gated on total CD11b+ graft) in the peripheral blood of secondary recipient mice (n = 8 mice per group). (G) Evolution (normalized to baseline) of B-cell chimera (CD45.2+ cells gated on total B220+ graft) in the peripheral blood of primary recipient mice (n = 7–9 mice per group). (H) Evolution (normalized to baseline) of B-cell chimera (CD45.2+ cells gated on total B220+ graft) in the peripheral blood of secondary recipient mice (n = 8 mice per group). (B, C, E–H) Error bars represent SEM. (I) Normalized CD45.2+ chimera in peripheral blood of tertiary recipient mice at 8 wk after transplantation. n = 7–9 mice per group. Error bars represent SD.
The aPKCζ- and aPKCλ-deficient HSCs did not demonstrate a defect in HSC self-renewal (Fig. 3 A and B) or skewed myeloid/lymphoid lineage repopulation (Fig. 3 E–H and Fig. S5) when assayed in the setting of serial competitive repopulation. Competitively transplanted WT or aPKCζ- and aPLCλ-deficient tertiary recipients showed similar levels of hematopoietic engraftment (Fig. 3I) without bias in the level of gene deletion when compared with primary or secondary recipients (Fig. S6 A–C). These data suggest that inducible loss of function of aPKCζ and aPKCλ in the adult hematopoietic system does not affect HSC self-renewal and lineage repopulation activity during serial BM transplantation.
aPKCζ and aPKCλ Are Dispensable in the HSC/P-Interaction with the Hematopoietic Microenvironment.
BM-HSC/P retention and mobilization are exquisitely controlled by a delicate network of molecular signals, which are generated by the hematopoietic microenvironment (33, 34). We analyzed whether the loss of function of aPKCζ and aPKCλ could result in defective interaction of HSC/P with the hematopoietic microenvironment and result in impaired HSC/P trafficking. Deficiency of aPKCζ and aPKCλ did not change the content of HSC/Ps present in the peripheral blood (Fig. 4A). aPKCζ- and aPKCλ-deficient mice also showed a normal response to G-CSF–induced HSC/P mobilization similar to the WT mice (Fig. S7), suggesting that aPKCζ and aPKCλ do not regulate HSC/P mobilization.
Fig. 4.
Deficiency of aPKCζ and aPKCλ does not affect interaction of HSC/P within the BM microenvironment. (A) Absolute numbers of CFU-Cs present in the peripheral blood of WT; Vav1-Cre or PKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre mice (n = 6–16 mice per group). Data represent average of two different experiments. (B) Experimental set up. 3 × 106 BMNCs CD45.2+ WT; Vav1-Cre or PKCζ−/−; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre mice, were noncompetitively transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ WT mice. CD45.2+ chimera was monitored in the peripheral blood of the recipient mice. (C) Absolute numbers of CD45.2+CD11b+-myeloid cells present in the peripheral blood of CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice after 8 wk of transplantation from B (n = 6–9 mice per group). (D) Absolute numbers of CD45.2+B220+ B-lymphoid cells present in the peripheral blood of CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice after 8 wk of transplantation from B (n = 6–9 mice per group). (E) Absolute numbers of CD45.2+CD3+ T-lymphoid cells present in the peripheral blood of CD45.1+ B6.SJLPtprca Pep3b/BoyJ mice after 8 wk of transplantation from B (n = 6–9 mice per group). (C–E) Error bars represent SD. (F) Experimental set up. The 2 × 106 BM cells from CD45.2+ WT; Vav1-Cre or aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre mice were mixed with 2 × 106 CD45.1+ B6.SJLPtprca Pep3b/BoyJ WT BM cells and competitively (1:1 ratio) transplanted into lethally irradiated primary mice. (G) Evolution of CD45.2+ chimera in the peripheral blood of recipient mice from F (n = 7–10 mice per group). (H) Adhesion (performed in triplicate) of WT; Vav1-Cre, PKCζ−/−; Vav1-Cre, aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre HSC/Ps to fibronectin (CH-296) in vitro. Error bars represent SEM. (I) Migration (performed in triplicate) of WT; Vav1-Cre, PKCζ−/−; Vav1-Cre, aPKCλΔ/Δ; Vav1-Cre or aPKCζ−/−λΔ/Δ; Vav1-Cre HSC/Ps toward CXCL12 in vitro. (G, I) Error bars represent SEM.
As mentioned earlier, self-renewal, proliferation, and survival of engrafted aPKCλ or aPKCζ/λ-deficient HSC was not significantly different from their WT counterparts. It is possible, however, that primary deficiency of aPKCζ and aPKCλ results in defective homing/engraftment in the transplanted mice. If this is true, in such case we would expect in the development of a BM failure syndrome. Therefore, CD45.2+ WT or aPKCζ- and aPKCλ-deficient BM were noncompetitively transplanted into lethally irradiated CD45.1+ B6.SJLPtprca Pep3b/BoyJ recipient mice and peripheral blood lineage repopulation was determined (Fig. 4B). Despite the complete deletion of aPKCλ alleles in the transplanted mice (Fig. S8), we did not see significant changes in the generation of donor-derived myelopoiesis or lymphopoiesis in the peripheral blood in the recipient mice (Fig. 4 C–E). Furthermore, competitive repopulation activities of WT or aPKCζ- and aPKCλ-deficient HSCs (Fig. 4F) were also similar (Fig. 4G). This finding confirmed that the critical phases of homing, survival, proliferation, and differentiation of aPKCζ- and aPKCλ-deficient HSC/P were preserved.
Finally, adhesion to the C terminus of fibronectin (CH-296), an important constituent of the BM microenvironment and migration toward CXCL12, a physiological chemoattractant, were similar for WT and aPKCζ- and aPKCλ-deficient HSC/Ps (Fig. 4 H and I). Collectively, these results indicate that aPKCζ and aPKCλ are dispensable for HSC/P trafficking within the hematopoietic microenvironment.
aPKCs Are Not Polarized in HSC and Deficiency of aPKCζ/λ Does Not Impair HSC Polarization In Contact with the Hematopoietic Microenvironment.
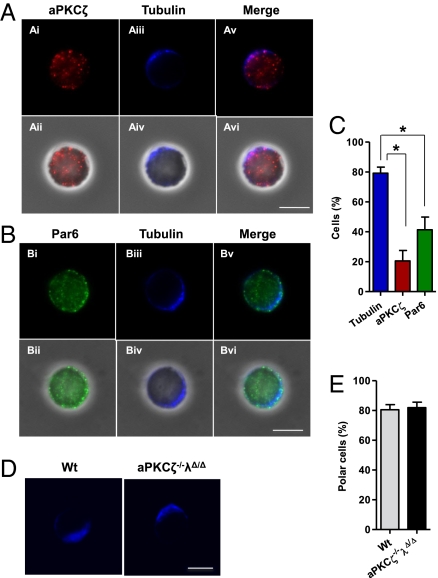
Our genetic data indicated that aPKCζ/λ does not play a functional role in the critical phases of mammalian HSC engraftment and hematopoiesis. We evaluated whether aPKCζ has a polarized pattern of distribution in the HSCs. In other model systems, asymmetric cell division is preceded by the activation of the aPKCζ-Par6-Par3 polarity complex and the realignment of the microtubule cytoskeleton (7, 11). We analyzed the distribution of aPKCζ and Par6 in WT HSCs in vitro after overnight culture on fibronectin, a major component of the hematopoietic microenvironment, which is responsible for integrin polarization and signaling (35). Although the intracellular localization of tubulin, a major constituent of the microtubular network, was highly polarized in isolated HSCs, which were in contact with fibronectin, the distribution of aPKCζ and Par6 was not proportional to the frequency of tubulin polarized HSCs (Fig. 5 A–C). In addition, the deficiency of aPKCζ/λ expression in HSCs did not impair microtubular network polarization (Fig. 5 D and E), indicating that aPKCζ/λ expression is dispensable in HSC polarization in the conditions tested. Together, these data indicate that aPKCζ/λ-downstream cell polarity signaling is dispensable in the determination of mammalian HSC activity and suggests that alternative molecular complexes are involved in stem cell asymmetry.
Fig. 5.
aPKCζ and Par6 are not polarized in HSC and aPKCs do not regulate HSC polarization. (A) Immunofluorescence pictures showing aPKCζ (red) along with tubulin (blue) localization in LT-HSCs. (Ai, Aii) aPKCζ is detected diffusely in the cell cytoplasm without any specific asymmetric distribution in most of LT-HSCs. (Aiii, Aiv) Most of LT-HSCs display a highly polarized distribution of the microtubule network. (Scale bar, 5 μm.) (B) Immunofluorescence pictures showing Par6 (green) along with tubulin (blue) localization in LT-HSCs. (Bi, Bii) Par6 is distributed throughout the cell body and beneath the cell membrane. Par6 localization is not polarized in most of LT-HSCs. (Biii, Biv) LT-HSCs display a highly polarized distribution of the microtubule network. (Scale bar, 5 μm.) (C) Bar diagram showing quantification of immunofluorescence analysis. Fifty to 100 LT-HSCs were singularly analyzed in each experiment (n = 4). Error bars represent SEM of the number of tubulin (79.7%), aPKCζ (21.53%), and Par6 (41.24%) polarized cells scored per sample. *P < 0.05. (D) Immunofluorescence pictures showing tubulin (blue) localization in LT-HSCs isolated from wild type or aPKCζ- and aPKCλ-deficient mice. Most of LT-HSCs display a highly polarized distribution of the microtubule network (n = 3–4 mice per group) (Scale bar, 5 μm.) (E) Bar diagram showing quantification of immunofluorescence analysis (n = 30–50 LT-HSCs from individual mouse). Error bars represent SD.
Discussion
We have explored whether aPKCζ and aPKCλ control HSC polarization in response to developmental and environmental cues, and if HSC polarization is required to produce appropriate numbers of stem cells and differentiated daughter cells. Previous reports using alternative RNA silencing or protein neutralization methods, which are not deprived of potential nontarget effects, suggested that the polarity-determining kinase aPKCζ, in particular, can positively regulate HSC activity (19, 20). To elucidate whether aPKCζ and aPKCλ play a role in HSC activity in vivo, we used combined constitutive, inducible, or tissue-specific gene deletion in vivo. A deficiency of aPKCζ, induced through targeted homologous recombination of embryonic stem cells, did not affect HSC activity and gross hematopoiesis in vivo, and there was no change in the frequency or content of HSC/P and colony-forming progenitors present in the BM. aPKCζ−/− HSCs showed normal survival and proliferation similar to the WT HSC in vivo, and competitive BM transplantation experiments suggested that HSC self-renewal and lineage repopulation ability in primary, secondary, and tertiary recipient mice was not altered because of deficiency of aPKCζ. aPKCζ was also dispensable in 5-FU–induced stressed hematopoiesis in vivo. These results indicate that aPKCζ does not play decisive role in murine HSC fate determination in vivo, contrary to previous reports (19, 20).
An alternative hypothesis was that aPKCλ might compensate for the loss of aPKCζ in vivo. Therefore, we determined the effect of a combined deletion of aPKCζ and aPKCλ in HSC in vivo. Because deletion of aPKCλ results in embryonic lethality (12), we generated mice that had constitutive hematopoietic-specific (Vav1-Cre) (26) and hematopoietic-inducible (Mx1-Cre) (23) deletion of aPKCλ. However, the loss of function of aPKCζ and aPKCλ, using Vav1-Cre and Mx1-Cre mice models, did not affect HSC activity during steady state and 5-FU–induced stressed hematopoiesis. Inducible genetic deletion of aPKCζ and aPKCλ in the adult hematopoietic system also did not affect HSC self-renewal and lineage repopulation activity. In addition, aPKCζ and aPKCλ were also dispensable for HSC/P homing, retention, adhesion, and migration, which are important parameters for HSC/P interaction within the hematopoietic microenvironment. Furthermore, neither a hematopoietic-specific deficiency of aPKCλ nor a combined deficiency of aPKCζ/λ affected embryonic lethality because of hematopoietic failure, and the aPKCζ/λ-deficient mice were born following a Mendelian ratio of inheritance. This finding is of interest because fetal hematopoiesis requires frequent cell cycling of HSCs and depends on a functionally different hematopoietic microenvironment compared with adult BM hematopoiesis (31, 32). Together, these data indicate that aPKCζ and aPKCλ do not regulate mammalian HSC activity in vivo.
HSC fate may depend on their polarized distribution, particularly when they are in contact within the BM stem-cell microenvironment. Unlike intestinal epithelial cells, which have distinct apical and basolateral cell surfaces, or intrinsically polarized neuronal cells, HSC polarization may not be morphologically apparent. Interestingly, in other systems, integrin-binding regulates cell polarization and determines cell division (11). In the BM, the vast majority of HSC are quiescent (32). We developed an in vitro assay where fibronectin-dependent forced microtubular network polarization could be efficiently assessed. In these conditions, our genetic models indicate that integrin-dependent binding of HSC to the hematopoietic microenvironment fails to polarize aPKCs and Par6, and a combined deficiency of aPKCζ and aPKCλ did not prevent polarization. This finding suggests that the existence of aPKCζ- and aPKCλ-dependent stem cell polarity is not a functional determinant for mammalian HSC activity. Conversely, aPKC signaling is implicated in the regulation of mammalian T-cell activity (12, 13), indicating that the contribution of aPKCζ/λ signaling in mammalian HSCs and in terminally differentiated cells is functionally distinguishable. These data underscore the importance of cell, tissue-microenvironment, and context-dependent regulation of evolutionary conserved signaling pathways.
Given that aPKCζ and aPKCλ are the core kinases within the cell-polarity complex in invertebrates and in other mammalian cells (7, 11, 36), it is an unexpectedly interesting finding that the activity of mammalian HSCs is not dependent on them, and may indicate the existence of an alternative signaling network defining HSC asymmetry with redundancy in mammalian stem cell polarity determination.
Materials and Methods
Information on animals, deletion of aPKCλ, LT-HSC repopulation, 5-FU administration may be found in SI Materials and Methods. For HSC/P assays, see SI Materials and Methods. RT-PCR analysis and statistical analysis are included in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. David Williams and Yi Zheng for critically reading this manuscript; the Cincinnati Children's Research Foundation Experimental Hematology and Cancer Biology Mouse, Flow Cytometry (NIH P30 DK090971), and Immunobiology Cores for services; and Margaret O'Leary for editing assistance. This study was supported in part by postdoctoral funding from The Institute of Cancer Research, United Kingdom/The Lady Tata Memorial Trust (to A.S.); and National Institutes of Health Grants R01-HL087159 (to J.A.C.), R01-AI072581 and R01-CA132847 (to J.M.), and R01-CA134530 (to M.D.-M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103132108/-/DCSupplemental.
References
- 1.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 2.Viloria-Petit AM, et al. A role for the TGFbeta-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci USA. 2009;106:14028–14033. doi: 10.1073/pnas.0906796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozdamar B, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 4.Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- 5.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Gönczy P. Mechanisms of asymmetric cell division: Flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- 7.Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol. 2003;15(1):67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 8.Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- 9.Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Ouyang Y, Somers WG, Chia W, Lu B. Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature. 2007;449(7158):96–100. doi: 10.1038/nature06056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang JQ, Leitges M, Duran A, Diaz-Meco MT, Moscat J. Loss of PKC lambda/iota impairs Th2 establishment and allergic airway inflammation in vivo. Proc Natl Acad Sci USA. 2009;106:1099–1104. doi: 10.1073/pnas.0805907106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 14.Smith CA, et al. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J. 2007;26:468–480. doi: 10.1038/sj.emboj.7601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell. 2008;135:161–173. doi: 10.1016/j.cell.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 2006;124:1241–1253. doi: 10.1016/j.cell.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Duncan AW, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 18.Wu M, et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1:541–554. doi: 10.1016/j.stem.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hope KJ, et al. An RNAi screen identifies Msi2 and Prox1 as having opposite roles in the regulation of hematopoietic stem cell activity. Cell Stem Cell. 2010;7(1):101–113. doi: 10.1016/j.stem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Petit I, et al. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest. 2005;115(1):168–176. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florian MC, Geiger H. Concise review: Polarity in stem cells, disease, and aging. Stem Cells. 2010;28:1623–1629. doi: 10.1002/stem.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitges M, et al. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 23.Cancelas JA, et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta A, Arnett J, Dunn S, Williams DA, Cancelas JA. Rac2 GTPase deficiency depletes BCR-ABL+ leukemic stem cells and progenitors in vivo. Blood. 2010;116:81–84. doi: 10.1182/blood-2009-10-247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daria D, et al. The retinoblastoma tumor suppressor is a critical intrinsic regulator for hematopoietic stem and progenitor cells under stress. Blood. 2008;111:1894–1902. doi: 10.1182/blood-2007-02-071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng CG, Weksberg DC, Taylor GA, Sher A, Goodell MA. The p47 GTPase Lrg-47 (Irgm1) links host defense and hematopoietic stem cell proliferation. Cell Stem Cell. 2008;2(1):83–89. doi: 10.1016/j.stem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghiaur G, et al. Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells. Blood. 2008;111:3313–3321. doi: 10.1182/blood-2007-08-110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan CT, McKearn JP, Lemischka IR. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990;61:953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- 32.Bowie MB, et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 34.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 35.Fournier-Thibault C, et al. Sonic hedgehog regulates integrin activity, cadherin contacts, and cell polarity to orchestrate neural tube morphogenesis. J Neurosci. 2009;29:12506–12520. doi: 10.1523/JNEUROSCI.2003-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.