Abstract
The majority of carbon sequestration at the Earth’s surface occurs in marine continental margin settings within fine-grained sediments whose mineral properties are a function of continental climatic conditions. We report very high mineral surface area (MSA) values of 300 and 570 m2 g in Late Cretaceous black shales from Ocean Drilling Program site 959 of the Deep Ivorian Basin that vary on subcentennial time scales corresponding with abrupt increases from approximately 3 to approximately 18% total organic carbon (TOC). The observed MSA changes with TOC across multiple scales of variability and on a sample-by-sample basis (centimeter scale), provides a rigorous test of a hypothesized influence on organic carbon burial by detrital clay mineral controlled MSA. Changes in TOC also correspond with geochemical and sedimentological evidence for water column anoxia. Bioturbated intervals show a lower organic carbon loading on mineral surface area of 0.1 mg-OC m-2 when compared to 0.4 mg-OC m-2 for laminated and sulfidic sediments. Although either anoxia or mineral surface protection may be capable of producing TOC of < 5%, when brought together they produced the very high TOC (10–18%) apparent in these sediments. This nonlinear response in carbon burial resulted from minor precession-driven changes of continental climate influencing clay mineral properties and runoff from the African continent. This study identifies a previously unrecognized land–sea connection among continental weathering, clay mineral production, and anoxia and a nonlinear effect on marine carbon sequestration during the Coniacian-Santonian Oceanic Anoxic Event 3 in the tropical eastern Atlantic.
Keywords: climate change, cretaceous ocean, source rocks, ocean dead zones
The burial of organic matter (OM) in sediments is a fundamental biogeochemical process, regulating among other things the CO2 balance of the ocean and atmosphere, and hence the temperature of the Earth’s surface. Although the dominant locus of OM burial is marine continental margin settings (1), the controls responsible for the observed variability of OM burial remain remarkably contentious with a strong bias toward oceanographic processes such as enhanced biological productivity (2–4) and exposure to oxidants (5–8). By contrast, some high-resolution continental margin marine records of organic carbon burial coupled to simulations argue for a more direct influence from continental and hydrological processes (3, 4, 9, 10). This is consistent with a growing body of evidence from modern environments identifying a strong control by terrestrial processes driven by freshwater, land-derived nutrients, and preservative effects of mineral surfaces (1, 11–14).
The relative contribution of the marine versus terrestrial influence is unresolved but likely holds the key to a more fundamental understanding of the underlying mechanisms and potential capacities of carbon sequestration in marine systems. The dynamic interaction among warming climate, changes in terrestrial runoff, and marine processes is evident today in the expansion of marine oxygen deficient (hypoxic) zones (e.g., ocean dead zones) (15). The relation among these influences has yet to be fully expressed in the present ocean, but the extent to which these processes can proceed is preserved in past records of greenhouse transitions. It is well known from these intervals that anomalous concentrations of organic carbon accumulated as black shale (5, 16) and were associated with massive disruption of marine ecosystems, ocean currents, marine nutrient, and element cycling leading to widespread oxygen deficiency within the water column, and global climate perturbations (17). Study of these records at sufficient temporal resolution can potentially provide insight into changes in marine carbon burial influence on CO2 sequestration and its feedback relevant to future climate.
Ancient organic rich deposits are commonly used to infer past oceanographic conditions (3–5); however, their composition is also determined by a series of mineral and diagenetic effects that complicate this inference (18, 19). Studies of modern sediment identify a dominance of particulate organic material at the sea floor (20). The origin and flux of these particles result from oceanographic conditions such as nutrient abundance. Much of this material, however, is remineralized within the first 10 cm of sediment with studies identifying < 10% of total organic carbon (TOC) present at 30 cm from the sea floor (6, 8, 12, 13). At this depth, TOC stabilizes and is comprised of > 90% OM that cannot be separated from the mineral matrix (1, 6, 11–13, 21). Because of its stability, this fraction is most likely to enter the geologic record (1, 12) and likely forms a critical component of ancient black shale (18). This is consistent with the OM found in most black shale, which occurs as an amorphous fraction requiring chemical separation from the mineral phase and contains only a minor particulate component (22).
The origin and paleoenvironmental implications of mineral associated OM in ancient and modern sediments are considerably less well understood than the more readily traceable particulate fraction. It is presently unclear how, when, and where organic compounds become physically or chemically associated with mineral surfaces (1) and what role the mineralogy of sediment and type of organic compounds play in what ultimately enters the sedimentary record. Although carbon isotope data indicate a dominantly marine origin for organic compounds associated with mineral surfaces, continental processes dictate the type (reactivity) and abundance of mineral surfaces, with subsequent modification of the mineral-OM fraction by diagenesis (1, 6, 8, 11–13, 21, 23, 24). Thus, this fraction may record significant biases induced by continental weathering and physical or chemical processes occurring between mineral surfaces and OM either in the water column or near-surface sediments that are independent of oceanographic processes influencing the particulate fraction.
The influence of oxidants on the mineral associated fraction of OM has also been less clearly demonstrated than it has on the particulate fraction. Deep sea sediments with long-term exposure to oxic waters have very low OM associated with a given mineral surface area (OM:MSA) implying that mineral surfaces can only slow remineralization when exposed to an unlimited oxidant supply (1). However, in continental margin settings where most carbon is buried, other factors may also be important in the mineral-stabilized portion of the sediment (6, 13). Although some studies show that OM loading on a given area of mineral surface is greater from within the oxygen minimum zone than from without (6, 8, 21), others do not (2, 11, 25). Variations in OM:MSA may alternatively be influenced by OM availability in ocean or pore water, reactivity of specific classes of organic compounds (26–29), sediment mineralogy (11, 13), and/or the effects of bioturbation (6, 7). The nanoscales at which mineral surfaces interact with OM make direct observation of the processes involved difficult; thus studies capable of isolating primary controls are important to understand better the dominant influences acting on this type of carbon burial, and thus the implications of organic carbon enrichment in ancient successions.
In this study we use high-resolution Cretaceous records from the equatorial African continental margin in order to identify the interplay between land and sea contributions to marine carbon burial under high pCO2 conditions. We specifically focus on abrupt, cyclical enrichments in organic carbon (3–16%) within Santonian continental margin sediments. These represent Oceanic Anoxic Event (OAE) 3 in which the relative influence of anoxia and terrestrial sediment control of MSA vary significantly in response to subtle natural shifts in central African climate during the interval associated with oceanic anoxic event III.
Results
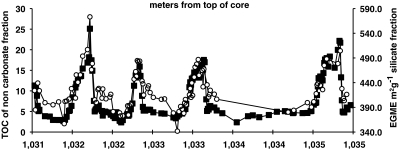
Centimeter-scale (equivalent to millennial-centennial scale) geochemical records employed on homogenous gray to black Cretaceous (Santonian) aged sediments from the tropical Deep Ivorian Basin (DIB) off west Africa [Ocean Drilling Program (ODP) site 959 (30)] reveal > 40 discrete meter-scale cycles of TOC that vary between approximately 3 and approximately 16% (31) (Fig. 1). Spectral analysis identifies a precessional timing (approximately 22 kys) of these cycles initiated with a stepwise rise to maximum TOC on cm scales (9).
Fig. 1.
TOC (squares) and MSA (circles) compared to distance below sea floor in ODP site 959 for four 22 ky cycles. MSA closely follows increase in TOC. Abrupt shifts in TOC from base level to > 20% occur over single samples, suggestive of a threshold. These results are presented for the siliciclastic portion of the sediment to remove the effects of dilution by other phases such as carbonate.
The recurring and systematic character of the TOC shifts argues for a single set of dominant controls that were capable of response on short time scales of probably centuries or less, and in a sufficiently delicate balance to be triggered by subtle differences in forcing from solar insolation. We expected that changes not directly (mechanistically) controlling OM preservation would fall out of phase with these clearly defined climate-driven geochemical cycles, whereas those that were directly involved would closely track the abrupt TOC changes in each cycle. Following the lead from studies of modern stabilized sediments, we hypothesized that a first-order relation between MSA and TOC (e.g., refs. 1, 12, 13, 21, and 32) would also occur in ancient successions and that the abrupt changes in TOC would provide a rigorous test of a mechanistic role MSA has on carbon preservation in the geologic record (18). Also following studies of modern sediments, we anticipated that OM:MSA should vary between oxic and suboxic conditions (6, 8). We thus focused on four of these meter-scale cycles to determine the detailed relation between redox conditions and changes in MSA through the sub-centimeter-scale steps in TOC evident within each cycle (Fig. 1).
To capture the frequency of change in the TOC record, MSA was measured at centimeter resolution in concert with evaluation of bioturbation, which was used as the primary evidence to determine the onset of strongly oxygen-depleted conditions in the benthic environment. We evaluated further redox chemistry using biomarker and trace metal data (33), though the biomarker measurements were at a lower resolution (meter scale) and used to determine the extremity of seawater redox conditions and not the rate of change relative to TOC.
We further refined our hypothesis to test for the influence of the smectitic class of hydratable 2∶1 clay minerals rather than fine-grained sediments in general because recent work shows that these minerals may be responsible for a significant enhancement in carbon preservation (11, 18, 19, 26, 27, 34). This may be because of their uniquely weak layer charge that allows organic compounds to access protective interlayer sites (34–36) and/or that they may coalesce around organic compounds from initially dispersed single layer crystallites [quasicrystal model (37)] in pore fluids to form nanocomposites. To test the role of smectite, we used the ethylene glycol monoethyl ether (EGME) method to measure MSA (see SI Text). Because the EGME method includes the interlayer surface area of smectitic clays as well as the external surface area, it provides a total surface area determination. This is in contrast to typical MSA measurements that determine only external surface and a minor portion of interlayer area using N2 adsorption with the assumption that interlayer sites are inaccessible to organic compounds (12, 13). A diagnostic property of smectite compared to the 2∶1 clay minerals with stronger layer charge such as illite is penetration of these interlayer sites by the polar ethylene glycol molecule within EGME, providing surface area that is an order of magnitude greater (approximately 750 m2 g) than illite (< 90 m2 g) or kaolinite (20 m2/g), and two orders greater than quartz silt (< 10 m2 g) or carbonate (< 13 m2 g) (38) no matter how finely ground (11). When smectite is present, changes in its abundance thus control total MSA determination because of this great disparity in surface area. The EGME method also provides an approximation of a single molecular monolayer coating of ethylene glycol on mineral surfaces when in equilibrium with an EGME vapor (39). We use this molecular monolayer coating to compare with the OM:MSA in our natural samples deposited under varying redox conditions (Fig. 2B).
Fig. 2.
(A) MSA before and after removal of OM by six bleach treatments (method discussed in SI Text). This relation that does not show a loss of MSA indicates that the EGME is not dissolving or interacting with kerogen within these samples. The slight gain in MSA may be attributed to the opening of pore space by OM removal. (B) TOC cross-plotted with MSA shows three distinct groupings of data coinciding with bioturbated, mixed bioturbated, and laminated and laminated intervals. Identified loading rate for each grouping is calculated using a regression within the group population (gray lines) with an overall relationship of r2 = 0.76, R2 = 0.43 (oxic facies), R2 = 0.58 (mixed facies), and R2 = 0.66 (reducing facies). The monolayer equivalent line was identified by measuring weight addition of ethylene glycol by a series of standards exposed to EGME (see text); it is included to show a reference line only and does not imply that OM in natural samples occurs in a monolayer coating. Pierre Shale samples from ref. 18 and modern clay size fraction separated from sea floor sediments from ref. 13.
MSA and TOC.
Our results (Fig. 1) identify a consistent and positive TOC to MSA relationship. Notably, shifts in TOC coincide with MSA even where TOC values increase up to 16% from one centimeter-spaced sample to the next. TOC and MSA are also related in second-order TOC variations of several percent superimposed on the major peaks. The very high 300 and 570 m2 g MSA values can result only from variations of smectite content, estimated to comprise between 30 and 60% of the noncarbonate fraction. We eliminated the possibility that EGME interacted with OM within the samples by demonstrating on a parallel split of samples with OM removed that MSA stayed the same or slightly increased with the loss of pore or interlayer occluding OM (Fig. 2A). Smectite presence was also independently confirmed using X-ray diffraction by showing broad peaks at approximately 6° two theta characteristic of mixed layer illite–smectite with likely inclusion of OM compounds in interlayer spaces (SI Text).
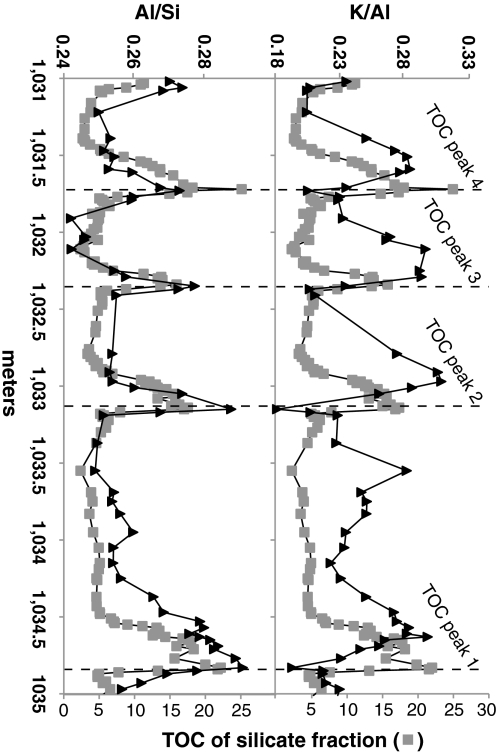
The Al/Si record provides further support for the association of clay minerals with OM in these sediments (Fig. 3) by indicating an increase of clay minerals relative to quartz and feldspar within each cycle of TOC enrichment. In contrast, K/Al is out of phase with TOC and MSA, reaching a minimum where these other measures reach a maximum (Fig. 3). K/Al records the relative amount of illitic layers in mixed layer smectite/illite, discrete illite, mica minerals, and feldspars relative to smectite (that has little K) and can change with weathering intensity independently from source clay compositional effects. Because multiple mineral phases contribute to K/Al and Al/Si, this relation is not as consistent as MSA, but it does indicate that nonexpandable illite and mica are more prevalent in the low TOC samples and that a mineralogical shift coincides with TOC cycles.
Fig. 3.
Elemental ratios of Al/Si and K/Al (31) compared to the TOC record against stratigraphic height. For Al/Si versus TOC R2 = 0.50. K/Al versus TOC shows no relationship. The Al/Si is used as a broad proxy for phyllosilicate to tectosilicates, whereas K/Al is used as a proxy for relative illite to smectite interlayers. Both ratios are also influenced by clay mineralogy, feldspar composition, and intensity of weathering alteration and are thus approximations.
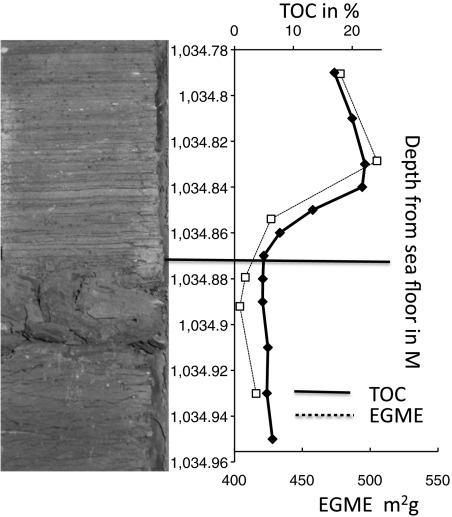
The abrupt shifts in smectite abundance are attributed to environmental changes rather than repetitive meter-scale depositional beds or current winnowing based on sedimentological evidence. The grain size remains constant through each cycle, and there is no evidence of erosional scours or traction, indicating that these finely laminated sediments are pelagic or hemipelagic (14) (Fig. 4). ODP site 959 was located on the seaward slope of the DIB in order to capture a pelagic record and distance it from continentally derived sediment gravity flows recorded on the opposite (landward) side of the basin (30). Mineralogical changes closely timed with changes in water column redox are consistent with previous studies interpreting changing paleoenvionmental conditions rather than dilution of OM by coarser clastic material in these sediments (9, 31, 33).
Fig. 4.
Core photograph from ODP site 959 showing the abrupt transition in TOC and MSA of the siliciclastic fraction compared to the evident bioturbated and laminated intervals. The laminated intervals are interpreted as the onset of anoxia and precede the rise in TOC and MSA.
Modern (stabilized) sediments record a consistent OM:MSA between 0.21 and 1 mg-OC m-2, and the calculated monolayer coating of organic compounds is approximately 0.86 mg-OC m-2 (1, 6, 12, 13). This range is consistent with other findings indicating that mineral surfaces have a more heterogeneous distribution of OM than a simple monolayer coating (e.g., ref. 40). The OM:MSA, however, potentially provides a useful reference for comparing the effects of different depositional settings on OM loading. Here we use a monolayer equivalent line for comparison to other studies, but do not intend to imply that natural OM forms a monolayer coating with clay mineral surfaces in these samples. Previously, this ratio has been determined by measuring MSA withN2 adsorption method (previously discussed) that cannot be used to determine accurately the hydratable interlayer sites of smectite critical to our hypothesis. BET data are thus not directly comparable to the total MSA determination reported here, and so we experimentally determined a “molecular monolayer” loading using ethylene glycol for total mineral surface area by exposing three smectitic clay standards, kaolinite and quartz (Fig. 2B) to EGME vapor (39). A regression was calculated from the gain in mass of a single molecular coating of ethylene glycol on MSA compared to the mass of the bulk sample for the suite of standards and a slope of 0.32 mg-OC m-2 identified as the “monolayer equivalent line” (MEL) (Fig. 2B).
ODP site 959 samples showed three distributions with respect to the MEL. Bioturbated samples fell well below the MEL and showed a similar slope from a linear regression calculated within the group that has a nonzero MSA intercept (Fig. 2B). Mixed laminated and bioturbated sediments plot close to the MEL and share a similar regression to the bioturbated samples and MEL. Laminated sediments with TOC values > 15% plot above the monolayer line with a higher OM:MSA. These three groups occur within stratigraphically distinct intervals of the TOC cycles, with the lower group precisely coinciding with the sharp break from the bioturbated (oxic) sediments (Fig. 4), the middle group comprised of the transition sediments showing intermittent bioturbation, and the high group occupying the laminated portions at the crest of the TOC and MSA cycles (Fig. 2B).
Redox and TOC.
Evidence for water column anoxia from laminated sediments, biomarkers, and redox element enrichments indicate periodic photic zone euxinia (33) that broadly coincided with TOC peaks (Fig. 1). Laminated samples comprising these peaks show a greater OM:MSA determined from a regression (0.7 mg-OC m-2) (Fig. 2B) than mixed laminated and bioturbated samples falling along the MEL (0.4 mg-OC m-2). The average carbon loading on mineral surface area is greater in the laminated (0.4 mg-OC m-2) and transition sediments (0.3 mg-OC m-2) than those in bioturbated intervals (0.1 mg-OC m-2).
Although redox conditions likely influenced the organic carbon loading of mineral surfaces, euxinia/anoxia alone cannot uniquely explain the exceptionally high TOC values or patterns of change. The recurring abrupt change from bioturbated to laminated sediment precedes the rise in TOC in each cycle studied (Fig. 4). Because redox is independent of MSA, it cannot account for the positive relation between MSA and TOC (Fig. 1), nor the terrestrial proxy indicators like Al/Si (Fig. 3) that match the abrupt rise in TOC in each geochemical cycle (31).
A primarily marine source for organic matter rather than terrigenous organic matter simply traveling with detrital clays is indicated by a strong positive relation between hydrocarbon yields (S2) and TOC (r = 0.97, n = 530) (14), a hydrogen index of between 400 and 720 mgHC/gTOC, a limited range of δ13C of bulk OM (-26.5‰ to -28‰), and a dominance of marine biomarkers (33). The mineral matrix shows strong fluorescence, which is common in clay-rich sediments (22) and is consistent with a dominance of amorphous OM (9). A particulate component comprised of algal cysts and limited occurrence of finely dispersed woody fragments is present, but comprises only a very minor fraction of the bulk TOC (14). These observations do not allow us to identify the site(s) where mineral surfaces and organic matter come into association, though OM and clay-rich nepheloid layers near the sea floor or pore fluids derived from partially remineralized particulate material seem most likely.
Discussion
These previously undescribed data show that not only is the seemingly homogenous mudstone interval comprising OAE 3 at ODP site 959 comprised of a surprisingly complex, and highly variable geochemical record of carbon burial (9), but variations in TOC directly coincide with changes in land-derived smectite and that the mineral surface-OM relation directly interacts with redox conditions. The meter to centimeter scale of variability with direct links to continental processes identifies a much more dynamic and land-connected system for carbon burial than the persistent and basin-wide condition of anoxia commonly considered for OAE 3 (16). We argue that the changes in MSA with TOC across multiple scales of variability, a broad range of TOC from 2–20% on a sample by sample centimeter scale provides a rigorous test of the hypothesized influence on TOC by MSA.
We propose that as in some modern (stabilized) sediments, that variations in water column redox conditions significantly modified the average amount of organic carbon associated with mineral surfaces imparting a primary oceanographic signal within these sediments. Water column anoxia was a recurring condition during supersource rock (TOC > 10%) formation phases and the excess of OM beyond a molecular monolayer ratio suggests physiochemical changes associated with strongly reducing and possibly sulfidic conditions. Potential mechanisms of increased carbon loading include redox modification of chemical sorption reactions (41), differences in organic compound concentration (21, 29, 42) and reactivity (7), polymerization of organic compounds facilitated by or on mineral surfaces (23), vulcanization reactions (1), or delamination of clay fundamental particles followed by later reformation of clay–organic nanocomposites (19, 21, 37).
These extremely organic carbon-rich samples show a roughly similar range in OM:MSA to modern stabilized sediments when interlayer sites of hydratable clay minerals are included (Fig. 2B). They are also consistent with data reported from the Cretaceous North American Western Interior Seaway (18). Contrary to modern sediments, however, regressions from the ancient examples show a positive MSA intercept at zero TOC (Fig. 2B) implying MSA that did not acquire OM or that subsequently lost OM. This is particularly evident for samples from oxic intervals, which also show conspicuously less OM than the intermittent suboxic and anoxic groupings. aAlthough changes in composition or concentration of OM in oxic waters could cause less carbon to be adsorbed to mineral surfaces, it neither explains the positive MSA intercept (Fig. 2B) nor is it likely to result in an OM:MSA so close to the slope of the monolayer regression. This curious similarity of slope in conjunction with a positive X-axis intercept in Fig. 2B implies the retention of a consistent amount of OM on each increment of mineral surface, but on only a portion of each sample. We prefer to interpret the “missing OM” implied by the positive X-axis intercept as OM that was less effectively shielded from oxidation by repetitive introduction of sea water or destruction of clay mineral or aggregate structure induced by bioturbation (7, 41). The retention of a similar rate of change of OM to MSA with the MEL and other groups, however, implies retention of a more refractory portion of mineral-associated OM. We speculate that this OM (ranging between 2 and 6% TOC) is located within interlayer sites, whereas the more readily oxidized fraction of OM was associated with clay mineral external surfaces and edges that can exceed 200 m2/g in smectitic sediments (43).
ODP site 959 trends indicate that smectite acts to preserve significant concentrations of OM in sediments, even in oxic intervals (3–6% TOC), but that supersource rock formation (> 15% TOC) only resulted when water column anoxia facilitated a higher organic carbon loading and retention on mineral surfaces. Although the drivers of anoxia are not immediately apparent in the DIB (9), the systematic lag between onset of anoxic conditions and an increase in smectite within precession-driven cycles points to an influence by terrestrial climate through runoff effects on the expansion of an oxygen minimum and supply of clay minerals. We consequently propose that supercarbon depositional events can be triggered when a background of smectite-rich sediment intersects expanding regions of suboxic waters.
The presence of smectite within this succession not only provides specific favorable mineralogical properties for carbon preservation, but its abundance is a function of specific climate conditions on the continents, directly linking continental climate change and surface weathering with marine carbon burial. Detrital clay minerals present in marine sediments are almost exclusively formed by precipitation in soils or by weathering of continental volcanic rocks. They are then exported to marine sediments via riverine erosion. We conclude that the very high MSA of the TOC-rich intervals in ODP 959 can occur only with a significant fraction of smectite. The similar ratio of OM:MSA between these high TOC sediments and similar modern (stabilized) continental margin sediments support an active role of smectite mineralogy for carbon sequestration. Because smectite is the product of a seasonal climate, small changes in continental climate that are capable of expanding this seasonal belt or placing a consistent flux of sediments in contact with expanding anoxic conditions can potentially trigger carbon burial events, amplifying an initial climate perturbation.
Where changes in continental and oceanographic conditions lead to the overlap of these two critical factors, strongly enhanced OM burial and related CO2 sequestration results in a negative feedback to global warming. The degree to which this process impacted regional and global climate depends on how widespread this overlap region was that developed along the margins of the tropical and subtropical ocean. In the case of Cretaceous tropical Africa, we surmise that subtle insolation-driven changes periodically shifted the intertropical convergence zone (ITCZ) (e.g., refs. 31 and 44), increasing rainfall and eroding formerly seasonally influenced and stable soils that formed smectite. Although this process would otherwise simply move the seasonal belt south for no net carbon burial increase in the ocean, in the case of the DIB it resulted in anomalous carbon burial because of the proposed mechanism, i.e., detrital smectite encountering an expanding zone of anoxic waters that formed in response to enhanced nutrient and freshwater runoff from tropical Africa in an overall warm equatorial ocean during Cretaceous greenhouse conditions.
The data in Fig. 1 confirm exceptional changes between two very different depositional modes over millimeter thicknesses implying very rapid response rates, likely on decadal time scales. The rapidity of onset and the profoundness of change recorded in the Cretaceous sediments strongly argue for repeated crossing of a threshold created by the coincidence of smectite deposition and anoxia in the DIB. These conditions were triggered by a minor increase in climate forcing from solar insolation related to precession that, in turn, shifted the position of the ITCZ to alter the regional hydrological cycle (44) in tropical/subtropical Africa.
Increasing depth and width of the oxygen minimum zone (“ocean dead zones”) forming in response to present global warming in low latitude marine regions (15, 45) may push environmental conditions closer to those identified in ODP 959. We can only speculate how close we are to a similar threshold given the much stronger CO2 forcing anticipated in this century, and where and over what area it may occur. Although supercarbon burial as observed in the Cretaceous Equatorial Atlantic is not significant today, we argue that as modern low latitude hypoxic areas expand with warming into areas of present smectite-rich mud deposition, the system may cross a threshold similar to the one observed in ODP site 959.
Materials and Methods
We used a standard polar molecule addition method (EGME) for mineral surface area determination following ref. 39. Details of application of this method to determine surface area of ancient sediments are addressed in SI Text and in ref. 18. In order to assure that organic matter did not dissolve or otherwise interact with EGME, we removed OM from samples split from the original powders and then compared MSA measurements determined on each split; details are provided in SI Text and results are in Fig. 2A.
Supplementary Material
Acknowledgments.
Analysis was assisted by Thomas Bristow and Keith Morrison. TRAX record 158. Funding supporting this work included NSF-OCE 0926953, ARC-DP110104367, Royal Society–Wolfston Research Merit award and a visiting fellowship to the University of Newcastle (to M.J.K.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. L.D. is a guest editor invited by the Editorial Board.
See Commentary on page 9729.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018670108/-/DCSupplemental.
References
- 1.Hedges JI, Keil RG. Sedimentary organic matter preservation: An assessment and speculative synthesis. Mar Chem. 1995;49:81–115. [Google Scholar]
- 2.Ganeshram RS, Calvert SE, Pedersen TF, Cowie GL. Factors controlling the burial of organic carbon in laminated and bioturbated sediments off NW Mexico: Implications for hydrocarbon preservation. Geochim Cosmochim Acta. 1999;63:1723–1724. [Google Scholar]
- 3.Mort H, et al. Phosphorus and the roles of productivity and nutrient recycling during oceanic anoxic event 2. Geology. 2007;35:483–486. [Google Scholar]
- 4.Nederbragt A, Thurow J, Vonhof H, Brumsack HJ. Modelling oceanic carbon and phosphorus fluxes: Implications for the cause of the late Cenomanian Oceanic Anoxic Event (OAE2) J Geol Soc London. 2004;161:721–728. [Google Scholar]
- 5.Arthur MA, Sageman BB. Marine black shales—Depositional mechanisms and environments of ancient deposits. Ann Rev Earth Pl Sci. 1994;22:499–551. [Google Scholar]
- 6.Hedges JI, et al. Sedimentary organic matter preservation: A test for selective degradation under oxic conditions. Am J Sci. 1999;299:529–555. [Google Scholar]
- 7.Hulthe G, Hulth S, Hall PO. Effect of oxygen on degradation rate of refractory and labile organic matter in continental margin sediments. Geochim Cosmochim Acta. 1998;62:1319–1328. [Google Scholar]
- 8.Keil JI, Cowie GL. Organic matter preservation through the oxygen-deficient zone of the NE Arabian Sea as discerned by organic carbon:mineral surface area ratios. Mar Geol. 1999;161:13–22. [Google Scholar]
- 9.Beckmann B, Flögel S, Hofmann P, Wagner T. Mid-Cretaceous African climate development and implications for the marine carbon cycle. Nature. 2005;437:241–244. doi: 10.1038/nature03976. [DOI] [PubMed] [Google Scholar]
- 10.Galy V, et al. Efficient organic carbon biurial in the Bengal fan sustained by the Himalayan erosional system. Nature. 2007;450:407–411. doi: 10.1038/nature06273. [DOI] [PubMed] [Google Scholar]
- 11.Ranson B, Kim D, Kastner M, Wainwright S. Organic matter preservation on continental slopes: Importance of mineralogy and surface area. Geochim Cosmochim Acta. 1998;62:1329–1345. [Google Scholar]
- 12.Mayer LM. Surface area control of organic carbon accumulation in continental shelf sediments. Geochim Cosmochim Acta. 1994;58:1271–1284. [Google Scholar]
- 13.Keil RG, Tsmakis E, Fuh C, Giddings C, Hedges JI. Mineralogic and textural controls on the organic composition of coastal marine sediments: Hydrodynamic separation using SPLITT-fractionation. Geochim Cosmochim Acta. 1994;58:879–893. [Google Scholar]
- 14.Beckmann B, Hofmann P, Wagner T. Linking Coniacian-Santonian (OAE3) black shale formation to African climate variability: A reference section from the eastern tropical Atlantic at orbital time scales (ODP site 959, off Ivory Coast/Ghana) In: Harris N, editor. Source Rock Development: Bioproductivity, Organic Preservation, or Sedimentation Rate? Vol 82. Tulsa, OK: Society for Sedimentary Geology; 2005. pp. 125–144. [Google Scholar]
- 15.Rabalais NN, Turner RE, Diaz RJ, Jusrtic D. Global change and eutrophication of coastal waters. ICES J Mar Sci. 2009;66:1528–1537. [Google Scholar]
- 16.Negri A, Ferretti A, Wagner T, Meyers PA. Phanerozoic organic-carbon-rich marine sediments: Overview and future research challenges. Palaeogeogr Palaeoclimatol Palaeoecol. 2009;273:218–227. [Google Scholar]
- 17.Jenkyns HC. Evidence for rapid climate change in the Mesozoic-Palaeogene greenhouse world. Philos Trans R Soc Lond A. 2003;361:1885–1916. doi: 10.1098/rsta.2003.1240. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy MJ, Pevear DR, Hill RJ. Mineral surface control of organic carbon in black shale. Science. 2002;295:657–660. doi: 10.1126/science.1066611. [DOI] [PubMed] [Google Scholar]
- 19.Salmon V, Derenne S, Lallier-Verges E, Largeau C, Beaudoin B. Protection of organic matter by mineral matrix in a Cenomanian black shale. Org Geochem. 2000;31:463–474. [Google Scholar]
- 20.Hartnett HE, Keil JI, Devol AH. Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature. 1998;391:572–574. [Google Scholar]
- 21.Arnarson T, Keil JI. Changes in organic matter-mineral interactions for marine sediments with varying oxygen exposure times. Geochim Cosmochim Acta. 2007;71:3545–3556. [Google Scholar]
- 22.Littke R, Baker DR, Rullkötter J. Deposition of petroleum source rocks. In: Welte DH, Horsfield B, Baker DR, editors. Petroleum and Basin Evolution. Berlin: Springer; 1997. pp. 271–334. [Google Scholar]
- 23.Collins MJ, Bishop AN, Farrimond P. Sorption by mineral surfaces: Rebirth of the classical condensation pathway for kerogen formation? Geochim Cosmochim Acta. 1995;59:2387–2391. [Google Scholar]
- 24.Huguet C, et al. Selective preservation of soil organic matter in oxidized marine sediments (Madeira Abyssal Plain) Geochim Cosmochim Acta. 2008;72:6061–6068. [Google Scholar]
- 25.Isaacs C. Depositional framework of the Monetery Formation, California. In: Isaacs C, RullKotter J, editors. The Monterey Formation: From Rocks to Molecules. New York: Columbia Univ Press; 2001. pp. 31–59. [Google Scholar]
- 26.Satterberg J, Arnarson T, Lessard E, Keil RG. Sorption organic matter from the four phytoplankton species to montmorillonite, chlorite, and kaolinite in seawater. Mar Chem. 2003;81:11–18. [Google Scholar]
- 27.Ding X, Henrichs SM. Adsorption and desporption of proteins and polyamino acids by clay minerals and marine sediments. Mar Chem. 2002;77:225–237. [Google Scholar]
- 28.Meyers PA, Quinn JG. Fatty acid-clay mineral assocaition in artificial and natural sea water solutions. Geochim Cosmochim Acta. 1971;35:628–632. [Google Scholar]
- 29.Alperin MJ, et al. Benthic fluxes and porewater conentration profiles of dissolved organic carbon in sediments from the North Carolina continental slope. Geochim Cosmochim Acta. 1999;63:427–448. [Google Scholar]
- 30.Mascle J, Lohmann GP, editors. Shipboard-Scientific-Party. Proceedings of the Ocean Drilling Program, Initial Reports. Vol. 159. College Station, TX: Ocean Drilling Program; 1996. pp. 65–150. [Google Scholar]
- 31.Hofmann P, Beckmann B, Wagner T. A millenial to centennial-scale record of African climate variability and organic carbon accumulation in the Coniacian-Santonian eastern tropical Atlantic (ODP site 959, off Ivory Coast/Ghana) Geology. 2003;31:135–138. [Google Scholar]
- 32.Keil RG, Montlucon DB, Prahl FG, Hedges JI. Sorptive preservation of labile organic material in marine sediments. Nature. 1994;370:549–552. [Google Scholar]
- 33.Wagner T, Sinninghe Damsté J, Beckmann B, Hofmann P. Euxinia and primary production in Upper Cretaceous eastern equatorial Atlantic surface waters fostered orbital-driven formation of marine black shales. Paleoceanography. 2004;19:PA3009. 10.1029/2003PA000898. [Google Scholar]
- 34.Williams LB, Canfield B, Voglesonger KM, Holloway JR. Organic molecules formed in a “primordial Womb”. Geology. 2005;33:913–916. [Google Scholar]
- 35.Sposito G, et al. Surface geochemistry of the clay minerals. Proc Natl Acad of Sci USA. 1999;96:3358–3364. doi: 10.1073/pnas.96.7.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theng BKG, Churchman GJ, Newman RH. The occurrence of interlayer clay-organic complexes in two New Zealand soils. Soil Sci. 1986;142:262–266. [Google Scholar]
- 37.Nadeau PH, Wilson MH, McHardy WJ, Tait JM. Interstratified clays as fundamental particles. Science. 1984;225:923–925. doi: 10.1126/science.225.4665.923. [DOI] [PubMed] [Google Scholar]
- 38.Suess E. Interaction of organic compounds with calcium carbonate-II. Organo-carbonate association in recent sediments. Geochim Cosmochim Acta. 1973;37:2435–2447. [Google Scholar]
- 39.Cihacek LJ, Bremner JM. A simplified ethylene glycol monoethyl ether procedure for assessment of soil surface area. Soil Sci Soc Am J. 1979;43:821–822. [Google Scholar]
- 40.Ransom B, Bennett RH, Baerwald R, Shea K. TEM study of in situ organic matter on continental margins: Occurrence and the “monolayer” hypothesis. Mar Geol. 1997;138:1–9. [Google Scholar]
- 41.Zhu Q, Aller RC, Fan Y. Two-dimensional pH distributions and dynamics in bioturbated marine sediments. Geochim Cosmochim Acta. 2006;70:4933–4949. [Google Scholar]
- 42.Henrichs SM. Early diagenesis of organic matter in marine sediments: Progress and perplexity. Mar Chem. 1992;39:119–149. [Google Scholar]
- 43.Churchman GJ, et al. Geotechnical properties indicating environmental uses for an unusal Australian bentonite. Appl Clay Sci. 2002;20:199–209. [Google Scholar]
- 44.Flögel S, Wagner T. Linking Mid-Cretaceous global climate modeling and geological marine proxy records—Insights to insolation-driven coupling of atmospheric circulation and ocean properties. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;235:288–304. [Google Scholar]
- 45.Stramma L, Johnson GC, Sprintall J, Mohrholz V. Expanding oxygen-minimum zones in the tropical oceans. Science. 2008;320:655–658. doi: 10.1126/science.1153847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.