Abstract
Habitat loss and disease are main drivers of global amphibian declines, yet the interaction between them remains largely unexplored. Here we show that paradoxically, habitat loss is negatively associated with occurrence, prevalence, and infection intensity of the chytrid fungus Batrachochytrium dendrobatidis (Bd) in amphibian populations in the tropics. At a large spatial scale, increased habitat loss predicted lower disease risk in amphibian populations across Costa Rica and eastern Australia, even after jointly considering the effect of potential biotic and abiotic correlates. Lower host-species richness and suboptimal microclimates for Bd in disturbed habitats are potential mechanisms underlying this pattern. Furthermore, we found that anthropogenic deforestation practices biased to lowlands and natural vegetation remaining in inaccessible highlands explain increased Bd occurrence at higher elevations. At a smaller spatial scale, holding constant elevation, latitude, and macroclimate, we also found a negative relationship between habitat loss, and both Bd prevalence and infection intensity in frog populations in two landscapes of the Brazilian Atlantic Forest. Our results indicate that amphibians will be disproportionately affected by emerging diseases in pristine environments, and that, paradoxically, disturbed habitats may act as shelters from disease, but only for the very few species that can tolerate deforestation. Thus, tropical amphibian faunas are threatened both by destruction of natural habitats as well as increased disease in pristine forests. To curb further extinctions and develop effective mitigation and restoration programs we must look to interactions between habitat loss and disease, the two main factors at the root of global amphibian declines.
Keywords: chytridiomycosis, species diversity, anurans, Neotropics, pathogen
Habitat loss and chytridiomycosis, a disease caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd), are two main causes of global amphibian declines (1–4). Habitat loss lowers amphibian species diversity by reducing natural habitats (1) and increasing population isolation (5), inbreeding (6), edge effects (7), and discontinuity between terrestrial and aquatic habitats (8). Disturbance to natural vegetation also changes ecosystem structure, shifting macro (9) and microclimates (10), and altering hydrological cycles (11). Thus, habitat loss may also influence amphibian susceptibility to disease by altering host-community structure, transmission pathways, and pathogen persistence and virulence (12). Bd is a water-borne epidermal pathogen with a broad host range among amphibians (13) and has been implicated in population declines and species extinctions worldwide (3, 4, 14–16). Bd prevalence and infection intensity are important predictors of disease risk and population die-offs (17) and, to some extent, can be modified by environmental factors. Bd prevalence varies with latitude (18), elevation (19), precipitation (18, 20, 21), and temperature (18, 20), presumably reflecting Bd optimal growth conditions (22, 23).
The most severe amphibian declines and extinctions have been observed in the Neotropics (15) and Australia (14). In both regions, Bd outbreaks occur primarily in pristine forests at high-elevation mountainous sites, such as the Talamancas in Central America (15, 24), the Tropical Andes in South America (15), and the Great Dividing Range in eastern Australia (25). In contrast, pathogen prevalence is lower and amphibian populations often remain stable at lowland sites (15, 19, 25). Because Bd optimal growth occurs at mild temperatures (17–25 °C) and high humidity (22, 23), these regional patterns of declines could result from the interaction among elevation, climate, and also habitat loss, all of which are potentially correlated. Temperature is negatively correlated with elevation. Elevation and habitat loss are often negatively correlated because of anthropogenic deforestation practices that are biased to the flatter and more accessible lowlands (26). Finally, tropical habitat loss increases average temperatures and may change precipitation patterns (11). In the case of Bd, most empirical studies have focused on the interaction of only two of these three critical components, elevation and climate, eclipsing the potential role of habitat loss and its cascading biotic and abiotic consequences on Bd epidemiology at both large and small spatial scales.
Here, we examined the effect of habitat loss on Bd infections in tropical amphibian populations at both large and small spatial scales. We hypothesized that habitat loss is negatively associated with Bd infections because of lower host-species richness and potentially suboptimal microclimate for Bd in disturbed habitats. First, we analyzed published surveys of Bd infections of Rain frog populations (Craugastor fitzingeri) in Costa Rica (20), and Stony Creek frog populations (Litoria lesueuri) in eastern Australia (18). For each sampling site we quantified the degree of habitat loss, measured as the percentage of nonnatural vegetation cover within 1-km buffers surrounding sampling locations. We tested the effect of habitat loss on pathogen occurrence, prevalence, and infection intensity using spatial autoregressions and path analysis, and accounted for potential effects of host-species richness, latitude, elevation, and bioclimatic metrics of temperature and precipitation. In a second study, replicated in two landscapes of the Brazilian Atlantic Forest, we compared Bd prevalence and infection intensity in populations of the Golden Lesser treefrog (Dendropsophus minutus) among sites with varying levels of habitat loss. At this smaller scale, we controlled for the effects of environmental factors by sampling populations with similar climate, elevation, and latitude, and used spatial autoregressions to test the relationship of habitat loss with Bd prevalence and infection intensity.
Results
Habitat Loss Predicts Bd Infections in Costa Rica and Australia.
Increased habitat loss predicted lower Bd occurrence in amphibian populations in Costa Rica (βAUTOLOG = −0.022, t = −1.953, P = 0.051). Our initial stepwise screening for explanatory variables selected habitat loss, amphibian species richness, elevation, temperature annual range, and precipitation of the driest quarter of the year as variables with high scores for explaining Bd occurrence. When jointly considering these effects in a model selection approach, including all possible models, habitat loss remained a strong predictor of pathogen occurrence (Table 1 and Table S1). In the best model, we found a negative effect of habitat loss and a positive effect of species richness on Bd occurrence.
Table 1.
Autologistic and conditional autoregressive models testing simultaneously the effects of habitat loss, amphibian species richness, and environmental factors on Bd occurrence in amphibian populations in Costa Rica, and on Bd prevalence and infection intensity in amphibian populations in Australia
| Term | βautolog/βcar | Std. coeff. | SE | t | VIF | P |
| Occurrence | ||||||
| Constant | −6.311 | 0 | 2.033 | −3.104 | — | 0.002 |
| Spatial autocovariate term - yW | 3.138 | 0.879 | 3.075 | 1.020 | — | 0.308 |
| i) Habitat loss | −0.030 | −3.769 | 0.012 | −2.430 | 1.020 | 0.015 |
| ii) Amphibian species richness | 0.098 | 4.224 | 0.042 | 2.318 | 1.020 | 0.020 |
| Prevalence | ||||||
| Constant | −15.980 | 0 | 33.360 | −0.479 | — | 0.636 |
| i) Habitat loss | −0.304 | −0.420 | 0.113 | −2.695 | 1.279 | 0.012 |
| ii) Amphibian species richness | 1.414 | 0.466 | 0.658 | 2.148 | 2.059 | 0.042 |
| iii) Latitude and maximum temperature warmest month PC1* | −36.959 | −2.179 | 11.699 | −3.159 | 2.470 | 0.004 |
| ii × iii | 0.796 | 1.439 | 0.390 | 2.039 | 1.355 | 0.052 |
| Infection intensity | ||||||
| Constant | 0.555 | 0 | 1.439 | 0.386 | — | 0.703 |
| i) Habitat loss | −0.017 | −0.416 | 0.006 | −2.626 | 1.025 | 0.014 |
| ii) Precipitation of the driest month | 0.043 | 0.544 | 0.011 | 4.001 | 1.025 | <0.001 |
Whole-model tests: Occurrence: (χ2 = 14.888, n = 125, P = 0.002); Prevalence: (F = 8.700, n = 31, r2OLS = 0.581, Predictor+Space r2 = 0.632, P < 0.001); Infection intensity: (F = 10.491, n = 31, r2OLS = 0.458, Predictor+Space r2 = 0.582, P < 0.001). Significant variables in the model are highlighted in bold. VIF stands for variance inflation factor and denotes colinearity in the model if higher than 10. Std. coeff stands for standard coefficient. Final models chosen based on Akaike Information Criterion.
*PC1 consolidating latitude and maximum temperature of the warmest month accounted for 96.00% of the variation in the original variables.
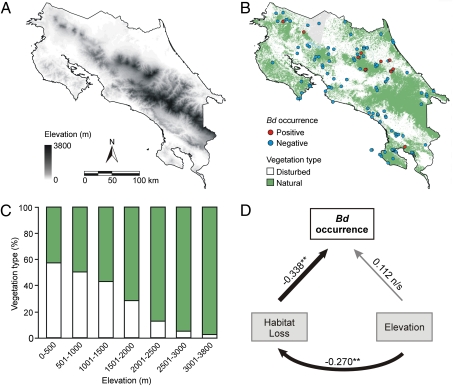
Anthropogenic deforestation is nonrandomly distributed throughout Costa Rica because habitats in flatter lowlands are disproportionately disturbed and, thus, natural vegetation persists at higher elevations (Fig. 1 A–C). This nonrandom pattern potentially confounds the effects of elevation and habitat loss on pathogen occurrence. We considered the influence of elevation on habitat loss in a binary response path analysis and showed that the effect of elevation on Bd occurrence is indirect through habitat loss (whole-model test: χ2[3] = 22.545, P < 0.001) (Fig. 1D).
Fig. 1.
(A) Elevation range and (B) spatial distribution of disturbed and natural vegetation throughout Costa Rica. Sampling sites are Bd-positive (red circles) and -negative (blue circles); vegetation types are disturbed (white) and natural (green); other land-cover classes are freshwater (light blue) and unclassified cloud cover (gray). (C) Percentage of disturbed (white) and natural vegetation (green) across the altitudinal gradient. (D) Path Analysis model showing the relative strength of habitat loss and elevation on Bd occurrence among amphibian populations. Numbers are standardized path coefficients (**P < 0.01). The thickness of the arrows represents the relative strength of the relationship.
Increased habitat loss also predicted lower Bd prevalence in amphibian populations across eastern Australia (βCAR = −0.346, t = −2.579, P = 0.015), in this case, without the confounding effects of elevation across sampling locations (18). The initial screening detected habitat loss, amphibian species richness, latitude, maximum temperature of the warmest month, and precipitation of the warmest quarter of the year as potential explanatory variables for Bd dynamics. Because latitude and maximum temperature of the warmest month were highly correlated (r = −0.920, P < 0.001), we included them in the model selection as a PC variable. When jointly considering all effects in model selection, habitat loss remained a strong negative predictor of Bd prevalence, amphibian species richness a positive predictor, latitude a positive predictor, and maximum temperature during the warmest month a negative predictor (Table 1 and Table S1). Habitat loss alone was not a significant predictor of Bd infection intensity (βCAR = −0.012, t = −1.596, P = 0.122); however, when considered jointly with other variables selected in the initial screening, habitat loss became a strong factor, explaining infection intensity together with precipitation of the driest month (Table 1 and Table S1). The inclusion of spatial autocorrelation in our analyses improved model fit for all reported models (Table 1).
Habitat Loss Predicts Bd Infections at Small Spatial Scale.
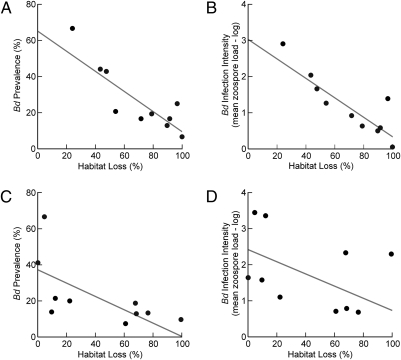
Habitat loss predicted lower Bd prevalence in amphibian populations in both Brazilian Atlantic Forest landscapes. As expected, none of the 19 bioclimatic variables explained Bd prevalence and infection intensity at this smaller spatial scale. We showed that habitat loss alone is a key factor predicting Bd prevalence (βCAR= −0.556, t = −5.074, P = 0.001) (Fig. 2A) and infection intensity (βCAR = −0.027, t = −5.564, P < 0.001) (Fig. 2B) in Araucaria Moist Forest, and although not statistically significant, the same trends were found for the Serra do Mar Coastal Forest [prevalence: βCAR = −0.371, t = −1.683, P = 0.136 (Fig. 2C); infection intensity: βCAR = −0.017, t = −1.520, P = 0.172 (Fig. 2D)]. Because host population density can differ across disturbance gradients, we examined the effect of amphibian capture rate (as a proxy for population density) on both Bd prevalence and infection intensity and found no significant relationships [Serra do Mar Coastal Forest (βCAR = −2.402, t = −1.306, P = 0.233; βCAR = −0.168, t = −1.966, P = 0.090), Araucaria Moist Forest (βCAR = −4.695, t = −1.475, P = 0.184; βCAR = −0.200, t = −1.324, P = 0.227)].
Fig. 2.
Effect of habitat loss on Bd prevalence and infection intensity across populations of D. minutus in two Brazilian Atlantic Forest landscapes: (A and B) Araucaria Moist Forest, southern Brazil and (C and D) Serra do Mar Coastal Forest, southeastern Brazil.
Discussion
We showed that habitat loss, a main cause of species extinctions worldwide (27), is negatively associated with Bd occurrence, prevalence, and infection intensity in tropical amphibian populations. This effect was evident even when jointly considering several known environmental correlates of pathogen growth and persistence (18–21, 24). We corroborated these results at a smaller geographic scale, where habitat loss predicted lower pathogen prevalence and infection intensity in populations with similar macroclimate, topography, and latitude. Thus, localized differences in natural vegetation cover may explain why neighboring populations often have highly contrasting Bd dynamics. Our path analysis showed clearly that elevation had indirect effects on Bd occurrence via habitat loss. Therefore, the role of habitat loss as a determinant of amphibian disease threat has likely been underestimated because of nonrandom deforestation practices relative to topography.
Our analyses demonstrate a negative relationship between habitat loss and disease risk on three continents and at two spatial scales, despite many studies showing the opposite pattern. Habitat loss disrupts natural ecosystems and often increases the risk of human and wildlife diseases (12, 28). Tropical deforestation coincides with a rise of malaria in Africa, Asia, and Latin America, independent of human population density (12, 29). Habitat change was positively associated with the emergence of bat-borne Nipah virus in Malaysia (30), cryptosporidiosis in Europe and North America, and food-borne illnesses globally (31). Bighorn sheep populations in the San Andres Mountains, New Mexico, suffered demographic declines because of severe habitat loss and fragmentation and were subsequently stricken by an epidemic of psoroptic scabies (28). A possible mechanism for this general pattern is that habitat loss limits individual movement and dispersal, which in turn increases disease risk because of elevated stress and contact rates among individuals in crowded patchy populations (32). Population-based models of disease dynamics indicate a potential trade-off in that habitat fragmentation can limit pathogen transmission between small and isolated populations, but it also increases genetic erosion of resistant alleles and reduces the probability of rescue events, increasing the demographic consequences of disease epidemics (33).
In the case of Bd, there is mounting evidence that humans have been playing a key role in spreading the pathogen (3, 34, 35), with infections often higher in areas of higher human footprint (36). Surveys in both temperate and tropical regions revealed that Bd detectability increased with human population density, and in the vicinity of port cities and the highways connecting them (37). Our results, however, show that anthropogenic habitat loss, specifically deforestation, can lower Bd infections in amphibians. This pattern was also observed in Litoria wilcoxii populations of Eastern Australia, where forested habitats harbored amphibians with higher pathogen prevalence then neighboring agricultural lands (38). Other forms of habitat change may also decrease Bd fitness: small populations of Litoria aurea persisted in heavy industrial and mining areas after a severe outbreak of chytridiomycosis, indicating that Bd may be sensitive to environmental contaminants (39). Likewise, in a pristine region of Eastern Australia where Bd was previously documented, forest-associated amphibians suffered local extinctions, but species richness remained high in adjacent urban areas (40). Combined, these examples and our results suggest that although humans may assist pathogen dispersal, anthropogenic habitat changes may also limit Bd persistence.
We propose two potential mechanisms for elevated disease risk in pristine environments: (i) lower Bd infection risk at disturbed habitats because of lower host-species richness, and (ii) suboptimal microclimatic conditions for Bd in disturbed habitats. Bd is one of the most host-generalist pathogens ever found, with over 350 amphibian host species documented to date (3, 13). Therefore, higher biodiversity and community complexity might amplify Bd infections, because greater diversity of host species can enhance pathogen transmission due to higher availability of susceptible hosts throughout the year. We found a positive relationship between amphibian species richness and Bd infections in Costa Rica and Australia, where the negative effects of habitat loss on amphibian species richness are well documented (41, 42). Thus, habitat loss may indirectly reduce Bd infections by lowering species richness in amphibian communities. Although we do not have data on species richness for our small-scale study, deforestation along riparian zones is shown to decrease amphibian species richness in the Brazilian Atlantic Forest (2), thus the same potential mechanism may apply for our smaller scale landscape studies. Different disease outcomes across natural populations may result from density-dependent host-pathogen dynamics (43); however, we found no significant effect of capture rate (a proxy for host population density) on Bd prevalence and infection intensity. Clearly, the relationship between biodiversity and disease dynamics is complex (44) and deserves further investigation.
A second potential mechanism for decreased Bd infections in disturbed habitats is suboptimal microclimatic conditions for the pathogen arising from habitat loss. Habitat loss changes microclimate (10), potentially shifting temperature and humidity to levels that limit Bd growth and persistence (22). This mechanism accounted for the rise of Sudden Oak Death in northern California, where the pathogen thrived in areas of natural vegetation cover with milder temperatures, higher understory humidity, and reduced temperature oscillations, resulting in higher inoculum load in infected trees in closed forest (45). Multiple studies have investigated the role of regional climate in Bd-induced amphibian declines (24, 46, 47). However, few studies to date have focused on how microclimate mediates host–pathogen interactions (48, 49), and more broadly, how large-scale climate relates to microclimates experienced by individual frogs. A recent study found that ponds with fewer surrounding trees reached higher temperatures, significantly reducing Bd infections in salamanders (49). Given the known temperature-dependence of host–pathogen interactions, another hypothesis is that temperature variability, rather than average temperature, is an important predictor of disease risk (47). Increased temperature variability reduces host-immunological response against pathogens (50). If habitat loss increases microclimatic variability, resulting in immunocompromised hosts, we would expect increased disease risk, a pattern opposite to the one we found. Conversely, if habitat loss increases temperature variability with extremes beyond the Bd optimal temperature range, this will result in reduced Bd infections. Therefore, the outcome for disease risk will depend on the relative effects of microclimatic conditions on the host and the pathogen.
We found that habitat loss reduced Bd prevalence and infection intensity in species that are wide-ranging habitat generalists and persist across disturbance gradients. These results raise the obvious question of how habitat loss and disease will impact Bd-susceptible amphibians with narrow ranges and habitat specialization. Most tropical amphibians are in fact specialists, with on average smaller geographic ranges, more patchy distributions, and more restricted microclimatic tolerances (51), limiting their ability to escape infections in habitats less suitable for Bd. Thus, habitat specialists, such as species in the genus Atelopus, potentially suffer negative effects because of both habitat loss and higher Bd exposure (15). In fact, Bd homogenizes tropical amphibian faunas by targeting endemic species (52), thus the interaction between habitat loss and disease may further increase the risk of extinction among habitat specialists.
In this age of biodiversity crisis, it is paradoxical that amphibians can be disproportionately affected by emerging diseases in pristine tropical forests, and that disturbed habitats might act as refuges from disease for the few species that can tolerate deforestation. Our findings support the hypothesis that habitat loss is a key factor in Bd epidemiology in the tropics, and that associated changes in host community structure and microclimate shifts may be the mechanisms behind this pattern. Therefore, epidemiological studies of amphibian declines should incorporate host population and community attributes as well as microclimate data to elucidate mechanisms that control disease and thus enhance conservation of wild populations. If deforestation is consistently biased to lower elevations in regions experiencing amphibian declines, our findings may have even greater implications for predicting Bd spread, especially in the context of environmental determinants and infection patterns at smaller spatial scales, those ecologically relevant at the individual level. The emerging field of spatial epidemiology, bridging landscape and disease ecology (53), provides a more complete picture of amphibian population declines and extinctions. Most tropical amphibians are threatened both by destruction of natural habitats and increased disease in pristine areas. Thus, our results indicate that to curb further extinctions and develop effective mitigation and restoration programs, we must look to interactions between habitat loss and disease: the two main factors at the root of the global decline of amphibians.
Materials and Methods
Large Spatial Scale Data.
We used published datasets of Bd occurrence among populations of the Common Rain frog (Craugastor fitzingeri, Craugastoridae) in Costa Rica (20), and Bd prevalence and infection intensity for populations of the Stony Creek frog (Litoria lesueuri complex, Hylidae) across eastern Australia (18). C. fitzingeri breeds terrestrially and L. lesueuri breeds in streams; both species are habitat generalists and populations persist with Bd (18, 20). Sampling sites for C. fitzingeri were distributed throughout Costa Rica (125 sites; 349 sampled frogs), ranging from sea level to elevations up to 2,110 m. Frogs were sampled between 1993 and 2008, after the arrival of Bd in that country (15). Bd infection in frogs was assessed using histological screening (20). Because of uneven sampling efforts across populations, we considered only occurrence (presence or absence) of Bd at each sampling site. Sampling sites for L. lesueuri ranged from northern Queensland to southern New South Wales (31 sites; 863 sampled frogs), along the eastern slope of the Great Dividing Range. To control for elevation and seasonality all sampling occurred at lowland sites (mean elevation 84.51 ±49.7 SD), and within a 42-d period during the spring of 2005. Australian frogs were swabbed in the field and Bd-screened using quantitative PCR (qPCR) (18). Bd infection was assessed at the individual level. Prevalence was estimated as the percentage of infected individuals per population, and infection intensity as the mean number of “zoospore DNA equivalents” for all individuals at each population (18).
We obtained land-cover information from Fondo Nacional de Financiamento Forestal for Costa Rica [30-m resolution; coverage period 1995–2005 (54)], and from the Bureau of Rural Sciences for Australia [100-m resolution; coverage period 1995–2000 (55)]. We acquired elevation data (90-m resolution) from the Consultative Group on International Agricultural Research Consortium for Spatial Information (56). Nineteen bioclimatic variables for both studies were extracted using Worldclim/Bioclim layers (1000-m resolution), available at http://www.worldclim.org/bioclim (57). These metrics of temperature and precipitation are averaged from 50-y records (1950–2000) from a dense network of climatic stations throughout the world (e.g., precipitation records from 47,554 locations, temperature from 24,542 locations). We did not include temperature variability or any variable accounting for climate change in our analyses because samples for Bd diagnosis were collected over short time periods, precluding longer-term temporal analyses of Bd dynamics. We obtained amphibian species richness at each sampling locations in Costa Rica and Australia by overlaying GIS shapefiles of species historical geographic ranges from the Global Amphibian Assessment (16).
We created habitat loss layers for Costa Rica and Australia as the percentage of nonnatural vegetation cover ranging from 0% to 100% at 1-km resolution. Land-cover types considered as nonnatural were urbanization, pasture, agriculture, and exotic crops. Natural vegetation types were primarily forest habitats. All variables were measured at 1-km pixel to maintain a consistent scale across the analyses.
Small Spatial Scale Data.
We sampled 20 populations of the Golden Lesser treefrog (Dendropsophus minutus, Hylidae) in two landscapes of the Brazilian Atlantic Forest in southern and southeastern Brazil [10 populations in Araucaria Moist Forest, State of Rio Grande do Sul (29° 24′ S, 50° 24′ W), and 10 populations in Serra do Mar Coastal Forest, State of São Paulo (23° 20′ S, 45° 12′ W)]. Maximum distance between sampling locations was 21.33 km for Araucaria Moist Forest and 18.88 km for Serra do Mar Coastal Forest. Our focal species is a habitat generalist that breeds in ponds during the austral summer.
We swabbed frogs in the field (537 sampled frogs; average of 26.85 frogs per site) with sterile swabs for later Bd quantification in the laboratory. We screened samples for Bd in singlicate using Taqman qPCR (58), extending the range of the standards to 1,000 zoospore DNA equivalents to determine the presence of Bd and infection intensity. For calculations of Bd prevalence, we categorized individuals as Bd-positive when zoospore equivalents were ≥ 1. We defined infection intensity as the mean number of “zoospore equivalents” per population. We calculated amphibian capture rate for each population based on individuals sampled per person-hour.
We assessed natural vegetation cover for each sampling site based on high-resolution satellite images from 2010 (SPOT, 2-m resolution). In each landscape, the selected study sites represented a gradient of natural vegetation cover. We calculated habitat loss as the percentage of nonnatural vegetation cover within a diameter of 600 m and 1 km surrounding each sampling site using ArcGIS 9.3.1 (59). To avoid effects of elevation and macroclimate we chose landscapes with low climatic and topographic variability (mean elevation of sampling sites for Araucaria Moist Forest 918.0 ± 10.2 m SD; for Serra do Mar Coastal Forest 929.9 ± 71.4 m SD). We extracted the same 19 bioclimatic variables (57) for each sampling site at 1-km scale. To avoid biases because of seasonality, we sampled continuously over 30 d in Serra do Mar Coastal Forest, and 41 d in Araucaria Moist Forest in 2009/2010.
Statistical Analyses.
To assess the relationship between habitat loss and Bd occurrence in amphibian populations among sampling sites in Costa Rica while accounting for spatial autocorrelation, we performed autologistic regressions (AUTOLOG). To investigate the relationship between habitat loss and Bd prevalence, and between habitat loss and infection intensity among sampling sites in Australia, we used conditional autoregressions (CAR). After this first univariate assessment, we used stepwise regressions (exclusion cutoff P < 0.10; inclusion cutoff P < 0.20) to screen for biotic and abiotic factors that potentially predict disease threat to be included in model selection procedures. For each analysis, we screened a total of 23 explanatory variables, including habitat loss, amphibian species richness, latitude, elevation, and the 19 bioclimatic temperature and precipitation metrics. Once important variables were identified for each dataset, we used principal components analysis to consolidate cross-correlated variables, and used the scores of the first PC axis as variables in the subsequent model selection procedure. We used AUTOLOG and CAR model selections, including selected explanatory variables and Bd (occurrence, prevalence, or infection intensity) as a response variable. We tested all possible models including interactions. Competing models were ranked based on Akaike Information Criterion (AIC). We reported the best fit model for each run. We assessed multicolinearity in each of the final models using a variance inflation factor.
We used binary response path analysis to statistically model causal relationships among elevation, habitat loss, and Bd occurrence in Costa Rica, providing information about the relative strength of the different paths. In the model, elevation influences habitat loss, and these two variables are allowed to influence Bd occurrence independently.
For the small-scale data, we used the same stepwise screening method to confirm that none of the 19 bioclimatic variables explain Bd prevalence and infection intensity in both landscapes when accounting for habitat loss (measured at 1-km scale). To quantify the effect of habitat loss on Bd prevalence and infection intensity for populations in the two landscapes of the Brazilian Atlantic Forest, we used single CARs. We log-transformed zoospore data (infection intensity) for the analyses. Because climatic variables were not included, final models for both landscapes included habitat loss measured within 600-m diameter buffers surrounding sampling locations. We also ran single CARs to quantify the effect of capture rate on both Bd prevalence and infection intensity. Capture rate was used as proxy for population density of D. minutus in our analyses of pathogen dynamics. In all analyses at both scales, sampling site was used as the replicate in statistical tests of Bd dynamics. We ran analyses using Spatial Analysis in Macroecology v4.0 (60) and Mplus v6.0 (61).
Supplementary Material
Acknowledgments
We thank M. F. K. Becker, H. W. Greene, C. F. B. Haddad, K. R. Lips, A. V. Longo, J. Rohr, K. Wagner, K.R.Z. laboratory members, and one anonymous reviewer for feedback on the manuscript; A. V. Longo for help with qPCRs; C. F. B. Haddad for field planning and sampling design; F. Adams and N. Alves for field support; and S. Sadigov and F. Vermeylen for statistical assistance. Research permits were provided by Instituto Chico Mendes–Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis/Brazil (Permit 20621-1 and 21.125-1), Instituto Florestal do Estado de São Paulo (Permit 260108-009.662), and the Cornell University Institutional Animal Care and Use Committee. Our work was funded by grants from the American Philosophical Society, Sigma Xi, Andrew Mellon Foundation, Department of Ecology and Evolutionary Biology at Cornell University, Mario Einaudi Center for International Studies, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Fulbright (Grant 2157-08) (to C.G.B.) and National Science Foundation Grant DEB-0542848 (to K.R.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.J.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014497108/-/DCSupplemental.
References
- 1.Cushman SA. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol Conserv. 2006;128:231–240. [Google Scholar]
- 2.Becker CG, Fonseca CR, Haddad CFB, Batista RF, Prado PI. Habitat split and the global decline of amphibians. Science. 2007;318:1775–1777. doi: 10.1126/science.1149374. [DOI] [PubMed] [Google Scholar]
- 3.Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 4.Wake DB, Vredenburg VT. Colloquium paper: Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arens P, et al. Genetic population differentiation and connectivity among fragmented Moor frog (Rana arvalis) populations in The Netherlands. Landscape Ecol. 2007;22:1489–1500. [Google Scholar]
- 6.Andersen LW, Fog K, Damgaard C. Habitat fragmentation causes bottlenecks and inbreeding in the European tree frog (Hyla arborea) Proc Biol Sci. 2004;271:1293–1302. doi: 10.1098/rspb.2004.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbina-Cardona JN, Olivares-Perez M, Reynoso VH. Herpetofauna diversity and microenvironment correlates across a pasture-edge-interior ecotone in tropical rainforest fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biol Conserv. 2006;132:61–75. [Google Scholar]
- 8.Becker CG, Fonseca CR, Haddad CFB, Prado PI. Habitat split as a cause of local population declines of amphibians with aquatic larvae. Conserv Biol. 2010;24:287–294. doi: 10.1111/j.1523-1739.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 9.Costa MH, Foley JA. Combined effects of deforestation and doubled atmospheric CO2 concentrations on the climate of Amazonia. J Clim. 2000;13:18–34. [Google Scholar]
- 10.Kapos V. Effects of Isolation on the Water Status of Forest Patches in the Brazilian Amazon. J Trop Ecol. 1989;5:173–185. [Google Scholar]
- 11.Webb TJ, Woodward FI, Hannah L, Gaston KJ. Forest cover-rainfall relationships in a biodiversity hotspot: The Atlantic forest of Brazil. Ecol Appl. 2005;15:1968–1983. [Google Scholar]
- 12.Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 13.Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009;63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- 14.Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:e72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IUCN Conservation International, NatureServe. Global Amphibian Assessment. 2010. Available at: http://www.iucnredlist.org/amphibians (accessed Dec. 2010)
- 17.Vredenburg VT, Knapp RA, Tunstall T, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriger KM, Pereoglou F, Hero JM. Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conserv Biol. 2007;21:1280–1290. doi: 10.1111/j.1523-1739.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 19.Brem FMR, Lips KR. Batrachochytrium dendrobatidis infection patterns among Panamanian amphibian species, habitats and elevations during epizootic and enzootic stages. Dis Aquat Organ. 2008;81:189–202. doi: 10.3354/dao01960. [DOI] [PubMed] [Google Scholar]
- 20.Puschendorf R, et al. Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: Proposing climatic refuges as a conservation tool. Divers Distrib. 2009;15:401–408. [Google Scholar]
- 21.Longo AV, Burrowes PA, Joglar RL. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis Aquat Organ. 2010;92:253–260. doi: 10.3354/dao02054. [DOI] [PubMed] [Google Scholar]
- 22.Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004;96:9–15. [PubMed] [Google Scholar]
- 23.Bustamante HM, Livo LJ, Carey C. Effects of temperature and hydric environment on survival of the Panamanian Golden Frog infected with a pathogenic chytrid fungus. Integr Zool. 2010;5:143–153. doi: 10.1111/j.1749-4877.2010.00197.x. [DOI] [PubMed] [Google Scholar]
- 24.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 25.Laurance WF, McDonald KR, Speare R. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conserv Biol. 1996;10:406–413. [Google Scholar]
- 26.Viana VM, Tabanez AAJ, Batista JLF. In: Tropical Forest Remnants: Ecology, Management and Conservation of Fragmented Communities. Laurance WF, Bierregaard RO, editors. Chicago: Univ. of Chicago Press; 1997. pp. 347–365. [Google Scholar]
- 27.Wilson EO. The Diversity of Life. New York: W. W. Norton & Company; 1992. [Google Scholar]
- 28.Boyce WM, Weisenberger ME. The rise and fall of psoroptic scabies in bighorn sheep in the San Andres Mountains, New Mexico. J Wildl Dis. 2005;41:525–531. doi: 10.7589/0090-3558-41.3.525. [DOI] [PubMed] [Google Scholar]
- 29.Vittor AY, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 30.Chua KB, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 31.Rose JB, et al. Climate variability and change in the United States: Potential impacts on water- and foodborne diseases caused by microbiologic agents. Environ Health Perspect. 2001;109(Suppl 2):211–221. doi: 10.1289/ehp.01109s2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott ME. The impact of infection and disease on animal populations: Implications for conservation biology. Conserv Biol. 1988;2:40–56. [Google Scholar]
- 33.McCallum H, Dobson A. Disease, habitat fragmentation and conservation. Proc Biol Sci. 2002;269:2041–2049. doi: 10.1098/rspb.2002.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James TY, et al. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog. 2009;5:e1000458. doi: 10.1371/journal.ppat.1000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan JAT, et al. Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2007;104:13845–13850. doi: 10.1073/pnas.0701838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams MJ, et al. Using occupancy models to understand the distribution of an amphibian pathogen, Batrachochytrium dendrobatidis. Ecol Appl. 2010;20:289–302. doi: 10.1890/08-2319.1. [DOI] [PubMed] [Google Scholar]
- 37.Murray KA, et al. Assessing spatial patterns of disease risk to biodiversity: Implications for the management of the amphibian pathogen Batrachochytrium dendrobatidis. J Appl Ecol. 2011;48:163–173. [Google Scholar]
- 38.Van Sluys M, Hero JM. How does chytrid infection vary among habitats? The case of Litoria wilcoxii (Anura, Hylidae) in SE Queensland, Australia. EcoHealth. 2009;6:576–583. doi: 10.1007/s10393-010-0278-1. [DOI] [PubMed] [Google Scholar]
- 39.Mahony M. In: Herpetology in Australia: A Diverse Discipline. Lunney D, Ayers D, editors. Mosman: Royal Zoological Society of New South Wales; 1993. pp. 257–264. [Google Scholar]
- 40.Lane A, Burgin S. Comparison of frog assemblages between urban and non-urban habitatsi n the upper Blue Mountains of Australia. Freshw Biol. 2008;50:2484–2493. [Google Scholar]
- 41.Bell KE, Donnelly MA. Influence of forest fragmentation on community structure of frogs and lizards in northeastern Costa Rica. Conserv Biol. 2006;20:1750–1760. doi: 10.1111/j.1523-1739.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 42.Parris KM, Lindenmayer DB. Evidence that creation of a Pinus radiata plantation in south-eastern Australia has reduced habitat for frogs. Acta Oecol. 2004;25:93–101. [Google Scholar]
- 43.Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA. 2010;107:9695–9700. doi: 10.1073/pnas.0912886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keesing F, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meentemeyer RK, Rank NE, Anacker BL, Rizzo DM, Cushman JH. Influence of land-cover change on the spread of an invasive forest pathogen. Ecol Appl. 2008;18:159–171. doi: 10.1890/07-0232.1. [DOI] [PubMed] [Google Scholar]
- 46.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci USA. 2010;107:8269–8274. doi: 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards-Zawacki CL. Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc Biol Sci. 2010;277:519–528. doi: 10.1098/rspb.2009.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raffel TR, Michel PJ, Sites EW, Rohr JR. What drives chytrid infections in newt populations? Associations with substrate, temperature, and shade. EcoHealth. 2011 doi: 10.1007/s10393-010-0358-2. 10.1007/s10393-010-0358-2. [DOI] [PubMed] [Google Scholar]
- 50.Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ. Negative effects of changing temperature on amphibian immunity under field conditions. Funct Ecol. 2006;20:819–828. [Google Scholar]
- 51.Brown JH, Stevens GC, Kaufman DM. The geographic range: Size, shape, boundaries and internal structure. Annu Rev Ecol Syst. 1996;27:597–623. [Google Scholar]
- 52.Smith KG, Lips KR, Chase JM. Selecting for extinction: Nonrandom disease-associated extinction homogenizes amphibian biotas. Ecol Lett. 2009;12:1069–1078. doi: 10.1111/j.1461-0248.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 53.Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: An emerging (or re-emerging) discipline. Trends Ecol Evol. 2005;20:328–336. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Calvo-Alvarado JC, Sánchez-Azofeifa A, Chong M, Castillo M, Jiménez V. Costa Rica Landcover Classification Based on Landsat Images ETM+ 2005. San José, Costa Rica: FONAFIFO-FUNTEC; 2006. Translated from Spanish. [Google Scholar]
- 55.Bureau of Rural Sciences, Agriculture Western Australia, New South Wales Land Information Centre. Agricultural Land Cover Change Datasets – Queensland and New South Wales Combined Data Containing Change, Landcover and Structural Vegetation at 100m Cell Size. Australia: Camberra; 2001. [Google Scholar]
- 56.Jarvis A, Reuter HI, Nelson A, Guevara E. Hole-filled seamless SRTM data V4. 2009. Available at: http://srtm.csi.cgiar.org (accessed July 2009)
- 57.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 58.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 59.ESRI. Arcview 9.3.1. Redlands, CA: 2009. [Google Scholar]
- 60.Rangel TF, Diniz JAF, Bini LM. SAM: A comprehensive application for Spatial Analysis in Macroecology. Ecography. 2010;33:46–50. [Google Scholar]
- 61.Muthén LK, Muthén BO. Mplus 6 User's Guide. Los Angeles, CA: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.