Abstract
Blood feeding by vector mosquitoes provides the entry point for disease pathogens and presents an acute metabolic challenge that must be overcome to complete the gonotrophic cycle. Based on recent data showing that coatomer protein I (COPI) vesicle transport is involved in cellular processes beyond Golgi–endoplasmic reticulum retrograde protein trafficking, we disrupted COPI functions in the Yellow Fever mosquito Aedes aegypti to interfere with blood meal digestion. Surprisingly, we found that decreased expression of the γCOPI coatomer protein led to 89% mortality in blood-fed mosquitoes by 72 h postfeeding compared with 0% mortality in control dsRNA-injected blood-fed mosquitoes and 3% mortality in γCOPI dsRNA-injected sugar-fed mosquitoes. Similar results were obtained using dsRNA directed against five other COPI coatomer subunits (α, β, β′, δ, and ζ). We also examined midgut tissues by EM, quantitated heme in fecal samples, and characterized feeding-induced protein expression in midgut, fat body, and ovary tissues of COPI-deficient mosquitoes. We found that COPI defects disrupt epithelial cell membrane integrity, stimulate premature blood meal excretion, and block induced expression of several midgut protease genes. To study the role of COPI transport in ovarian development, we injected γCOPI dsRNA after blood feeding and found that, although blood digestion was normal, follicles in these mosquitoes were significantly smaller by 48 h postinjection and lacked eggshell proteins. Together, these data show that COPI functions are critical to mosquito blood digestion and egg maturation, a finding that could also apply to other blood-feeding arthropod vectors.
Keywords: Dengue RNAi, serine protease, oocyte vitellogenin
Mosquitoes are responsible for transmitting two of the most widespread human pathogens in the world, namely Plasmodium falciparum, which causes malaria, and Dengue virus, the underlying cause of Dengue hemorrhagic fever. Although a variety of strategies have been developed to control the spread of these pathogens, it is clear that a multipronged approach will be needed to combat the inevitable insecticide and drug resistance that characterizes mosquito-borne diseases. For example, spraying of broad-spectrum insecticides and the distribution of pyrethroid-treated bed nets have proven effective; however, insecticide resistance develops, and pleiotropic effects on nontarget organisms are problematic (1). Moreover, whereas transgenic lines of pathogen-resistant mosquitoes offer great promise (2), implementation of this strategy requires additional development of genetic drive mechanisms (3) as well as identification of additional gene targets that offer increased flexibility in the design of transgenic lines. Because reducing mosquito populations in areas of high disease transmission has worked well in the past to reduce human infection rates, we have focused our efforts on discovering protein targets associated with blood meal metabolism, which could be used to develop biological and chemical approaches to vector control (4–7).
Aedes aegypti is the primary vector of dengue and Yellow Fever viruses, and it has spread rapidly into highly populated urban areas, which has led to a rise in cases of dengue hemorrhagic fever (8, 9). Blood feeding is required for completion of the gonotrophic cycle, and most Ae. aegypti mosquitoes lay ∼100 eggs at a time during up to five gonotrophic cycles over their short lifetime. Because 80% of the dry weight of a mosquito blood meal consists of protein, primarily hemoglobin, albumin, and Ig, proteolytic digestion of the blood meal is essential to nutrient acquisition for egg production. We have previously shown that reduced expression of selective abundant serine proteases in Ae. aegypti midgut epithelial cells leads to decreased digestion of albumin protein, defects in ovarian development, and a significant 30% reduction in egg production during the first and second gonotrophic cycles (6). In an attempt to further reduce blood meal protein digestion and fecundity, we have now focused our efforts on inhibiting vesicle transport processes that are required for secretion of multiple digestive enzymes, peritrophic matrix proteins, and other blood meal-metabolizing proteins into the midgut lumen.
Three vesicle transport systems have been described in eukaryotic cells and shown to be required for protein trafficking between the endoplasmic reticulum (ER), Golgi apparatus, and plasma membrane (10). The coatomer protein (COP) complexes COPI (11) and COPII (12) are primarily responsible for vesicle transport between the ER and Golgi, whereas clatharin complexes transport proteins between the plasma membrane, endosomes, and trans-Golgi network (13). Although most in vitro reconstitution experiments (14–16) and genetic analyses using model organisms (17, 18) support this model of COPI, COPII, and clatharin vesicle transport in eukaryotic cells, recent studies suggest that not all COPI, COPII, and clatharin-coated vesicles follow this simple scheme. For example, in addition to retrograde vesicle transport of ER-resident proteins from the Golgi to the ER, COPI vesicles have also been implicated in anterograde processes, including the secretion of cytosolic proteins (19), delivery of proteins to the plasma membrane (20), and cargo delivery to lipid droplets (21, 22). Although cell-based studies have provided valuable information about the basic mechanisms of cytosolic vesicle transport, less is known about the role of vesicle transport systems in mediating complex physiological processes at the organismal level.
We chose to investigate protein components in the COPI vesicle transport system as potential targets for vector control based on three recent findings. First, whole-genome RNAi screens in Drosophila cell lines identified γ-, α-, and β′COPI, ADP-ribosylation factor 79 (Arf79), and Golgi brefeldin A resistant guanine nucleotide exchange factor 1 (GBF1) as essential genes for vesicle transport (23) and lipid droplet metabolism (21, 22), both of which could be required for blood meal metabolism in mosquitoes. Second, Baum et al. (24) found that feeding β′COPI dsRNA to the Western corn rootworm Diabrotica virgifera was the most lethal dsRNA that they tested. Third, we identified α-, β-, and β′COPI as the most abundant proteins differentially associated with rough endoplasmic reticulum (RER) whorls in the midguts of unfed and fed Ae. aegypti mosquitoes (25). This latter result suggests that feeding-induced synthesis and secretion of some midgut proteases may be controlled by COPI processes.
We describe results here of experiments designed to identify the role of seven COPI coatomer proteins (α, β, β′, γ, δ, ε, and ανδ ζ) in mediating blood meal digestion in adult female Ae. aegypti mosquitoes. We found that blood feeding dramatically increased mortality rates in mosquitoes lacking an intact COPI coatomer complex and moreover, that delayed COPI defects blocked egg maturation and subsequent blood feeding by gravid female mosquitoes.
Results
Loss of COPI Coatomer Protein Expression Is Lethal to Blood-Fed Mosquitoes.
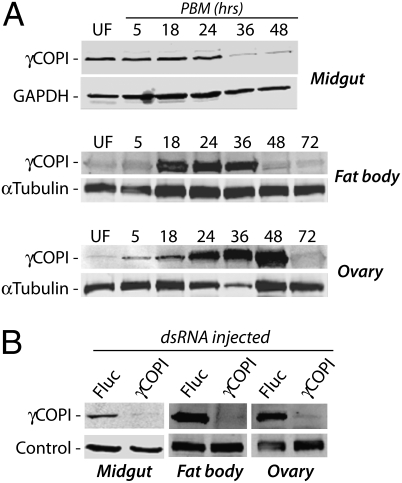
We initially characterized the γCOPI coatomer protein, because this subunit is a central component of the COPI coatomer F subcomplex (26) and interacts directly with the COPI regulatory proteins GBF1 (27) and ArfGAP2 (28). As shown in Fig. 1A, γCOPI protein is expressed in Ae. aegypti midgut epithelial cells before blood feeding; however, by 36 h postblood meal (PBM), the level of γCOPI protein is reduced and remains low at 48 h PBM. In contrast, γCOPI protein in fat body tissue is induced by blood feeding, with the highest level of expression between 18 and 36 h PBM. Similarly, γCOPI protein expression in ovary tissue is also induced by blood feeding; however, peak expression is 24–48 h PBM, which coincides with rapid follicular expansion (29). Fig. 1B shows that injection of 400 ng γCOPI dsRNA or the same amount of Firefly luciferase (Fluc) dsRNA, which was used as a negative control, resulted in significant RNAi-mediated knockdown at the protein level in pooled midgut, fat body, and ovary tissues.
Fig. 1.
γCOPI protein expression in mosquito midgut, fat body, and ovary tissues. (A) Western blot analysis of γCOPI coatomer protein expression in midgut, fat body, and ovary tissue of blood fed WT female Ae. aegypti mosquitoes at various times postblood meal (PBM). Expressions of GAPDH and α-tubulin were used as protein-loading controls, with each lane containing protein extracts from an equivalent number of mosquitoes. (B) Knockdown of γCOPI coatomer protein expression in midgut, fat body, and ovary tissues of mosquitoes injected 3–4 d earlier with 400 ng γCOPI or Fluc dsRNA. Midgut tissues were collected before blood feeding, whereas fat body and ovary tissues were collected 24 h PBM. Western blotting was done using the antigen-specific γCOPI and either GAPDH antibody (midgut) or α-tubulin antibody (fat body and ovary) to control for protein loading.
Because the knockdown level of γCOPI protein expression could be variable in individual mosquitoes within the pooled samples, we used quantitative RT-PCR (qRT-PCR) to quantitate γCOPI transcript levels in single mosquito midguts 3 d postinjection (PI) using 100 or 400 ng of dsRNA (Table S1). These analyses revealed that 90–100% of γCOPI dsRNA-injected mosquitoes displayed >80% decrease in γCOPI transcript levels using 100 and 400 ng dsRNA, with 0% mortality as a consequence of dsRNA injection for >120 h PI. Note that blood meal size, as determined by a BSA Western blotting assay (6), ranged from 90% to 100% of that of Fluc dsRNA-injected control mosquitoes, indicating that initial blood feeding and engorgement were similar in all of the dsRNA-injected mosquitoes (Table S1).
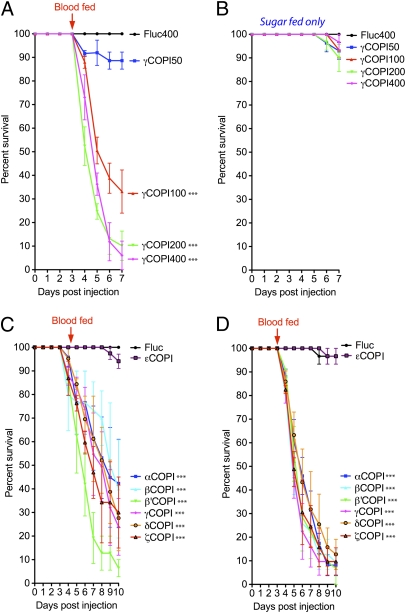
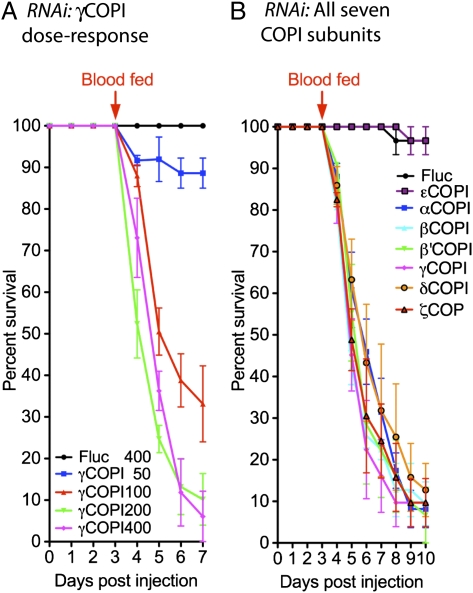
Based on our previous analysis of in vivo blood protein digestion in Ae. aegypti (6), we expected that knockdown of γCOPI expression would inhibit the secretion of proteases into the midgut lumen and thereby, delay blood digestion and egg deposition. However, we were surprised to find that injecting 100–400 ng γCOPI dsRNA at 2 d postadult emergence and then, blood feeding 3 d later led to a dramatic feeding-induced mortality (50–84%) within 24–72 h PBM (6 d PI) as shown in Fig. 2A. In contrast, there was 0% mortality in biological replicas of blood-fed Fluc dsRNA-injected mosquitoes and sugar-fed γCOPI dsRNA-injected mosquitoes for up to 120 h PI (Fig. 2B). Based on Kaplan–Meier survival analysis, mortality was significantly higher in blood-fed mosquitoes that were injected with 100, 200, and 400 ng γCOPI dsRNA (P < 0.001) compared with mosquitoes injected with Fluc dsRNA (400 ng).
Fig. 2.
Blood feeding induced mortality in mosquitoes injected with dsRNA targeting six different COPI coatomer subunits. (A) Survival curves of mosquitoes injected with γCOPI dsRNA (50, 100, 200, or 400 ng) or Fluc dsRNA (400 ng) at 2 d posteclosion, blood fed 3 d later (arrow), and then followed for 4 d PBM. Kaplan–Meier survival analysis showed that γCOPI knockdown caused significant mortality in blood-fed mosquitoes using 100, 200, or 400 ng γCOPI dsRNA, compared with mosquitoes injected with 400 ng Fluc dsRNA (***P < 0.001, log-rank analysis). Injection with 50 ng γCOPI dsRNA did not significantly increase mortality compared with Fluc dsRNA-injected mosquitoes. The number of surviving mosquitoes each day for 7 d PI is shown as mean ± SEM using data from three independent biological experiments. (B) Survival analysis of γCOPI (50, 100, 200, or 400 ng) or Fluc dsRNA (400 ng) -injected mosquitoes maintained on sugar for 7 d PI shown as mean ± SEM using data from three independent biological experiments. (C) Survival curves of mosquitoes injected with 100 ng COPI subunit-specific dsRNA or Fluc control dsRNA. Kaplan–Meier survival analysis shows that knockdown of six different COPI subunits (α, β, β′, δ, γ, and ζ) caused significant mortality (***P < 0.001, log-rank analysis) compared with Fluc dsRNA-injected mosquitoes. The number of surviving mosquitoes each day for 10 d PI is shown as mean ± SEM using data from three independent biological experiments. (D) Survival curves of mosquitoes injected with 400 ng COPI subunit-specific dsRNA or Fluc control dsRNA. The data were collected, plotted, and analyzed the same as in C.
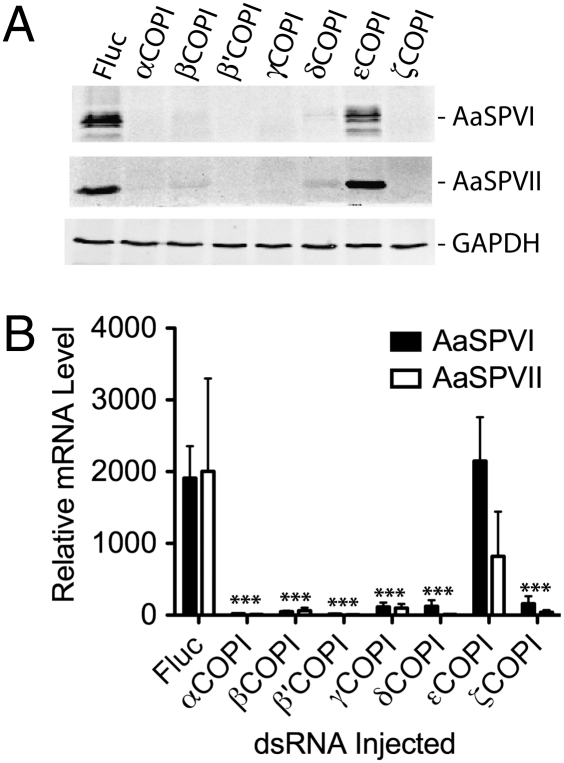
Studies have shown that the COPI coatomer complex consists of seven distinct protein subunits called α, β, β′, δ, γ, ε, and ζ (26). Therefore, we next tested the effect of knocking down the expression of each individual subunit to see if blood feeding-induced mortality effect was unique to the γCOPI subunit. Decreased expression of five of the other six coatomer proteins (α, β, β′, δ, and ζ) led to significant blood feeding-induced mortality (P < 0.001) over a 7-d period PBM when using either 100 (58–94% mortality) or 400 ng (88–95% mortality) of COPI dsRNA (Fig. 2). The one exception was the εCOPI subunit, which like Fluc dsRNA, showed no effect on mortality, although injection of 400 ng εCOPI dsRNA reduced transcript levels 91.3% in 100% of the injected mosquitoes (Table S1). Importantly, mortality was shown to be significantly higher in blood-fed mosquitoes injected with 100 ng β, β′, δ, γ, or ζCOPI dsRNA compared with mosquitoes from the same experiments that were maintained on a sugar alone (Table S2). Kaplan–Meier survival analysis revealed that decreased expression of the β′ subunit was significantly more lethal than decreased expression of any other COPI subunit when 100 ng dsRNA were injected (Table S2).
COPI Deficiency Causes Biological Defects at Organismal, Tissue, and Cellular Levels.
Although the data in Fig. 2 clearly showed that knocking down COPI coatomer protein expression led to blood feeding-induced mortality, the underlying cause of this accelerated death was not obvious. Therefore, we analyzed the effects of COPI knockdowns on midgut biology, because blood feeding involves the female mosquito ingesting a blood meal within minutes that is equal to her own body weight. We reasoned that deficiencies in the COPI transport system in midgut epithelial cells could directly or indirectly inhibit the mosquito's ability to cope with the anatomical and metabolic stress of blood feeding.
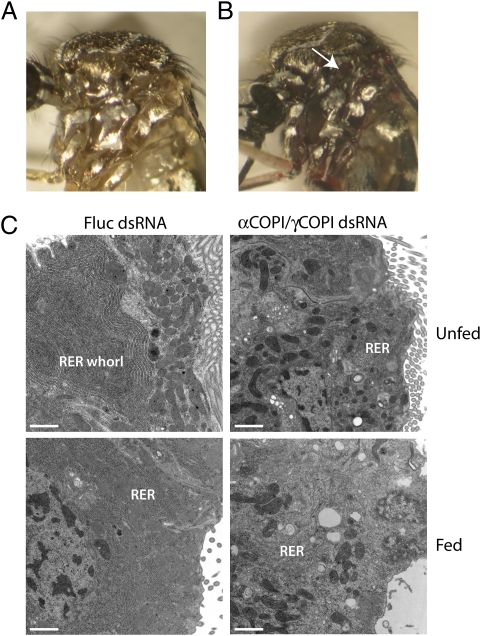
One of the most striking phenotypes in γCOPI-deficient blood-fed mosquitoes was a red coloring in the thoracic segment (compare Fig. 3A with Fig. 3B), an indication that blood from the midgut had leaked into the hemocoel. Indeed, routine midgut dissections of blood-fed γCOPI dsRNA-injected mosquitoes revealed that the midgut epithelial cell lining was extremely fragile. It was possible, therefore, that pressure exerted on the midgut as a result of engorgement caused the epithelial cell lining to rupture and allowed blood to escape into the lumen. To examine midgut epithelial cells in more detail, we performed EM using midgut sections isolated from unfed and amino acid-fed mosquitoes that had been injected with either an equal mixture of αCOPI/γCOPI dsRNA or Fluc dsRNA. As shown in Fig. 3C, midgut epithelial cells of unfed Fluc dsRNA-injected mosquitoes contained large RER whorls as previously reported (30); however, these cellular membrane structures were absent from the midgut epithelial cells of αCOPI/γCOPI-deficient mosquitoes. In addition, the RER whorls disassembled by 2 h after feeding in the Fluc dsRNA-injected mosquitoes, whereas midgut epithelial cells in the αCOPI/γCOPI dsRNA-injected mosquitoes were shown to contain extended regions of swollen endoplasmic reticulum, similar to those regions observed in mosquitoes injected with only αCOPI dsRNA (25). The observed differences in cytosolic membrane organization in midgut epithelial cells were not associated with global changes in protein expression based on SDS/PAGE analysis of midgut protein extracts isolated from Fluc and COPI dsRNA-injected mosquitoes (Fig. S1).
Fig. 3.
Decreased γCOPI expression induces phenotypic changes at the organismal and cellular levels in blood-fed mosquitoes. (A) Representative photo of a blood-fed WT mosquito 24 h PBM. (B) Representative photo of a blood-fed γCOPI dsRNA-injected mosquito 24 h PBM. The red color in the thoracic segment indicates that blood from the midgut has entered the hemocoel (arrow). (C) Representative photos from EM analysis of midgut epithelial cells obtained from female mosquitoes injected 3 d earlier with Fluc dsRNA (800 ng) or a mixture of αCOPI dsRNA (400 ng) and γCOPI dsRNA (400 ng). Unfed refers to sugar-fed mosquitoes, and fed denotes that mosquitoes were fed an artificial amino acid meal 2 h before dissection. Representative RER and an RER whorl are labeled. (Scale bar: 0.5 microns.)
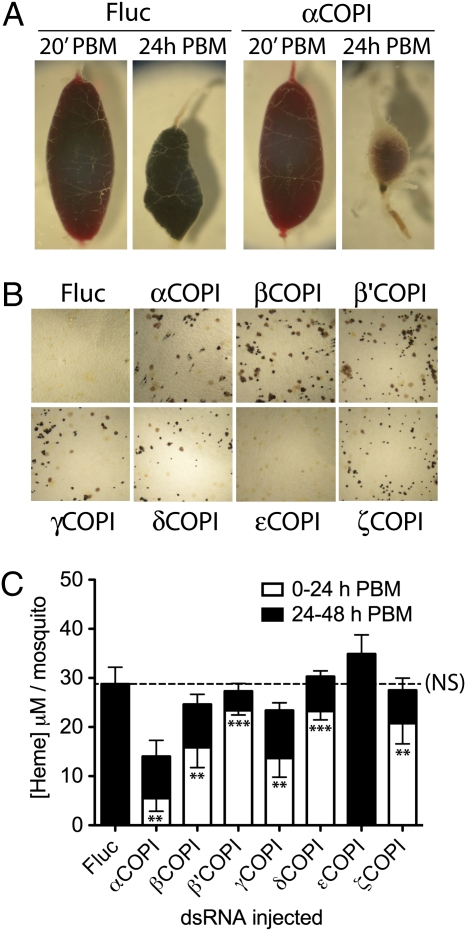
During the preparation of the midgut protein extracts, we noticed that midguts isolated from the COPI dsRNA-injected mosquitoes 24 h PBM were smaller and lighter in color than those isolated from the Fluc dsRNA-injected mosquitoes, and moreover, they lacked an intact peritrophic matrix. As shown in Fig. 4A, this difference in midgut phenotype was not seen immediately after blood feeding comparing Fluc with αCOPI dsRNA-injected mosquitoes, which was consistent with our quantitation of midgut BSA levels showing that COPI dsRNA injections did not interfere with normal blood feeding (Table S1). Midguts dissected from β-, β′-, δ-, γ-, and ζCOPI dsRNA-injected mosquitoes had the same phenotype as the αCOPI dsRNA-injected mosquitoes, whereas midguts removed from εCOPI dsRNA-injected mosquitoes were comparable with the midguts of Fluc dsRNA-injected mosquitoes (Fig. S2).
Fig. 4.
Premature blood meal excretion in COPI dsRNA-injected mosquitoes. (A) Representative photos of whole mosquito midguts removed from Fluc and αCOPI dsRNA-injected mosquitoes at 20 min and 24 h PBM. Phenotypes similar to αCOPI dsRNA-injected mosquitoes were observed in midguts isolated from β-, β′-, γ-, δ-, and aνδ ζCOPI dsRNA-injected mosquitoes, whereas midguts isolated from εCOPI dsRNA-injected mosquitoes were similar to midguts from Fluc dsRNA-injected mosquitoes (Fig. S2). (B) Representative photos of filter paper removed 24 h PBM from vials containing five fully engorged blood-fed mosquitoes that had been injected 4 d earlier with 400 ng indicated dsRNA. (C) Quantitation of heme in feces collected from vials containing five fully engorged blood-fed mosquitoes that had been injected 4 d earlier with 400 ng indicated dsRNA. Heme levels in the vials were determined during the first 24 h PBM (white bars) and the second 24 h PBM (black bars). Data show means ± SEM from three independent experiments. Student t test analysis showed significant differences between the amount of heme in feces collected 0–24 h PBM from COPI dsRNA-injected mosquitoes compared with the amount of heme in feces collected from Fluc dsRNA-injected mosquitoes during the same time period (***P < 0.001). There was no significant (NS) difference between the total amount of heme collected from 0 to 48 h in COPI dsRNA-injected mosquitoes compared with Fluc dsRNA-injected mosquitoes.
In addition to leakage of blood into the hemocoel (Fig. 3B), we also observed a large amount of undigested blood in the feces of α-, β-, β′-, δ-, γ-, and ανδ ζCOPI dsRNA-injected mosquitoes within 24 h PBM compared with Fluc and εCOPI dsRNA-injected mosquitoes (Fig. 4B). To quantitate the extent of premature defecation in the COPI dsRNA-injected mosquitoes, we measured fecal heme levels during the first 24 h PBM and again during the period 24–48 h PBM. As shown in Fig. 4C, the α-, β-, β′-, δ-, γ-, and ανδ ζCOPI dsRNA-injected mosquitoes deposited significantly more heme during the first 24 h PBM than the Fluc dsRNA-injected mosquitoes, although the total amount of heme deposited over the entire 48 h period was similar. Not surprisingly, we observed significant decreases in egg production in surviving blood-fed mosquitoes that were injected with 100 ng or more of α-, β-, β′-, δ-, γ-, and ανδ ζCOPI dsRNA, which was consistent with the presence of immature oocytes in the ovaries (Fig. S3).
COPI Function Is Required for Feeding-Induced Midgut Protease Expression.
The observed premature defecation in COPI dsRNA-injected mosquitoes could be caused by inhibition of midgut protease synthesis and/or secretion into the midgut lumen. Therefore, we analyzed expression of two Ae. aegypti midgut serine proteases VI and VII (AaSPVI and AaSPVII) at 24 h PBM in mosquitoes that had been injected with 400 ng COPI or Fluc dsRNA at 3 d before feeding. As shown in Fig. 5A, decreased α-, β-, β′-, γ-, δ-, and ζCOPI expression in blood-fed mosquitoes led to inhibition of both AaSPVI and AaSPVII protein expression. qRT-PCR analysis of individual dsRNA-injected mosquitoes showed that protease protein expression was inhibited at the transcriptional level (Fig. 5B). Although the mechanism of transcriptional induction of AaSPVI and AaSPVII gene expression in blood-fed mosquitoes is not understood, it could be that one or more membrane-dependent signaling pathways are compromised in COPI dsRNA-injected mosquitoes. Importantly, decreased AaSPVI and AaSPVII expression in COPI dsRNA-injected mosquitoes was specific to blood feeding-induced events, because global gene expression and protein synthesis were similar in all samples (Fig. S1).
Fig. 5.
COPI coatomer protein function is required for induction of late-phase protease expression in blood-fed mosquitoes. (A) Western blot analysis of AaSPVI and AaSPVII serine protease protein expression 24 h PBM in midgut epithelial cells of blood-fed mosquitoes that had been injected with 400 ng indicated dsRNA species 4 d earlier. Protein loading was monitored using an anti-GAPDH antibody. (B) qRT-PCR analysis of AaSPVI and AaSPVII transcript levels 24 h PBM in midgut epithelial cells of blood-fed mosquitoes that had been injected with the indicated dsRNA species (400 ng) 4 d earlier. Data show protease transcript levels as the mean value ± SEM in 10 individual mosquitoes using ribosomal protein S7 transcript levels as the internal control. Student t test analysis showed that AaSPVI and AaSPVII transcript levels in α-, β-, β′-, γ-, δ-, and aνδ ζCOPI dsRNA-injected mosquitoes were significantly lower than in Fluc and εCOPI dsRNA-injected mosquitoes (P < 0.001).
Secretion of Eggshell Proteins from Follicular Epithelial Cells Is COPI-Dependent.
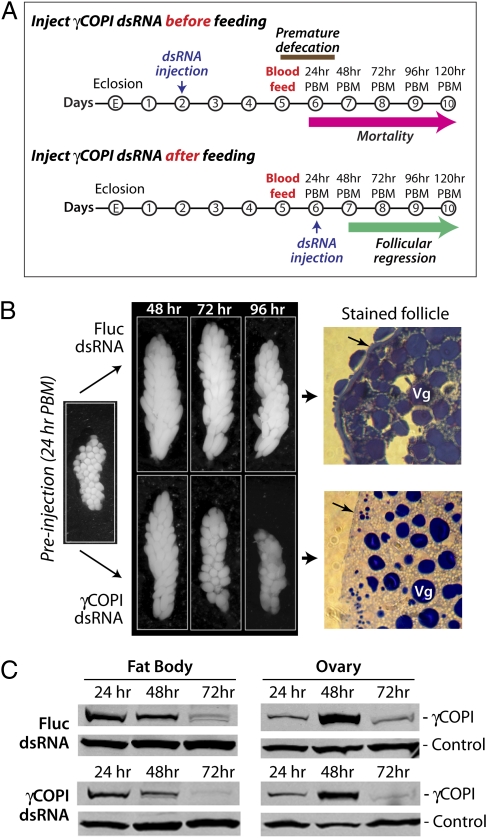
Intrathoracic injection of COPI dsRNA blocks expression of COPI coatomer proteins in not only midgut tissue but also in fat body and ovary tissue (Fig. 1B). However, because blood-fed mosquitoes are unable to complete the gonotrophic cycle when COPI dsRNA is injected before blood feeding (Fig. S3), it is not possible to investigate the role of COPI vesicle transport in oocyte maturation using this approach. Therefore, as illustrated in Fig. 6A, we injected γCOPI dsRNA after blood feeding (24 h PBM) and analyzed oocyte maturation and fecundity at 48–120 h PBM (24–96 h PI). We found that ovaries from Fluc and γCOPI dsRNA-injected mosquitoes were similar at 48 h PBM, but by 72 h PBM, ovaries from γCOPI dsRNA-injected mosquitoes were reduced in size (Fig. 6B). At 96 h PBM, the ovaries had regressed considerably, and average follicle size was 40% smaller in γCOPI dsRNA-injected mosquitoes compared with Fluc dsRNA-injected mosquitoes (Fig. S4). The γCOPI-deficient mosquitoes were provided with oviposition substrate but failed to lay any eggs, and they were unable to blood feed a second time, suggesting that a γCOPI deficiency in the ovaries impacts reproductive capacity beyond the first gonotrophic cycle (Fluc dsRNA-injected mosquitoes were not given oviposition substrate).
Fig. 6.
Knocking down γCOPI coatomer protein expression 24 h PBM inhibits follicular development and blocks eggshell protein secretion. (A) Timeline showing when γCOPI or Fluc dsRNA was injected relative to blood feeding. (B) Photographs are shown of representative ovaries dissected from γCOPI and Fluc dsRNA-injected mosquitoes at 48–96 h PBM, which corresponds to 24–72 h PI (timeline in A). Also shown are photographs of representative follicles obtained from γCOPI or Fluc dsRNA-injected mosquitoes at 72 h PBM (48 h PI) that were imbedded in epoxy resin and stained with toluidine blue and basic fuchsin. The location of the blue staining proteinaceous eggshell layer in a follicle from a Fluc dsRNA-injected mosquito is identified by an arrow, which is clearly missing in the follicle from the γCOPI dsRNA-injected mosquito (Vg, vitellogenin granules). (C) Western blot of γCOPI protein expression in fat body and ovary tissue isolated at various times PBM from mosquitoes injected with Fluc or γCOPI dsRNA at 24 h PBM as in B.
This observed difference in follicular shape and size could be caused by reabsorption within the ovaries. However, SDS/PAGE analysis of an ovarian protein extract as well as Western blotting using an antivitellogenin antibody indicated that the stark morphological differences were not reflected at the protein level (Fig. S4). Instead, it seemed that the smaller follicle size in γCOPI dsRNA-injected mosquitoes was caused by water loss, because toluidine blue staining revealed that regressed oocytes lacked an eggshell protein layer at 72 h PBM (Fig. 6B). Note that the size of vitellogenin granules is similar in follicles isolated from Fluc and γCOPI dsRNA-injected mosquitoes, which is consistent with a vitellogenin Western blot (Fig. S4). National Institutes of Health ImageJ analysis of the Western blot shown in Fig. 6C showed a ∼50% decrease in γCOPI protein at 72 h PBM (48 h PI) in the fat body and ovary of γCOPI dsRNA-injected mosquitoes, suggesting that the follicular regression phenotype may be directly or indirectly caused by a deficiency in γCOPI function.
Discussion
Blood meal metabolism is a challenging feat for female mosquitoes. Nutrients from this large protein-rich liquid meal must be converted into raw materials and used to produce a clutch of eggs within 3 d. Although protein trafficking and vesicle transport mechanisms in the mosquito midgut, fat body, and ovary tissues have not been well-characterized, completion of the gonotrophic cycle clearly relies on these processes to metabolize the blood meal. For example, blood digestion is initiated by rapid and efficient secretion of proteolytic enzymes and peritrophic matrix components from midgut epithelial cells into the lumen, which is followed by uptake of amino acids, oligopeptides, and lipids through membrane-bound transporter proteins. Moreover, fat body cells secrete vitellogenins into the hemolymph where oocytes are able to import them using mechanisms that rely on endosomes and vesicles. Last, ovarian nurse cells and follicular epithelial cells secrete components used by developing eggs to complete embryogenesis after the egg is fertilized and deposited. Defects in protein trafficking and vesicle transport systems in any one of these vital tissues would restrict blood meal metabolism and fecundity.
Developmental studies in Drosophila (31, 32) and silkworms (33) have shown that the COPI vesicle transport system is required for tube expansion during morphogenesis, and RNAi screens in the Drosophila S2 cell line identified COPI coatomer proteins as essential components of protein secretion (23) and lipid droplet formation (21, 22). Based on these studies as well as our own data showing that decreased expression of Ae. aegypti midgut serine proteases [AaSPVI, AaSPVII, and Ae. aegypti Late Trypsin (AaLT)] results in significantly lower egg production (6), we designed experiments to investigate the role of COPI, COPII, and clatharin transport pathways in blood meal digestion and metabolism. In the data reported here, we show that knocking down expression of any one of six COPI coatomer proteins (α, β, β′, γ, δ, and ζ) results in blood feeding-induced mortality in a dsRNA dose-dependent manner. Moreover, we found that blood feeding-induced mortality in COPI dsRNA-injected mosquitoes was preceded by leakage of blood into the hemocoel, reorganization of rough endoplasmic reticulum membrane structures, premature defecation, and inhibition of midgut serine protease expression at the transcriptional level. These phenotypes are dramatically different from the phenotypes recently reported in miR-275–depleted Ae. aegypti mosquitoes (34) and Anopheles gambiae mosquitoes with reduced catalase expression (35).
The molecular structure of several COPI coatomer protein complexes has recently been reported (36–38), which together with molecular structures of COPII and clatharin protein complexes, provides a working model for assembly of the heptameric COPI coatomer complex (39). Because six of seven subunits are thought to form interactions needed for the vesicle coat and induce membrane budding (α, β, β′, γ, δ, and ζ), the loss of any one of these subunits would likely disrupt the entire complex and thereby, block COPI functions. Indeed, studies have shown that mutations or RNAi knockdown of the α, β, β′, γ, δ, and ζ COPI subunits often results in a similar phenotype, whereas loss of the εCOPI subunit is not detrimental (21, 22). These results are similar to what we found in our study (Figs. 2, 4, and 5). A dispensable role of the εCOPI subunit in mediating COPI functions most likely relates to its proposed role as a targeting protein rather than an integral component of the vesicle protein coat (36). We found a significant difference between the blood feeding-induced mortality rate of mosquitoes injected with 100 ng β′COPI dsRNA and blood-fed mosquitoes injected with 100 ng α-, β-, γ-, δ−, or ζCOPI dsRNA (Table S2). These results are consistent with the unique function of the β′COPI subunit in facilitating the formation of the underlying triskelion structure within the lattice of the vesicle coat (37, 39) and similar to a report showing that ingestion of β′COPI dsRNA by corn rootworm larvae is the most lethal dsRNA sequence tested (24).
What causes blood feeding-induced mortality in COPI-deficient Ae. aegypti mosquitoes? Based on the observations that blood from the midgut appears in the hemocoel (Fig. 3B) and the undigested blood meal is prematurely defecated within 24 h PBM (Fig. 4C), it seems likely that the physiological or anatomical stress of blood feeding results in a fatal midgut injury. One possibility is that membrane reorganization and disassembly of RER whorl structures in unfed COPI-deficient mosquitoes (Fig. 3C) as well as the appearance of swollen ER in midguts of these same mosquitoes after feeding contribute to weakening of the single cell layer of midgut epithelium. Styers et al. (19) reported that depletion of βCOPI from human HeLa cells using RNAi resulted in fusion of the ER-Golgi intermediate compartment (ERGIC), trans-Golgi network (TGN), and the Golgi into a single large compartment, which in some cases, caused cell death. They found that global protein synthesis was not altered in the βCOPI RNAi-treated cells and that most other cellular organelles seemed to function normally. We also observed selective effects of COPI knockdown in dsRNA-injected mosquitoes based on EM (Fig. 3C) and SDS/PAGE (Fig. S1) analyses.
Consistent with the idea that the mosquito midgut epithelium was damaged in COPI dsRNA-injected mosquitoes as a consequence of blood feeding, cytological studies of An. gambiae mosquitoes showed that midgut epithelial cells were reduced in thickness by 80% after blood feeding (40). This striking morphological change is associated with increased actin expression, which the study by Sodja et al. (40) suggests may provide structural support to the engorged midgut epithelium. Therefore, if loss of COPI function altered the ability of midgut epithelial cells to undergo morphological changes required to offset increased pressure within the lumen, then the epithelial cell layer could rupture. This proposal is supported by a study showing that the COPI regulatory protein Arf1 (Arf79 in Ae. aegypti) has a role in regulating actin cytoskeleton dynamics (41).
The data in Fig. 6 show that γCOPI dsRNA injection at 24 h PBM permitted blood meal digestion and vitellogenesis; however, mean follicular length was decreased by 40% at 96 h PBM. Follicular regression seemed to be the result of water loss, because there was no qualitative or quantitative difference in the spectrum of ovary proteins present in the γCOPI and Fluc dsRNA-injected mosquitoes (Fig. S4C). This conclusion was supported by histological analysis of the developing oocytes, which indicated that defective follicles lacked a layer of eggshell proteins and most likely, were also devoid of the associated hydrophobic wax layer (Fig. 6B). Based on the timing of the dsRNA injection and what is known about oocyte maturation in mosquitoes (29), we suggest that vitellogenesis is essentially complete in Ae. aegypti by 48 h PBM and that eggshell protein secretion from follicular epithelial cells may be COPI-dependent. It should be possible to use RNAi approaches to investigate a number of other processes governing mosquito oocyte maturation and egg development, which were previously thought to be intractable because of low knockdown efficiencies in ovary tissues that occur when dsRNA is injected before blood feeding.
Materials and Methods
Rearing Mosquitoes.
Ae. aegypti mosquitoes (Rockefeller strain) were used in all experiments and reared as previously described (6). Mosquitoes were blood fed using an artificial feeder containing either bovine blood purchased from Pel-Freez Arkansas LLC or human blood donated by American Red Cross. Blood-fed mosquitoes were inspected by light microscopy, and only fully engorged females were used in the studies described here.
dsRNA Synthesis and Microinjection.
Gene-specific dsRNA was designed, synthesized, and injected as described (6). Briefly, Ae. aegypti COPI coatomer subunit ortholog sequences were retrieved from GenBank, and appropriate gene-specific oligonucleotide primers were designed with a T7 RNA polymerase promoter sequence on the 5′ end (Table S3). After cDNA amplification, the gene-specific PCR products were cloned into the pGEM-T easy vector (Promega), and the plasmid insert was sequenced using a 3730XL DNA Analyzer (Applied Biosystems) by the Genomics Analysis Technology Core facility (University of Arizona). Fluc was used to synthesis nonspecific dsRNA, which served as an injection control in each experiment. Synthesis of dsRNA was performed in vitro using the MEGAscript RNAi Kit (Ambion), purified, and resuspended in nuclease-free water at a concentration of 1–5 μg/μL before storage at −80 °C. Cold-anesthetized 2-d-old sugar-fed female mosquitoes or blood-fed female mosquitoes (24 h PBM) were injected with 50–400 ng dsRNA using a Nanoject II microinjector (Drummond) essentially as described (6). The microinjector was mounted on an MM33 micromanipulator (Märzhäuser Wetzlar) and operated hands-free by an electric foot peddle. This setup provides excellent control of needle insertion in or near the right mesothoracic spiracle to a shallow depth of no more than ∼0.2 mm, which we find is critical. To maximize control over the depth of needle penetration, we placed each cold-anesthetized mosquito on a small foam pad and raised it up to the mounted microneedle with two hands to properly insert the needle using the aid of a dissecting microscope. By adjusting the concentration of the dsRNA stock solution to maintain injection volumes under 210 nL and bringing the anesthetized mosquitoes up to the stationary needle using the foam pad rather than inserting the microneedle into the stationary mosquito, we routinely achieve 100% survival of all injected mosquitoes with >90% knockdown in >90% of injected mosquitoes (Table S1). Injected mosquitoes are allowed to recover for 48–72 h in an inverted netted cup placed over a small cotton pad containing 10% sucrose, which provides easy access to food without the need to fly.
Western Blotting.
Midgut, fat body, and ovary tissues of cold-anesthetized mosquitoes were dissected in 1× PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) under a dissecting microscope. Tissues were manually homogenized with a pellet pestle (Kontes) in either nondenaturing homogenization buffer (50 mM Tris·HCl, pH 8.0, 10 mM CaCl2) or lysis buffer (12 mM sodium deoxycholate, 0.2% SDS, 1% triton X-100, protease inhibitor mixture dissolved in 1× PBS). Protein extracts were stored at −80 °C until analysis by SDS/PAGE and Western blotting using a 12% acrylamide resolving gel and 3% stacking gel. The resolved proteins were electrophoretically transferred to a nitrocellulose membrane (Odyssey Nitrocellulose; LI-COR Biosciences). The membranes were blocked with 4% nonfat dry milk for 1 h and incubated with primary antibodies in 4% skim milk in 1× PBS containing 0.1% Tween 20 overnight at 4 °C. Rabbit polyclonal antibodies directed against Ae. aegypti γCOPI were prepared by GenScript using a peptide with the amino acid sequence NH3-CDPTTGLPDSDEGY-COOH. Rabbit polyclonal antibodies directed against the Ae. aegypti midgut proteases AaSPVI, AaSPVII, and AaLT have been previously described (6). Anti-GAPDH antibody was obtained from Abcam, anti–α-tubulin monoclonal antibody was obtained from Developmental Studies Hybridoma Bank, and antivitellogenin antibody was provided by Alexander Raikhel (University of California, Riverside, CA). The dilutions of the primary antibodies were as follows: γCOPI (1:2,000), AaSPVI (1:1,000), AaSPVII (1:1,000), AaLT (1:200), vitellogenin (1:5,000), GAPDH (1:500), and α-tubulin (1:2,000). The secondary antibodies were either IRDye 800CW goat anti-rabbit secondary antibody (1:10,000; LI-COR Biosciences) or IRDye 800CW goat anti-mouse secondary antibody (1:10,000; LI-COR Biosciences), both of which were incubated with the filter for 1 h. Fluorescence of immunoreactive protein bands was directly visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences).
Measuring Knockdown Efficiency of dsRNA.
Knockdown efficiency of each injected dsRNA was verified by real-time qRT-PCR using gene-specific qRT-PCR primers (Table S3) as described (6). Briefly, cDNA was prepared from DNase I pretreated total RNA isolated from individual dsRNA-injected mosquitoes that were either sugar- or blood-fed (24 h PBM). qRT-PCR was performed using PerfeCTa SYBR Green FastMix, ROX (Quanta BioSciences), and the 7300 Real-Time PCR System (Applied Biosystems). Ribosomal protein S7 transcript levels were used as an internal control for normalization of mRNA yields in all samples. The knockdown efficiency of gene-specific dsRNA was determined using Fluc dsRNA-injected mosquitoes as a control.
Microscopy.
Mosquito midguts used for transmission EM were fixed for 1 h at room temperature (2.5% glutaraldehyde, 2% formaldehyde, 0.1 M Pipes buffer, pH 7.4), transferred to 1% osmium tetroxide for 1 h, washed in deionized water, and stained with 2% aqueous uranyl acetate for 30 min. Specimens were dehydrated with ethanol dilutions, infiltrated with Spurr's resin, and flat-embedded at 60 °C. Longitudinal sections (50 nm) were prepared using a Leica UC2T ultramicrotome and placed onto uncoated 150-mesh copper grids followed by counterstaining with lead citrate. Midgut sections were viewed with an FEI CM12S electron microscope operated at 80 kV, and TIFF mages (8 bit) were captured using an AMT 4M pixel camera. Dissected ovaries used for light microscopy were fixed in 2.5% glutaraldehyde in 0.1 M Pipes buffer (pH 7.4) for 60 min at 4 °C and washed in 0.1 M Pipes buffer before imbedding in LX112 epoxy resin. Thin sections (0.5 μm) from the embedded tissues were stained with toluidine blue and basic fuchsin.
Fecal Heme Measurements.
Fully engorged blood-fed mosquitoes were verified by light microscopy, and pools of five fed mosquitoes were immediately transferred to glass vials with a meshed cap. Mosquitoes were provided 10% sugar on cotton pads for the duration of the experiment. After 24 h, mosquitoes were transferred to a clean vial and maintained for another 24 h (48 h PBM). Because some COPI dsRNA-injected mosquitoes died within 24 h PBM, dead mosquitoes were replaced by live mosquitoes that were injected with the same COPI dsRNA for inclusion in the second 24-h sampling period. Some vials contained filter paper at the bottom to collect feces for photodocumentation, whereas the majority of glass vials were used directly for heme measurements based on a modified pyridine hemochrome method (42, 43). Briefly, fecal samples were dissolved in 50 mM NaOH, and an aliquot was added to a 20% pyridine solution in 100 mM NaOH. A few crystals of sodium dithionite were added to the solution to reduce the heme and form the pyridine hemochrome. The pyridine hemochrome was measured immediately on a CARY 50 UV visible spectrophotometer (Varian). Sample heme concentration was calculated from the absorbance at 556 nm minus the absorbance at 700 nm using an extinction coefficient of 32 mM−1 cm−1.
Statistical Analysis.
The appropriate statistical analysis was used to compare data from Fluc and COPI dsRNA-injected mosquitoes to determine whether RNAi-mediated knockdown of COPI coatomer subunits had a significant effect on mortality, heme deposition, protease gene expression, and reproduction, (GraphPad Software Inc). Specifically, Kaplan–Meier analysis with a log-rank significance test was used to analyze mortality data in Fig. 2, ANOVA with multiple comparisons was used to analyze heme deposition (Fig. 4C), and unpaired Student t test was used to analyze the effect of RNAi-mediated knockdown on fecundity (Fig. 5B), protease gene expression (Fig. 6C), and follicle length (Fig. S4).
Supplementary Material
Acknowledgments
We thank Mary Hernandez for rearing mosquitoes, Weston Stover and Ada Lee for help with analyzing mortality rates in the initial knockdown experiments, and Alex Raikhel for the Ae. aegypti vitellogenin antibody. This work was supported by National Institutes of Health Grants R01AI031951 and R01AI046541 (to R.L.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 9737.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102637108/-/DCSupplemental.
References
- 1.Enayati A, Hemingway J. Malaria management: Past, present, and future. Annu Rev Entomol. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JM, Taylor CE. Malaria control with transgenic mosquitoes. PLoS Med. 2009;6:e20. doi: 10.1371/journal.pmed.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knols BG, Bossin HC, Mukabana WR, Robinson AS. Transgenic mosquitoes and the fight against malaria: Managing technology push in a turbulent GMO world. Am J Trop Med Hyg. 2007;77(Suppl 6):232–242. [PubMed] [Google Scholar]
- 4.Brandon MC, et al. TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2008;38:916–922. doi: 10.1016/j.ibmb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isoe J, Zamora J, Miesfeld RL. Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect Biochem Mol Biol. 2009;39:68–73. doi: 10.1016/j.ibmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isoe J, Rascón AA, Jr, Kunz S, Miesfeld RL. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2009;39:903–912. doi: 10.1016/j.ibmb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaraffia PY, et al. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2008;105:518–523. doi: 10.1073/pnas.0708098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen CC, Beebe NW. The dengue vector Aedes aegypti: What comes next. Microbes Infect. 2010;12:272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Whitehorn J, Farrar J. Dengue. Br Med Bull. 2010;95:161–173. doi: 10.1093/bmb/ldq019. [DOI] [PubMed] [Google Scholar]
- 10.Paknikar KM. Landmark discoveries in intracellular transport and secretion. J Cell Mol Med. 2007;11:393–397. doi: 10.1111/j.1582-4934.2007.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck R, Rawet M, Wieland FT, Cassel D. The COPI system: Molecular mechanisms and function. FEBS Lett. 2009;583:2701–2709. doi: 10.1016/j.febslet.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Nakano A. Mechanisms of COPII vesicle formation and protein sorting. FEBS Lett. 2007;581:2076–2082. doi: 10.1016/j.febslet.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhausen T. Imaging endocytic clathrin structures in living cells. Trends Cell Biol. 2009;19:596–605. doi: 10.1016/j.tcb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostermann J, et al. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- 15.Rowe T, et al. COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D, Hay JC. Reconstitution of COPII vesicle fusion to generate a pre-Golgi intermediate compartment. J Cell Biol. 2004;167:997–1003. doi: 10.1083/jcb.200408135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney GE, Bowen NJ. p24 proteins, intracellular trafficking, and behavior: Drosophila melanogaster provides insights and opportunities. Biol Cell. 2004;96:271–278. doi: 10.1016/j.biolcel.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Fromme JC, Orci L, Schekman R. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 2008;18:330–336. doi: 10.1016/j.tcb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Styers ML, O'Connor AK, Grabski R, Cormet-Boyaka E, Sztul E. Depletion of beta-COP reveals a role for COP-I in compartmentalization of secretory compartments and in biosynthetic transport of caveolin-1. Am J Physiol Cell Physiol. 2008;294:C1485–C1498. doi: 10.1152/ajpcell.00010.2008. [DOI] [PubMed] [Google Scholar]
- 20.Rennolds J, et al. Cystic fibrosis transmembrane conductance regulator trafficking is mediated by the COPI coat in epithelial cells. J Biol Chem. 2008;283:833–839. doi: 10.1074/jbc.M706504200. [DOI] [PubMed] [Google Scholar]
- 21.Beller M, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bard F, et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439:604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- 24.Baum JA, et al. Control of coleopteran insect pests through RNA interference. Nat Biotechnol. 2007;25:1322–1326. doi: 10.1038/nbt1359. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Isoe J, Day WA, Miesfeld RL. Alpha-COPI coatomer protein is required for rough endoplasmic reticulum whorl formation in mosquito midgut epithelial cells. PLoS One. 2011;6:e18150. doi: 10.1371/journal.pone.0018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Béthune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- 27.Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009;10:58–64. doi: 10.1038/embor.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–3917. doi: 10.1093/emboj/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquardt WC, editor. Biology of Disease Vectors. 2nd Ed. London: Elsevier; 2005. [Google Scholar]
- 30.Rudin W, Hecker H. Functional morphology of the midgut of Aedes aegypti L. (Insecta, Diptera) during blood digestion. Cell Tissue Res. 1979;200:193–203. doi: 10.1007/BF00236412. [DOI] [PubMed] [Google Scholar]
- 31.Grieder NC, et al. gammaCOP is required for apical protein secretion and epithelial morphogenesis in Drosophila melanogaster. PLoS One. 2008;3:e3241. doi: 10.1371/journal.pone.0003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaram SA, et al. COPI vesicle transport is a common requirement for tube expansion in Drosophila. PLoS One. 2008;3:e1964. doi: 10.1371/journal.pone.0001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, et al. Silkworm coatomers and their role in tube expansion of posterior silkgland. PLoS One. 2010;5:e13252. doi: 10.1371/journal.pone.0013252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryant B, Macdonald W, Raikhel AS. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2010;107:22391–22398. doi: 10.1073/pnas.1016230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magalhaes T, Brackney DE, Beier JC, Foy BD. Silencing an Anopheles gambiae catalase and sulfhydryl oxidase increases mosquito mortality after a blood meal. Arch Insect Biochem Physiol. 2008;68:134–143. doi: 10.1002/arch.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsia KC, Hoelz A. Crystal structure of alpha-COP in complex with epsilon-COP provides insight into the architecture of the COPI vesicular coat. Proc Natl Acad Sci USA. 2010;107:11271–11276. doi: 10.1073/pnas.1006297107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C, Goldberg J. Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats. Cell. 2010;142:123–132. doi: 10.1016/j.cell.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu W, Lin J, Jin C, Xia B. Solution structure of human zeta-COP: Direct evidences for structural similarity between COP I and clathrin-adaptor coats. J Mol Biol. 2009;386:903–912. doi: 10.1016/j.jmb.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 39.Harrison SC, Kirchhausen T. Structural biology: Conservation in vesicle coats. Nature. 2010;466:1048–1049. doi: 10.1038/4661048a. [DOI] [PubMed] [Google Scholar]
- 40.Sodja A, Fujioka H, Lemos FJ, Donnelly-Doman M, Jacobs-Lorena M. Induction of actin gene expression in the mosquito midgut by blood ingestion correlates with striking changes of cell shape. J Insect Physiol. 2007;53:833–839. doi: 10.1016/j.jinsphys.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers KR, Casanova JE. Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol. 2008;18:184–192. doi: 10.1016/j.tcb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry EA, Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair PR, Gorman N, Jacobs JM. Measurement of heme concentration. Curr Protoc Toxicol. 2001;8:8.3. doi: 10.1002/0471140856.tx0803s00. [DOI] [PubMed] [Google Scholar]