Abstract
During the early stages of HIV-1 replication the conical capsid composed of p24CA protein dissociates from the rest of the cytoplasmic viral complex by a process called uncoating. Although proper uncoating is known to be required for HIV-1 infection, many questions remain about the timing and factors involved in the process. Here we have used two complementary assays to study the process of uncoating in HIV-1–infected cells, specifically looking at the timing of uncoating and its relationship to reverse transcription. We developed a fluorescent microscopy-based uncoating assay that detects the association of p24CA with HIV-1 viral complexes in cells. We also used an owl monkey kidney (OMK) cell assay that is based on timed TRIM-CypA–mediated restriction of HIV-1 replication. Results from both assays indicate that uncoating is initiated within 1 h of viral fusion. In addition, treatment with the reverse transcriptase inhibitor nevirapine delayed uncoating in both assays. Analysis of reverse transcription products in OMK cells revealed that the generation of early reverse transcription products coincides with the timing of uncoating in these assays. Collectively, these results suggest that some aspect of reverse transcription has the ability to influence the kinetics of uncoating.
Keywords: early events, retrovirus
After the HIV membrane fuses with the target cell membrane a poorly defined sequence of events occurs to establish infection. Viral fusion results in release of the viral genome into the cytoplasm of the target cell. The two viral genomic RNAs and associated proteins are initially contained within a conical capsid structure composed of p24CA protein. For infection to progress the capsid structure disassembles from the viral complex in a process referred to as uncoating. It is not clear whether all p24CA molecules dissociate from the complex or whether a subset remains. The released reverse transcription complex (RTC) matures into a double-stranded DNA containing preintegration complex (PIC), which is transported into the nucleus, where it integrates into the chromosomal DNA of the target cell. Although some of these early steps in HIV replication have been well described, other steps, such as uncoating, remain enigmatic. In addition, the interplay between these early stages of infection remains unclear. Therefore, the goal of this study was to investigate the uncoating step of HIV-1 replication, specifically looking at the timing of uncoating after viral fusion and the relationship between uncoating and reverse transcription.
There are a multiple models for the timing and cellular location of HIV-1 uncoating. An early model suggested that uncoating occurs immediately after viral fusion on the basis of the absence of significant amounts of p24CA from purified RTC/PIC preparations (1–3). Unfortunately, poor capsid stability confounds the isolation of these structures from cells (4). However, the ability of TRIM5α and TRIM-CypA to restrict HIV infection via interactions with the intact conical capsid reveals that this structure persists for some time after fusion (5–8). Another model suggests that during uncoating p24CA is not shed from the RTC but rather that the intact capsid docks at a nuclear pore, as has been observed for other viruses (9, 10). It has also been proposed that uncoating takes place as the reverse transcribing viral genome is transported toward the nucleus. This model is based on fluorescence microscopy observations that both cytoplasmic RTCs associated with or without p24CA can be found on microtubules (11). Collectively, these data suggest that uncoating occurs at some maturation point during delivery of the viral genome to the nucleus. However, it is unclear whether uncoating occurs early or late during genome generation and migration.
A number of studies suggest that the process of uncoating is modulated by viral and cellular factors. A seminal study by Forshey et al. (12) identified mutations in p24CA that reduced viral infectivity and altered capsid stability in an in vitro assay. These p24CA mutations also exhibited a differential ability to saturate restriction mediated by rhesus TRIM5α or owl monkey TRIM-CypA, compared with wild-type capsid (7, 8). Because only an intact capsid can compete for TRIM protein binding, these results support the observation that mutations in p24CA can stabilize or destabilize the HIV-1 capsid. Most of these p24CA mutant viruses also displayed defects in reverse transcription in vivo (12). These experiments indicated that determinants in HIV p24CA play a role in conical capsid stability and that the correct timing of uncoating is important for HIV-1 infection. Furthermore, these studies suggest that there is a relationship between uncoating and efficient reverse transcription. In support of this idea, another study suggested that reverse transcription is required for uncoating on the basis of fluorescent microscopy observations (9). Together these data indicate that uncoating is not merely an event upstream of reverse transcription but rather that there is an interplay between uncoating and reverse transcription of the viral genome.
Results
Development of an in Situ Uncoating Assay.
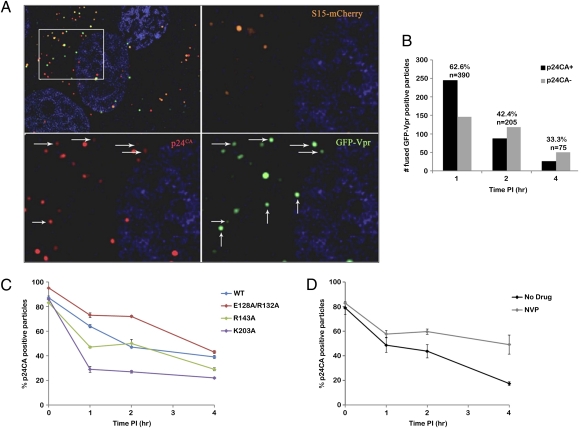
To gain insight into the process of uncoating we used in situ fluorescent staining as previously described (13, 14). VSV-g pseudotyped HIV is labeled with two fluorescent proteins to distinguish between virions that have productively entered the cytoplasm through a fusion event from those that remain in endosomes (13). The viral core is labeled with GFP-Vpr, which allows detection of cell-associated virions (11). The viral membrane is labeled with S15-mCherry, which is lost upon productive fusion of the virion (13, 15). For the in situ uncoating assay, dual-labeled virions are synchronized for infectivity by spinoculation at a temperature that is not permissive for fusion (16). After spinoculation, the inoculation media is exchanged for warm media and cells are incubated at 37 °C. The negative control is inhibition of fusion with the drug bafilomycin A (BafA) (17). At various times after infection cells are fixed and stained for p24CA. The experiment is then imaged, and the cell-associated virus is counted in each image. To measure uncoating, viral complexes that have productively fused (GFP-positive and mCherry-negative) are counted and classified as either coated (associated with p24CA) or uncoated (not associated with detectable p24CA; Fig. 1A).
Fig. 1.
Results from the in situ uncoating assay. (A) Top Left: One Z-section from a 1 h time point. The outlined box is enlarged in the other three panels to show S15-mCherry, GFP-Vpr, and p24CA signals. Fused virions are punctate signals that are GFP positive and mCherry negative, and were classified as associated with p24CA (horizontal arrows) or not associated with p24CA (vertical arrows). (B) Number of coated and uncoated fused virions counted at 1, 2, and 4 h post-infection. Above each bar is the percentage of coated viral particles and the total number of virions counted. (C) In situ uncoating assay was conducted on wild-type and p24CA mutants. Graphed is the percentage of coated viral particles at each time point. Error bars denote SE among three independent experiments. (D) In situ uncoating assay was conducted in the presence or absence of nevirapine (NVP). Graphed is the percentage of coated viral particles at each time point. Error bars denote the SE among two independent experiments.
Analysis of fixed cells 1 to 4 h post-infection showed that the number of fused GFP-Vpr–labeled particles decreased over time, as previously observed (Fig. 1B) (13). One hour after infection, p24CA was detected in 62.6% of fused particles, and this amount steadily declined to 4 h post-infection (Fig. 1B). Conversely, 85% of HIV-1–labeled particles contained p24CA at 4 h when fusion was inhibited with BafA (Fig. S1). Therefore, we conclude that p24CA remains associated with the HIV-1 core after fusion and is lost over time.
We next wanted to validate the in situ assay using viruses with mutations in p24CA. Three mutants were examined that previously have been described to alter capsid stability and reduce HIV-1 replication (12, 18). The mutants tested had a capsid that was either more stable (E128A/R132A), half as stable (R143A), or less stable (K203A) than wild-type HIV-1 capsid in biochemical assays (12). For each mutant, dual-labeled VSV-g pseudotyped virus was produced with incorporation of both fluorescent proteins similar to wild type. There was a general correlation between results of the in situ uncoating assay and capsid stability data from biochemical assays, such that E128/R132A uncoated more slowly than wild type, and K203A uncoated more rapidly than wild type, whereas R143A displayed an intermediate phenotype (Fig. 1C). These results validate the use of the in situ uncoating assay to detect changes in the rate of uncoating. Although these alterations in uncoating kinetics are only two- or threefold, viral infectivity is significantly decreased, indicating that the correct timing of uncoating is important for viral replication (12).
Effect of Reverse Transcription on Uncoating.
Some models propose a relationship between uncoating and reverse transcription (9, 11). Therefore, we tested whether inhibiting reverse transcription could affect uncoating kinetics by conducting the in situ uncoating assay in the presence or absence of the nonnucleotide reverse transcriptase inhibitor nevirapine. In the control infection, the number of capsid-associated complexes decreased over time, as in previous experiments (Fig. 1D). In contrast, nevirapine treatment caused the association of p24CA with GFP-Vpr–labeled viral complexes to persist over time (Fig. 1D). BafA controls showed no evidence of uncoating (Fig. S1). These results suggest that when reverse transcription is inhibited, p24CA remains associated with HIV-1 cores for longer times after fusion. Therefore, we propose that when reverse transcription is impaired, uncoating is delayed.
Cyclosporine A Washout Assay to Study Uncoating.
We decided to confirm the results of the in situ uncoating assay using an alternative approach adapted from studies on the kinetics of HIV-1 restriction by TRIM-CypA, a protein that has two characteristics that enable its use to test uncoating (19). First, TRIM-CypA inhibits HIV-1 replication by recognizing and binding to the intact conical capsid, implying that only replication of coated virions is inhibited (5–7). Second, the binding of TRIM-CypA to the viral capsid can be blocked by the drug cyclosporine A (CsA), therefore allowing virions to replicate in TRIM-CypA–expressing cells when CsA is present (5, 20, 21).
For this assay, owl monkey kidney (OMK) cells that endogenously express TRIM-CypA were spinoculated with a GFP reporter virus (VSV-g pseudotyped HIV-GFP) in the presence of CsA (16). After spinoculation, plates were incubated at 37 °C for 30 min to allow cell recovery and fusion of bound virus. Afterward, virus-containing media was exchanged for warm media, and CsA was washed out of the zero time point reactions by media exchange. CsA washout continued in the same manner at each additional time point. When CsA was washed out any virus that had an intact capsid, or had not uncoated, became susceptible to TRIM-CypA restriction and should not be able to infect the cell. However, virions that had progressed through the life cycle to the point where they lacked an intact capsid (uncoated) were no longer sensitive to TRIM-CypA restriction and could infect the cell. Cells were harvested 2 d after infection and subjected to flow cytometry to determine the percentage of infected cells. The percentage of GFP-positive cells was averaged for each triplicate reaction and graphed (Fig. 2 and Fig. S2). Because only uncoated particles can infect the cell during TRIM-CypA restriction, the percentage of GFP-positive cells at each washout time point should be representative of the percentage of uncoated virions.
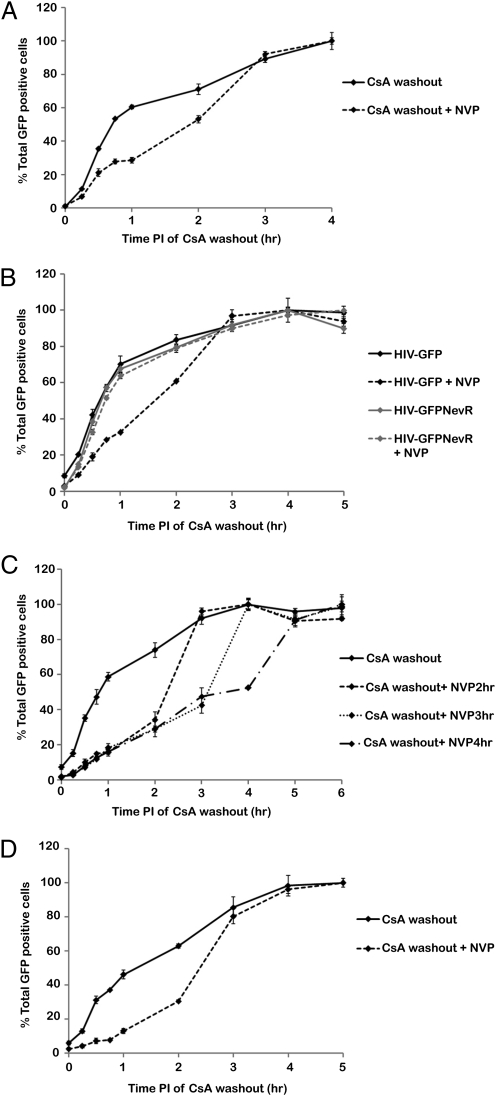
Fig. 2.
Results from the CsA washout assay. (A) CsA washout assay was conducted with or without a 2 h nevirapine (NVP) treatment. Graphed is the percentage of GFP-positive cells at each time of CsA washout normalized by setting the percentage at 4 h to 100%. Shown is a representative experiment from eight independent experiments. Error bars denote SE. (B) CsA washout assay was conducted using HIV-GFP or HIV-GFPNevR with or without a 2 h nevirapine treatment. Shown are normalized data of a representative experiment from four independent experiments. Error bars denote SE. (C) CsA washout assay was conducted using HIV-GFP without nevirapine treatment or with nevirapine treatment for 2, 3, or 4 h. Shown are normalized data of a representative experiment from four independent experiments. Error bars denote SE. (D) CsA washout assay was conducted using R7X4-GFP with or without a 2 h nevirapine treatment. Shown are normalized data of a representative experiment from three independent experiments. Error bars denote SE.
In the CsA washout assay the percentage of GFP-positive cells increased over time, leveling off 4 to 5 h after infection (Fig. S2). This assay reveals that the majority of particles uncoat by 2 h post-infection. These data were then normalized by setting the percentage of GFP-positive cells at 4 h after infection to 100% (Fig. 2A). The half-life of uncoating for this representative experiment is at 42 min post-infection. The overall percentages of GFP-positive cells varied slightly from experiment to experiment, so we averaged the time of 50% uncoating from eight independent experiments. We found that the half-life of uncoating occurred at an average of 39 min post-infection (Table 1).
Table 1.
Half-lives of uncoating and fusion in the CsA washout assay
| Virus | Uncoating (min) | Uncoating + NVP (min) | Fusion (min) | Normalized uncoating (min) |
| HIV-GFP | 39.12 (4.14) | 117.87 (8.21) | 15.81 (3.02) | 23.31 |
| HIV-GFPNevR | 38.94 (4.02) | 36.25 (3.13) | ND | ND |
| R7X4-GFP | 73.5 (4.72) | 142.57 (1.99) | 48.33 (0.19) | 25.17 |
Time after infection at which 50% of cells were GFP positive in the normalized CsA washout assay was calculated from multiple independent experiments and averaged for HIV-GFP, HIV-GFPNevR, and R7X4-GFP. The half-life of viral fusion for HIV-GFP and R7X4-GFP was calculated and averaged. SE is in parenthesis. Normalized half-life of uncoating was calculated by subtracting the half-life of fusion from uncoating for HIV-GFP and R7X4-GFP. ND, not determined.
Effect of Inhibition of Reverse Transcription on Uncoating in the CsA Washout Assay.
To determine whether inhibition of reverse transcription can alter the rate of uncoating, the CsA washout assay was conducted in the presence or absence of nevirapine. However, because the ultimate readout of this assay is infected GFP-positive cells, nevirapine could not be present in the culture media indefinitely. Therefore, we performed a nevirapine washout assay and determined that HIV infectivity could be partially recovered after nevirapine treatment (Fig. S3). We decided to use a 2 h nevirapine treatment because ≈50% of the infectivity could be recovered. The CsA washout assay was then performed in which nevirapine was present in the inoculation and culture media and was washed out at the 2 h time point. Treatment with nevirapine produced a shift in the washout assay curve compared with no treatment, with the percentage of GFP-positive cells leveling off 4 to 5 h after infection (Fig. 2A and Fig. S2). Similarly, the half-life of uncoating increased to 1 h and 52 min post-infection (Fig. 2A). From eight experiments we calculated that 50% of virions uncoated by an average of 1 h and 58 min post-infection when exposed to nevirapine for 2 h (Table 1).
To control for nonspecific effects of nevirapine, the CsA washout assay with nevirapine treatment was conducted using a nevirapine-resistant virus. The Y181C mutation of reverse transcriptase that generates nevirapine resistance was cloned into HIV-GFP to create HIV-GFPNevR (Fig. S2) (22, 23). HIV-GFPNevR uncoated with similar kinetics as HIV-GFP in the absence of nevirapine, and 50% of virions uncoated by 39 min post-infection (Fig. 2B, Table 1, and Fig. S2). Unlike HIV-GFP, HIV-GFPNevR displayed similar uncoating kinetics and half-lives of uncoating with and without nevirapine treatment (Fig. 2B, Table 1, and Fig. S2). These results indicate that the effect of nevirapine on HIV reverse transcriptase to inhibit reverse transcription is responsible for the alteration in uncoating kinetics rather than a side effect of nevirapine on another cellular or viral factor.
Uncoating in the CsA washout assay is defined as a loss of sensitivity to TRIM-CypA restriction. It is possible that reverse transcription within a coated viral complex could proceed to a point at which the intact capsid becomes resistant to TRIM-CypA restriction, which would mimic uncoating in the CsA washout assay. In this case, nevirapine treatment would also affect the rate of uncoating, similar to that observed in Fig. 2. To test this idea we performed the CsA washout assay using a virus with Q63/67A mutations in p24CA to uncouple the processes of uncoating and reverse transcription. This mutant virus has previously been shown to uncoat more slowly compared with wild type in the in situ uncoating assay, but proceeds through reverse transcription at a rate similar to wild-type virus (Fig. S4) (12, 14, 24). In the CsA washout assay Q63/67A uncoated much more slowly than wild type (Fig. S4). These results suggest that progression through reverse transcription is not sufficient to make the viral capsid resistant to TRIM-CypA restriction.
Next we examined the effects of longer nevirapine treatments on the timing of uncoating. Treatment with nevirapine for 3 and 4 h produced similar shifts in the washout assay curve as observed after 2 h of treatment (Fig. 2C and Fig. S2). For all time points, most of the remaining virus became resistant to TRIM-CypA restriction after 1 additional hour. The consistent resistance to TRIM-CypA restriction within 1 h of nevirapine removal is consistent with the model that reverse transcription influences uncoating.
Finally, we used the CsA washout assay to determine the effect of reverse transcription on uncoating of wild-type HIV-1. The CsA washout assay was conducted with replication competent R7X4-GFP virus. Similar to HIV-GFP, the percentage of GFP-positive cells increased over time, leveling off by 5 h after infection (Fig. 2D and Fig. S2). Treatment with nevirapine also delayed uncoating of R7X4-GFP (Fig. 2D and Fig. S2). The average half-life of uncoating for R7X4-GFP was 74 min post-infection, and nevirapine treatment increased this average half-life to 2 h and 23 min post-infection (Table 1).
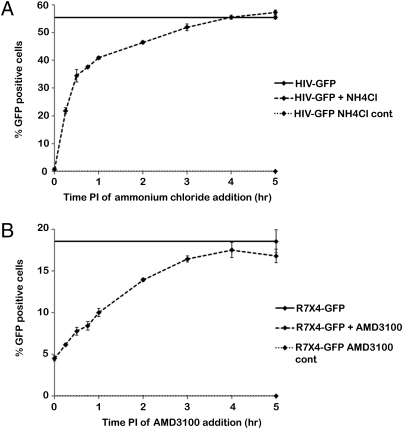
Interestingly, the half-life of uncoating for R7X4-GFP was greater than that for HIV-GFP, but both viruses displayed a similar increase in the half-life in response to nevirapine treatment. Therefore, we hypothesized that differences in viral fusion may account for the apparent increased half-life of uncoating in R7X4-GFP. To determine the half-life of viral fusion in the OMK cell assay system fusion inhibitor add-in assays were conducted similar to the CsA washout assay, except CsA was maintained in the culture media and the inhibitor was added at various times after infection. The average half-life of the VSV-g–mediated fusion reaction was 15 min in an ammonium chloride add-in assay using HIV-GFP (Fig. 3A and Table 1). The average half-life of the HIV Env-mediated fusion reaction was 48 min in an AMD3100 add-in assay using R7X4-GFP (Fig. 3B and Table 1). This 33-min difference in fusion largely accounts for the differences in half-life of uncoating when comparing HIV-GFP and R7X4-GFP virus (Table 1). These results demonstrate that the kinetics of uncoating as determined with the CsA washout assay are essentially the same if fusion is mediated by either VSV-g or wild-type HIV envelope.
Fig. 3.
Viral fusion in OMK cells. (A) Ammonium chloride add-in assay was conducted to examine VSV-g–mediated viral fusion in OMK cells. (B) AMD3100 add-in assay was conducted to examine HIV Env-mediated viral fusion in OMK cells. For both A and B, controls were continuous drug treatment and no treatment. Shown is a representative experiment from three independent experiments. Error bars denote SE.
Progression of Reverse Transcription During the Time Frame of Uncoating.
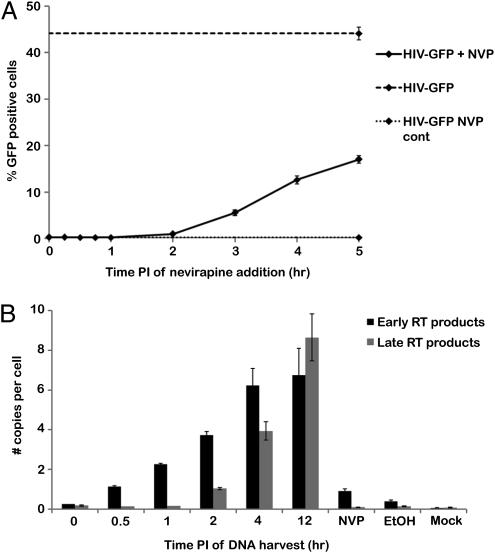
Finally, we wanted to gain insight into the extent of reverse transcription required to affect uncoating kinetics. To determine whether completion of reverse transcription correlated with uncoating we performed a nevirapine add-in assay with HIV-GFP virus in OMK cells. This assay was conducted similar to the CsA washout assay, except CsA was maintained in the culture media and nevirapine was added at various times after infection. Addition of nevirapine completely inhibited infection up to 1 h after infection, with a slight increase in the percentage of infected cells (0.5%) by 2 h post-infection (Fig. 4A). Because completion of reverse transcription is necessary for nevirapine resistance these data demonstrate that a small percentage of virions completed reverse transcription by 2 h post-infection. If we compare these data to the CsA washout assay, completion of reverse transcription occurs later than the majority of uncoating (Fig. 2).
Fig. 4.
Assays to correlate reverse transcription with uncoating. (A) Nevirapine (NVP) add-in assay was performed in OMK cells. Controls were continuous treatment with nevirapine and no treatment. Shown is a representative experiment from three independent experiments. Error bars denote SE. (B) Real-time PCR analysis was conducted on OMK cells infected with HIV-GFP. Controls were ethanol treatment, nevirapine treatment, and mock infection. Shown is a representative experiment from three independent experiments. Error bars denote SE among three reactions.
We also performed real-time PCR analysis for HIV-1 cDNA products indicative of early and late reverse transcription under conditions identical to the CsA washout assay. Cells were harvested at times corresponding to those used in the CsA washout assay. At 12 h after infection, cells were harvested for an additional experimental time point and the nevirapine, ethanol, and mock-infected control reactions. DNA was isolated and subjected to real-time PCR analysis using primers for early and late reverse transcription products. A small amount of early reverse transcription products were found over background levels at the 0 h time point, likely due to natural endogenous reverse transcription, and this amount increased until 4 h post-infection (Fig. 4B) (25). Late reverse transcription products remained at background levels until 2 h post-infection. The late reverse transcription product results correlate with the nevirapine add-in data (Fig. 4A), whereas the increase in early reverse transcription products coincides with uncoating in the CsA washout assay (Fig. 2). Together, these data suggest that early stages in reverse transcription, rather than late stages or completion of reverse transcription, may influence the rate of uncoating.
Discussion
Much of what is currently known about HIV-1 uncoating has come from biochemical and mutation-based analyses, but the process of uncoating within infected cells is poorly defined. Here we have used two different assays to study the kinetics of uncoating in HIV-1–infected cells. The assays complement each other when considering the strengths and weaknesses of each for detecting HIV-1 uncoating in cells.
The in situ uncoating assay directly detects uncoating by visualizing the association of p24CA with cytoplasmic viral complexes. We validated the assay using p24CA mutants and found a general correlation between results of the in situ uncoating assay and biochemical assays (Fig. 1C). Interestingly, the most striking differences in uncoating between these mutants and wild-type occurred at earlier time points, suggesting that alterations in the rate of uncoating early after fusion may severely impact viral replication. However, there are limitations in the in situ uncoating assay due to the dual labeling system required to observe these complexes. Because dual-labeled virions are generated by cotransfection it is not possible to label the membrane of all particles. We use viral preparations that have S15-mCherry membrane labeling of 80–90%, but viral particles that lack a membrane label are counted as fused coated particles in the assay, which could elevate the number of coated particles counted at each time point. GFP-Vpr–labeled virions have never been observed within the nucleus, indicating that the majority of GFP-Vpr is lost from the viral complex in the cytoplasm. This loss of signal makes it difficult to quantify all uncoated viral complexes in the cytoplasm, so the number of uncoated virions may be greater than what is counted. Because of these limitations the in situ assay is the more qualitative of the two uncoating assays we used.
The in situ assay will quantify viral particles that will not lead to infectious events. It has previously been shown that only a subset of cytoplasmic virions progress to productive infection. For example, approximately one third of reverse transcribed genomes that reach the nucleus integrate into the target cell genome (26). In contrast, the readout of the CsA washout assay is infectivity. Therefore, only virions that result in productive infection are counted in this assay. Because the CsA washout assay can be done in a high-throughput manner, more time points can be analyzed and the experiment can be repeated multiple times. Therefore, we consider the CsA washout assay a highly quantitative assay. However, in this assay uncoating is defined by a loss of sensitivity to TRIM-CypA restriction. Because it is not known how the process of uncoating occurs, it is possible that the cytoplasmic HIV-1 complex could become resistant to restriction by TRIM-CypA before uncoating is completed.
Despite these differing limitations, both uncoating assays revealed similar results. In HeLa cells and OMK cells substantial uncoating occurred within 1 h of viral fusion (Figs. 1 and 2). Results from both assays also suggest that reverse transcription facilitates uncoating. In the in situ assay inhibition of reverse transcription delayed the process of uncoating, as detected by an increased association of p24CA with cytoplasmic HIV-1 complexes (Fig. 1D). Likewise in the CsA washout assay, infection in the presence of nevirapine for 2 h slowed the process of uncoating and increased the half-life to almost 2 h from 40 min (Fig. 2 and Table 1). Uncoating also could be delayed with nevirapine treatment for 4 h in both assays (Figs. 1D and 2C). Finally, we observed a similar effect of nevirapine treatment on the uncoating of replication competent HIV-1, despite a shift in the timing of uncoating due to differences in fusion kinetics (Figs. 2D and 3 and Table 1).
The data presented here support the model that uncoating occurs as the reverse transcribing viral genome is transported to the nucleus. Completion of reverse transcription, and therefore resistance to nevirapine treatment, is not observed until at least 2 h post-infection (Fig. 4A). Furthermore, generation of early reverse transcription products, but not late reverse transcription products, correlates with the half-life of uncoating (Fig. 4B and Table 1). Indeed, further experiments are needed to determine how reverse transcription facilitates uncoating, but these preliminary analyses suggest that early products of reverse transcription may be involved. Reverse transcription brings about morphological changes within the viral genome. The viral genomic RNAs can participate in complex intrastrand basepairing and folding to form a compact structure. In contrast, double-stranded DNA forms a more rigid structure that requires interaction with cellular factors to achieve efficient bending. During early steps of reverse transcription DNA-RNA hybrid molecules may form a more rigid extended structure, which in turn could provide an outward force to destabilize the capsid. Because uncoating still proceeds in the absence of reverse transcription (some viral complexes become resistant to TRIM-CypA restriction during nevirapine treatment) it is clear that other factors, either viral or cellular in origin, play a role in the process. The uncoating assays used in this study and the general method of using multiple uncoating assays should be valuable in identifying and characterizing the role of such factors.
Materials and Methods
A more detailed description of the following methods is available in SI Materials and Methods.
Cells, Viruses, and Pharmaceuticals.
HeLa, 293T, and OMK cells were maintained under standard conditions. Viruses were produced using PEI transfection of 293T cells. HIV-GFPNevR proviral plasmid was cloned by site-directed mutagenesis and verified by DNA sequencing. CsA was used at 2.5 μM. Nevirapine was used at 5 μM. Ammonium chloride was used at 50 mM. AMD3100 was used at 10 μM.
In Situ Uncoating Assay.
The in situ uncoating assay was conducted as previously described (14).
CsA Washout Assay.
OMK cells were plated in 96-well dishes. Each experimental or control reaction was performed on triplicate wells. Cells were spinoculated with GFP reporter virus in the presence of CsA and 10 μg/mL polybrene for 1.5 h at 16 °C (HIV-GFP or HIV-GFPNevR) or 23 °C (R7X4-GFP, temperature-arrested state of fusion). Cells were incubated at 37 °C for 30 min, inoculation media was exchanged for warm media, and CsA was washed out of the zero time point reaction by media exchange. Washout continued at various times after infection. A parallel experiment was also subjected to nevirapine treatment by inclusion of the drug in the inoculation and warm media. After 2 h nevirapine was removed from all reactions by media exchange. Controls included ethanol washout, DMSO treatment, continuous treatment with CsA or ethanol, continuous treatment with nevirapine and CsA, and continuous treatment with DMSO and CsA. Two days after infection cells were harvested with 100 μL trypsin and fixed with 100 μL fix (4:1, 1× PBS:10% formaldehyde). The percentage of GFP-positive cells was determined by flow cytometry (Accuri C6 flow cytometer), averaged for each triplicate reaction, and SE was calculated. The time of 50% uncoating was determined by a best fit line and times were averaged from multiple independent experiments for HIV-GFP (eight), HIV-GFPNevR (four), and R7X4-GFP (three).
Viral Fusion Assay and Nevirapine Add-In Assay.
OMK cells were infected with HIV-GFP or R7X4-GFP similar to the CsA washout assay. Media containing fusion or reverse transcriptase inhibitors and CsA was added at various times after infection. The percentage of GFP-positive cells was determined as in the CsA washout assay. The half-life of viral fusion was determined by a best fit line, and times were averaged from three independent experiments.
Real-Time PCR Analysis.
OMK cells were infected as in the CsA washout assay, and cells were harvested at various times after infection. Real-time PCR was performed as previously described with primers for early and late reverse transcription products and B-actin (24, 26–28).
Supplementary Material
Acknowledgments
We thank Dr. Paul Bieniasz and Theodora Hatziioannou for OMK cells; Dr. Chris Aiken for proviral plasmids; the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program for the AG3.0 antibody and nevirapine; Dr. Chris Rold for CsA washout assay development; Z. Kelley and Zia Okocha for technical assistance; and Dr. Kelly Fahrbach and Cindy Danielson for critical review of the manuscript. This work was supported by NIH Grants RO1 AI47770 and P50 GM082545 (to T.J.H.), an American Society of Microbiology Robert D. Watkins Graduate Research Fellowship (to O.P.), and the James B. Pendleton Charitable Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014522108/-/DCSupplemental.
References
- 1.Bukrinsky MI, et al. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fassati A, Goff SP. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J Virol. 2001;75:3626–3635. doi: 10.1128/JVI.75.8.3626-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MD, Farnet CM, Bushman FD. Human immunodeficiency virus type 1 preintegration complexes: Studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forshey BM, Aiken C. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J Virol. 2003;77:4409–4414. doi: 10.1128/JVI.77.7.4409-4414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian S, Luban J. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forshey BM, Shi J, Aiken C. Structural requirements for recognition of the human immunodeficiency virus type 1 core during host restriction in owl monkey cells. J Virol. 2005;79:869–875. doi: 10.1128/JVI.79.2.869-875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Aiken C. Saturation of TRIM5 alpha-mediated restriction of HIV-1 infection depends on the stability of the incoming viral capsid. Virology. 2006;350:493–500. doi: 10.1016/j.virol.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Arhel NJ, et al. HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 2007;26:3025–3037. doi: 10.1038/sj.emboj.7601740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 11.McDonald D, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forshey BM, von Schwedler U, Sundquist WI, Aiken C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J Virol. 2002;76:5667–5677. doi: 10.1128/JVI.76.11.5667-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell EM, Perez O, Melar M, Hope TJ. Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell. Virology. 2007;360:286–293. doi: 10.1016/j.virol.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita M, Perez O, Hope TJ, Emerman M. Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells. PLoS Pathog. 2007;3:1502–1510. doi: 10.1371/journal.ppat.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodgers W. Making membranes green: Construction and characterization of GFP-fusion proteins targeted to discrete plasma membrane domains. Biotechniques. 2002;32:1044–1046. 1048, 1050–1051. doi: 10.2144/02325st05. [DOI] [PubMed] [Google Scholar]
- 16.O'Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Schwedler UK, Stray KM, Garrus JE, Sundquist WI. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J Virol. 2003;77:5439–5450. doi: 10.1128/JVI.77.9.5439-5450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Caballero D, Hatziioannou T, Zhang F, Cowan S, Bieniasz PD. Restriction of human immunodeficiency virus type 1 by TRIM-CypA occurs with rapid kinetics and independently of cytoplasmic bodies, ubiquitin, and proteasome activity. J Virol. 2005;79:15567–15572. doi: 10.1128/JVI.79.24.15567-15572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nisole S, Lynch C, Stoye JP, Yap MW. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci USA. 2004;101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towers GJ, et al. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat Med. 2003;9:1138–1143. doi: 10.1038/nm910. [DOI] [PubMed] [Google Scholar]
- 22.Richman D, et al. Human immunodeficiency virus type 1 mutants resistant to nonnucleoside inhibitors of reverse transcriptase arise in tissue culture. Proc Natl Acad Sci USA. 1991;88:11241–11245. doi: 10.1073/pnas.88.24.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman DD, et al. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68:1660–1666. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Dornadula G, Pomerantz RJ. Natural endogenous reverse transcription of HIV type 1. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S93–S95. [PubMed] [Google Scholar]
- 26.Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci USA. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JL, et al. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J Virol. 2006;80:9754–9760. doi: 10.1128/JVI.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.