Abstract
Female promiscuity can generate postcopulatory competition among males, but it also provides the opportunity for exploitation of rival male ejaculates. For example, in many insect species, male seminal fluid proteins (Sfps) transferred in a female's first mating stimulate increased fecundity and decreased receptivity to remating. Subsequent mates of females could potentially take advantage of the effects of the first male's Sfps and strategically reduce investment in their own ejaculate. We compared postmating responses (fecundity and sexual receptivity) of Drosophila melanogaster females after their first (virgin) matings (V), to the responses of females remating (M) 24 h after their first mating. The results show that M matings fail to boost fecundity and, thus, males are unlikely to gain fitness from transferring Sfps whose sole function—in V matings—is fecundity-stimulation. However, males can protect their likelihood of paternity in M matings through the transfer of receptivity-inhibiting Sfps. The levels of a fecundity-stimulating Sfp (ovulin) were significantly lower in M females relative to V females, at the same time point shortly after the end of mating. In contrast, the levels of a key receptivity-inhibiting Sfp (sex peptide) were the same in M and V females. These results support the hypothesis that males can adaptively tailor the composition of proteins in the ejaculate, allowing a male to take advantage of the fecundity-stimulating effects of the previous male's ovulin, yet maintaining investment in sex peptide. Furthermore, our results demonstrate sophisticated protein-specific ejaculate manipulation.
Keywords: strategic ejaculation, male accessory gland, sexual selection, intersexual interaction, reproduction
Female sexual promiscuity creates an arena for sperm competition and other forms of postcopulatory sexual selection (1, 2). Promiscuity may also provide the opportunity for males to exploit the effects of rival males’ ejaculates (3, 4). This opportunity arises because, in many species, the ejaculate not only is essential for fertilization but also can influence female postmating behavior and physiology in ways that promote male reproductive success. For example, in insects, products of the male accessory glands can have a variety of effects in the mated female, including stimulating fecundity, promoting sperm storage, and inhibiting receptivity to remating (reviewed in refs. 5–8). In mammals, functions of seminal fluids in the mated female can include stimulating ovulation, promoting sperm motility, mediating sperm storage, and protecting sperm through suppression of immune defense (9, 10; reviewed in ref. 11). If these male-induced effects persist beyond the time by which a female remates, then her next mate could exploit the effects of her earlier mates’ ejaculates. A male could thereby reduce his own mating costs by decreasing investment in particular components of his own ejaculate.
Recently developed theoretical models make specific predictions about ejaculate exploitation. For example, Hodgson and Hosken (4) argue that if the ejaculate of the first male to mate with a female improves sperm survival within the female's reproductive tract, males mating with recently mated females could benefit through both increased sperm survival and decreased investment in the ejaculate components causing this effect. Similarly, game theoretic analyses predict that, under certain conditions, males mating with recently mated females should exploit the fecundity-stimulating effects of a previous male's ejaculate (3). These theoretical predictions have never been tested empirically.
Two assumptions of ejaculate exploitation hypotheses are: (i) males can potentially benefit from effects of the ejaculates of rival males, and (ii) males have the ability to adjust their production or transfer of specific ejaculate components to exploit the effects of a previous male's ejaculate. Evidence consistent with the first assumption is provided by recent empirical studies. For example, in Drosophila melanogaster, the seminal fluid of one male can affect both sperm survival and offspring production of rival males. Viability of sperm from one D. melanogaster male is higher upon in vitro exposure to seminal fluid from another male than it is in the absence of exposure to seminal fluid (12). Furthermore, the number of progeny produced by the last male to mate with a female increases when the first (spermless) male to mate transfers the seminal fluid protein (Sfp), Acp36DE (13), presumably because of Acp36DE's effect of facilitating sperm storage (14). In crickets (Teleogryllus oceanicus), the viability of embryos sired by one male can be enhanced by the ejaculate effects of a rival male (15). These empirical studies provide evidence that a male can potentially benefit from the effects of a rival male's ejaculate. The second assumption of the ejaculate exploitation hypothesis—that males have the ability to adjust the specific ejaculate components that they transfer to females to exploit a rival male's ejaculate—has not previously been tested.
In the present study, we tested the main prediction of the ejaculate exploitation hypothesis: that males strategically adjust specific ejaculate components in such a manner as to exploit the ejaculate effects of a rival male. We chose D. melanogaster as our study subject for two reasons. First, the reproductive biology of D. melanogaster meets the conditions under which Alonzo and Pizzari's (3) model predicts that ejaculate exploitation will occur: males have information on whether they are mating with a virgin or mated female (16) and the additional fecundity a female experiences from remating is low [at least within the first 3–6 d after the initial mating (17–19)]. Second, the functions of specific components of the D. melanogaster ejaculate are well-characterized (reviewed in refs. 6, 20, and 21). Of specific relevance to the current study, fecundity is elevated primarily by two male Sfps—ovulin and sex peptide (SP)—in first matings of female D. melanogaster (17, 22, 23). Oogenesis is largely stalled in virgin females because of feedback-inhibition from accumulated mature oocytes in the ovary (24); ovulin lifts this blockage by causing the ovulation of these oocytes (25). Ovulin's effect on ovulation persists for only ∼24 h after mating (22, 26). SP maintains oogenesis at a high level (27) and remating every 3 to 6 d is sufficient to maximize female fecundity (e.g., refs. 17–19). Thus, in matings with females that have mated recently (within 3 d), provision of additional fecundity-stimulating Sfps may be, at least partially, redundant. SP also inhibits sexual receptivity in virgin females, whereas ovulin does not (17, 22, 23); however, the fecundity and receptivity effects of SP on previously mated females have not been tested directly (28). Therefore, in this study, we first tested whether remating—and specifically SP transferred during remating—affects the fecundity and receptivity of recently mated females. We then tested whether males adjust the ovulin and SP components of the ejaculate that are transferred to females in a manner consistent with the predictions of ejaculate exploitation hypotheses.
Results and Discussion
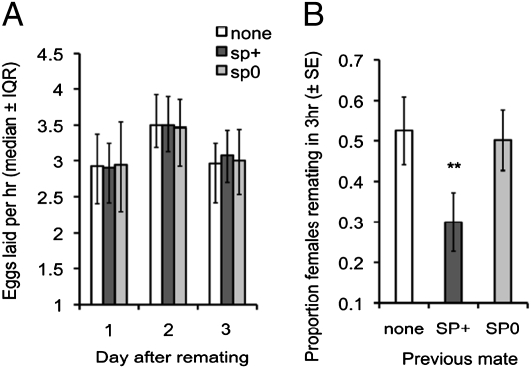
As expected, based on previous studies (e.g. refs. 17–19), we found that remating does not elevate fecundity in recently mated (1 d earlier) females, irrespective of the presence of SP (1 d postremating, F2,204 = 0.38, P = 0.69; 2 d postremating, F2,100 = 0.21, P = 0.81; 3 d postremating, F2,100 = 1.06, P = 0.35; summed total of 1–3 d postremating, F2,100 = 0.16, P = 0.85) (Fig. 1A). However, female sexual receptivity is significantly reduced by remating (1 d postremating, χ22 = 10.03, P = 0.007), an effect entirely attributable to SP (SP+ vs. no remating, χ21 = 7.01, P = 0.008; SP+ vs. SP0, χ21 = 6.78, P = 0.009; SP0 vs. no remating, χ21 = 0.157, P = 0.69) (Fig. 1B). Thus, when mating with recently mated females, males are unlikely to gain from transferring Sfps whose sole function is fecundity-stimulation, such as ovulin. In contrast, males mating with a recently mated female can delay future sperm competition and protect their paternity by maintaining the amount of receptivity-inhibiting SP transferred.
Fig. 1.
Postmating responses to remating of previously mated females. (A) Female fecundity (eggs per hour; median ± interquartile range) over 3 d following remating (1 d after an initial wild-type mating) to a male that transfers a complete ejaculate (SP+), an SP-null male (SP0) (23), or no male (none). n = 31–38 females per treatment. (B) Female receptivity (percentage of remating; mean ± SEM) 1 d following remating: same treatments as A. n = 58–80 females per treatment. **P < 0.01.
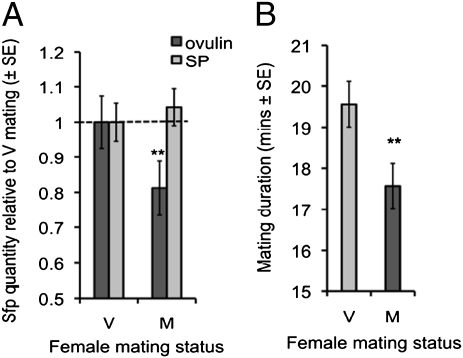
Based on the above findings and on ejaculate exploitation hypotheses, we predicted that males should adaptively reduce allocation of ovulin to recently mated (M) females compared with virgin (V) females. In contrast, males should maintain SP investment in M females to inhibit sexual receptivity (see above and Fig. 1B) and protect paternity. Using an ELISA method (29, 30), we found that ovulin levels in the reproductive tract of females shortly following M matings are significantly reduced compared with in V matings at the same time point (F1,137 = 9.02, P = 0.003) (Fig. 2A), an effect associated with reduced mating duration (F1,197 = 10.47, P = 0.0014) (Fig. 2B). However, we found no significant differences in SP levels at this time in the reproductive tracts of M versus V matings (F1,168 = 0.06, P = 0.80) (Fig. 2A). These data show that Sfps can be manipulated in a protein-specific manner. Moreover, the data support our predictions that males would reduce ovulin, but not SP transfer, to M females.
Fig. 2.
Male mating investment in previously mated (M) and virgin (V) females. (A) Quantity of ovulin and SP transferred to M and V females (mean ± SEM). Values shown are relative to the quantity transferred to V females. n = 68–89 females per treatment. (B) Mating duration of M and V matings (mean ± SEM). n = 68–89 mating pairs per treatment. **P < 0.01.
Our results suggest that second males can take advantage of first-male fecundity-stimulation, permitting a strategic reduction in ovulin allocation. SP levels are maintained, indicating that ejaculate allocation and ejaculate exploitation can be protein-specific. The mechanism by which males could adjust the molecular composition of their ejaculate is currently unclear. One plausible mechanism is through differential gene expression. Expression levels of specific Sfps vary with the perceived level of sperm competition (31). Furthermore, SP and ovulin expression varies among cell type within the male Sfp-producing accessory glands (32, 33). Moreover, at least some components of the ejaculate appear to be transferred sequentially to the female (34), so differential secretion or transfer is plausible. The ability to plastically adjust specific protein quantities in the ejaculate means that the costs to males of mating with previously inseminated females (e.g., sperm competition) may be partially ameliorated because of the second male's ability to exploit the effects of the previous male's Sfps. Thus, models of male reproductive investment (35, 36) need to be reconsidered to take into account such reductions in costs to males mating with previously mated females (3, 4).
The inability of males to further elevate fecundity in M females suggests that reduced ovulin transfer by males is adaptive because this reduction should decrease the male mating costs (37, 38) by allowing the conservation of Sfps for future mating events. A potential alternative is that a reduction in ovulin transfer [or of other fecundity-stimulating Sfps that were not quantified in this experiment (39)] is the reason why males fail to elevate fecundity in recently mated females (i.e., if males did maximize their transfer of fecundity-stimulating Sfps they might be able to further increase female fecundity). However, this explanation is unlikely given that (i) there would be clear fitness benefits to males in increasing female fecundity further were it possible; (ii) there are likely to exist physiological limits that inhibit females from exceeding a certain egg-production rate in a given environment; and (iii) the fecundity-stimulating effects of ovulin are likely a result of the “unblocking” of accumulated mature oocytes from the ovary (25) and this will only be relevant in virgin females.
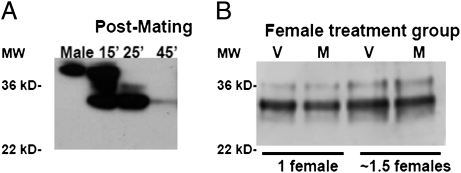
It is also possible that females could exert direct influence on ovulin levels, either by affecting the amount of ovulin transferred by males or by manipulating the fate of ovulin once it is transferred. However, several lines of evidence suggest that direct female effects are unlikely to be the major explanation for our findings. Ovulin levels in the female reproductive tract decline over time (29, 32). Some ovulin may leave the female reproductive tract and enter the hemolymph (32), but the ovulin present in the reproductive tract is proteolytically cleaved into smaller products (Fig. 3A) (40). Using Western blotting we found no evidence for differences in ovulin cleavage rates between V and M females: at 25 min after the start of mating, the same time point as for measurements of Sfp levels, M and V females were at identical stages of ovulin cleavage (Fig. 3B). Ovulin levels could potentially be affected by female “ejaculate ejection” [release of sperm and seminal fluids from the female reproductive tract after mating (41, 42)] if this had protein-specific effects. However, such ejection generally occurs much later after mating (∼3 h) (41) and visual inspection of female reproductive tracts during dissections confirmed that ejaculates were present (i.e., had not been ejected) in all but 1 of the 196 samples. The single (V) sample that apparently lacked an ejaculate was excluded from further analysis. “Sperm release” [release of stored sperm from the sperm storage organs into the bursa of the reproductive tract during mating (41, 42)] would not affect our results because we use the entire reproductive tract in our ELISAs. A final possibility is that females are able to influence the amount of Sfps that a male transfers in a protein-specific manner: for example, if completion of ovulin transfer occurs later than that of SP transfer and if M females terminate ejaculate transfer earlier than V females. However, it would be difficult to distinguish male- and female-mediated effects on the amount of ovulin transferred without disabling the female to prevent her from influencing Sfp transfer, which could lead to other, unintended, effects. Thus, although potential protein-specific female-mediated effects on Sfp levels cannot be unequivocally excluded, we believe that the most parsimonious proximate explanation for lower levels of ovulin in M females is reduced male transfer.
Fig. 3.
Ovulin processing in mated females. (A) Western blot showing intact ovulin in the male accessory glands and sequential processing of ovulin in mated females with time since mating. Male lane: one-fifth of a male equivalent; female lanes: two female reproductive tracts per lane. Proteins were separated on a 10.6% SDS-polyacrylamide gel and probed with antiovulin antibody. Prepared by N. Buehner (Cornell University, Ithaca, NY). (B) Western blot showing ovulin processing at 25 min after the start of mating in reproductive tracts of females that were previously mated (M) and females that were not previously mated (V). Proteins were separated on a 12% SDS-polyacrylamide gel and probed with antiovulin antibody.
Although mating duration was not the focus of our study, it is related to male reproductive success in D. melanogaster: the duration of a female's first mating is positively associated with her latency until remating (43, 44) but not with the number of sperm received (44, 45). Thus, longer first matings are associated with higher male reproductive success, presumably because of greater receptivity inhibition. However, our results demonstrate that the relationship between mating duration and receptivity inhibition is not a result of a general increase in SP transfer in longer matings, because mating duration changes independently of the amount of SP transferred (Fig. 2). We also found that the mating duration was longer for a female's first mating than for a female's second mating, results that are broadly consistent with some studies on D. melanogaster (45, 46) but at odds with two others (47, 48). The differences between studies in mating duration patterns may be because of differences in D. melanogaster strains, experimental methods, or housing conditions before the assays (43, 49). For example, the duration of a female's first mating is influenced both by exposure of the male to rival males before mating and exposure of the pair to extrapair males during mating (43, 49). It appears that in this species the relationship between female mating status and mating duration may be specific to genetic background and the particular mating environment. However, a recent cross-taxa meta-analysis showed that proxies for ejaculate investment, such as mating duration and ejaculate mass, are greater in matings with virgins than with mated females (50), a pattern consistent with our finding that mating duration and allocation of some components of the ejaculate may be reduced in M matings. Intriguingly, the same study (50) also found no evidence for increased sperm numbers transferred to virgin relative to mated females, suggesting that ejaculate investment in response to mating status may often specifically involve changes in nonsperm components, such as Sfps.
Conclusion
Across a wide range of taxa, studies have established unequivocally that males can strategically allocate sperm based on the relative risk or intensity of sperm competition (35, 51). However, only recently have researchers begun to investigate nonsperm aspects of ejaculate allocation theoretically (36) and empirically (30, 52, 53). Moreover, the idea that males can potentially exploit the ejaculates of rival males is a recent one that has previously received only theoretical attention (3, 4). Our results, together with previous studies (13), show that male D. melanogaster have the opportunity to exploit rival ejaculates. Furthermore, our results indicate that males may do so by tailoring the Sfp composition of their ejaculate in a protein-specific manner, suggesting an extraordinary level of sophistication in ejaculate strategies. It will now be important to determine whether such protein-specific allocation strategies are taxonomically widespread. More theoretical and empirical studies are required to determine the evolutionary consequences of such strategies for intersexual and intrasexual interactions.
Materials and Methods
Stocks.
Unless otherwise specified, flies were from a Dahomey wild-type stock (28, 30). SP0 (sp0/Δ130) and SP+ (sp+,sp0/Δ130) males (23) were backcrossed into Dahomey, as previously described (28).
Mating Experiments.
For all experiments, fly food was supplemented by live yeast granules. Before the experiment, 10 to 20 flies were maintained in same-sex vials. Flies were 3- to 5-d-old virgins on the day of the first mating in all experiments. For all matings, females were placed with two males without anesthesia.
Effects of Mating and SP on Fecundity and Receptivity in Previously Mated Females.
Day 0: females were placed individually in vials. Day 1: females were mated; after matings males were discarded. Day 2: females were transferred to individual fresh vials and given the opportunity to remate to either SP0 or SP+ males or they were not remated (randomly assigned). After remating, females were transferred to egg-laying vials for 24 h (1 d postremating, n = 58–80) and then either exposed to males for 3 h to measure receptivity (n = 67–81), or transferred to egg-laying vials for 2 d more (2 and 3 d postremating) to measure fecundity (n = 31–38). For the receptivity measurements, nonremating females were temporally interspersed with the other treatments to avoid time-of-day biases. The experiment was performed in two blocks. Fecundity for 2 and 3 d postremating was measured from one block only.
Sfp Quantities in V and M Females.
Day 0: females were placed in individual vials and randomly assigned to M or V treatment. Day 1: M females were mated, V females were not. After matings, all females were transferred to individual fresh vials. Day 2: males were added to each female-containing vial, except for a subset of M females which were not remated to analyze background Sfps remaining from Day 1 [redesignated as Background (B) females]. Mating duration was recorded (n = 99–100 per treatment). Females were flash-frozen 25 min after the start of mating (30). Mean mating duration in this study was 18.5 min (range, 6–32 min; 5% quantile = 12 min, 95% quantile = 25 min). Pairs that mated for less than 10 min or longer than 25 min were excluded from the study. B females, which were not exposed to males on day 2, were also flash-frozen. Matings and freezings of B females were interspersed between treatments to avoid time-of-day biases. The experiment was performed in two blocks. Ovulin and SP levels in female reproductive tracts of females were measured using ELISAs (n = 68–89), as previously described (29, 30). We calculated the quantity of Sfps transferred to females as the quantity present in the reproductive tract minus the average background quantity present in B females. Using data unadjusted for background Sfps (i.e., the total quantity present from both matings in M females) would not alter our conclusions (Fig. 2A) that less ovulin was transferred to M females than V females and that there was no difference in the amount of SP transferred to M and V females (unadjusted data: ovulin, F1,137 = 5.83, P = 0.017; SP, F1,168 = 1.15, P = 0.28).
Ovulin Processing in V and M Females.
Once in the reproductive tract of mated females, ovulin is processed into smaller cleavage products and becomes undetectable using standard methods (i.e., Western blotting, ELISA) (Fig. 3A) by 2 to 3 h after the start of mating (in contrast to SP, which disperses much more slowly) (29, 54). Our antibody detects cleaved ovulin, but the presence of dramatic differences in the ovulin processing rate between V and M females could potentially confound results. To check whether this was the case, we compared the rate of ovulin processing at 25 min after the start of mating. To do so, we killed females at 25 min after the start of mating by flash-freezing them in liquid nitrogen. We placed the frozen females on ice and dissected their lower reproductive tracts (including the uterus, sperm storage organs, oviducts, and parovaria) in a physiological saline. We ground the female reproductive tracts in 2× SDS sample buffer (25 mM Tris-HCl pH 6.8, 20% glycerol, 4% SDS, 10% β-mercaptoethanol, 0.001% Bromophenol blue) and then boiled them for 4 min. Proteins were separated on one-dimensional SDS-polyacrylamide gels. Full-length ovulin and ovulin processing products were visualized using standard Western blotting (54).
Data Analysis.
Sfp transfer, mating duration, receptivity, and fecundity data from 1 d postremating were analyzed using linear mixed-effects models in R. Receptivity data were analyzed using generalized linear mixed-effects models, specifying a binomial distribution. Mating treatment was the fixed effect (SP+, SP0, or none for the first experiment and M or V for the second experiment). For Sfp data, ELISA plate nested within block was the random effect. For other analyses, block alone was the random effect. For post hoc comparisons, Tukey tests in mixed-effects models used the multcomp package in R. Fecundity data from 2 and 3 d postremating were analyzed with one-way ANOVAs. Extreme outliers (Grubb's test, P < 0.001) were excluded from further analysis (1 M datapoint for mating duration, 1 V and 1 M datapoint for ovulin data). Fecundity and mating duration data were Box-Cox transformed to improve normality.
Acknowledgments
We thank M. Bloch-Qazi, H. J. Brockmann, J. Perry, T. Pizzari, T. Chapman, and two anonymous reviewers for valuable input related to this manuscript; and N. Buehner, C. Tan, R. Zuckerman, D. Smith, and S. Tonick for assistance with experiments. N. Buehner prepared the Western blot in Fig. 3A. This work was funded by the Human Frontier Science Program, Lloyd’s Tercentenary Foundation, and in part by National Institutes of Health Grants F32GM074361 and R01-HD038921.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Birkhead TR, Møller AP. Sperm Competition and Sexual Selection. London: Academic Press; 1998. [Google Scholar]
- 2.Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton, Chichester: Princeton University Press; 1996. [Google Scholar]
- 3.Alonzo SH, Pizzari T. Male fecundity stimulation: Conflict and cooperation within and between the sexes: Model analyses and coevolutionary dynamics. Am Nat. 2010;175:174–185. doi: 10.1086/649596. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson DJ, Hosken DJ. Sperm competition promotes the exploitation of rival ejaculates. J Theor Biol. 2006;243:230–234. doi: 10.1016/j.jtbi.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: Identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 7.Gillott C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu Rev Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 8.Wolfner MF. The gifts that keep on giving: Physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. [DOI] [PubMed] [Google Scholar]
- 9.Adams GP, Ratto MH, Huanca W, Singh J. Ovulation-inducing factor in the seminal plasma of alpacas and llamas. Biol Reprod. 2005;73:452–457. doi: 10.1095/biolreprod.105.040097. [DOI] [PubMed] [Google Scholar]
- 10.Gwathmey TM, Ignotz GG, Mueller JL, Manjunath P, Suarez SS. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol Reprod. 2006;75:501–507. doi: 10.1095/biolreprod.106.053306. [DOI] [PubMed] [Google Scholar]
- 11.Poiani A. Complexity of seminal fluid: A review. Behav Ecol Sociobiol. 2006;60:289–310. [Google Scholar]
- 12.Holman L. Drosophila melanogaster seminal fluid can protect the sperm of other males. Funct Ecol. 2009;23:180–186. [Google Scholar]
- 13.Chapman T, Neubaum DM, Wolfner MF, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc Biol Sci. 2000;267:1097–1105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-González F, Simmons LW. Paternal indirect genetic effects on offspring viability and the benefits of polyandry. Curr Biol. 2007;17:32–36. doi: 10.1016/j.cub.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Tompkins L, Siegel RW, Gailey DA, Hall JC. Conditioned courtship in Drosophila and its mediation by association of chemical cues. Behav Genet. 1983;13:565–578. doi: 10.1007/BF01076402. [DOI] [PubMed] [Google Scholar]
- 17.Chapman T, et al. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priest NK, Galloway LF, Roach DA. Mating frequency and inclusive fitness in Drosophila melanogaster. Am Nat. 2008;171:10–21. doi: 10.1086/523944. [DOI] [PubMed] [Google Scholar]
- 19.Fowler K, Partridge L. A cost of mating in female fruitflies. Nature. 1989;338:760–761. [Google Scholar]
- 20.Chapman T. The soup in my fly: Evolution, form and function of seminal fluid proteins. PLoS Biol. 2008;6:e179. doi: 10.1371/journal.pbio.0060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravi Ram K, Wolfner MF. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol. 2007;47:427–445. doi: 10.1093/icb/icm046. [DOI] [PubMed] [Google Scholar]
- 22.Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci USA. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- 25.Chapman T, Herndon LA, Heifetz Y, Partridge L, Wolfner MF. The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc Biol Sci. 2001;268:1647–1654. doi: 10.1098/rspb.2001.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heifetz Y, Lung O, Frongillo EA, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- 27.Soller M, Bownes M, Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur J Biochem. 1997;243:732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- 28.Fricke C, Wigby S, Hobbs R, Chapman T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J Evol Biol. 2009;22:275–286. doi: 10.1111/j.1420-9101.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- 29.Sirot L, Buehner N, Fiumera A, Wolfner M. Seminal fluid protein depletion and replenishment in the fruit fly, Drosophila melanogaster: An ELISA-based method for tracking individual ejaculates. Behav Ecol Sociobiol. 2009;63:1505–1513. doi: 10.1007/s00265-009-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wigby S, et al. Seminal fluid protein allocation and male reproductive success. Curr Biol. 2009;19:751–757. doi: 10.1016/j.cub.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedorka KM, Winterhalter WE, Ware B. Perceived sperm competition intensity influences seminal fluid protein production prior to courtship and mating. Evolution. 2011;65:584–590. doi: 10.1111/j.1558-5646.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 32.Monsma SA, Harada HA, Wolfner MF. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev Biol. 1990;142:465–475. doi: 10.1016/0012-1606(90)90368-s. [DOI] [PubMed] [Google Scholar]
- 33.Styger D. Drosophila melanogaster. 1992. Molecular analysis of sex peptides in. (Translated from German). PhD Thesis (University of Zurich, Zurich) [Google Scholar]
- 34.Lung O, Wolfner MF. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol. 2001;31:543–551. doi: 10.1016/s0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 35.Wedell N, Gage MJG, Parker GA. Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol. 2002;17:313–320. [Google Scholar]
- 36.Cameron E, Day T, Rowe L. Sperm competition and the evolution of ejaculate composition. Am Nat. 2007;169(6):E158–E172. doi: 10.1086/516718. [DOI] [PubMed] [Google Scholar]
- 37.Partridge L, Farquhar M. Sexual activity reduces lifespan of male fruitflies. Nature. 1981;294:580–582. [Google Scholar]
- 38.Dewsbury DA. Ejaculate cost and male choice. Am Nat. 1982;119:601–610. [Google Scholar]
- 39.Saudan P, et al. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur J Biochem. 2002;269:989–997. doi: 10.1046/j.0014-2956.2001.02733.x. [DOI] [PubMed] [Google Scholar]
- 40.Park M, Wolfner MF. Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev Biol. 1995;171:694–702. doi: 10.1006/dbio.1995.1315. [DOI] [PubMed] [Google Scholar]
- 41.Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 42.Snook RR, Hosken DJ. Sperm death and dumping in Drosophila. Nature. 2004;428:939–941. doi: 10.1038/nature02455. [DOI] [PubMed] [Google Scholar]
- 43.Bretman A, Fricke C, Hetherington P, Stone R, Chapman T. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav Ecol. 2010;21:317–321. [Google Scholar]
- 44.Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: The relation between sperm transfer and copulation duration. Evolution. 2000;54:534–542. doi: 10.1111/j.0014-3820.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 45.Lüpold S, Manier MK, Ala-Honkola O, Belote JM, Pitnick S. Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity, and age. Behav Ecol. 2011;22:184–191. [Google Scholar]
- 46.Pavković-Lučić S, Kekić V. Influence of mating experience on mating latency and copulation duration in Drosophila melanogaster females. Russ J Genet. 2009;45:875–877. [PubMed] [Google Scholar]
- 47.Friberg U. Male perception of female mating status: Its effect on copulation duration, sperm defence and female fitness. Anim Behav. 2006;72:1259–1268. [Google Scholar]
- 48.Singh SR, Singh BN. Female remating in Drosophila: Comparison of duration of copulation between first and second matings in six species. Curr Sci. 2004;86:465–470. [Google Scholar]
- 49.Bretman A, Fricke C, Chapman T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc Biol Sci. 2009;276:1705–1711. doi: 10.1098/rspb.2008.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly CD, Jennions MD. Sexual selection and sperm quantity: Meta-analyses of strategic ejaculation. Biol Rev Camb Philos Soc. 2011 doi: 10.1111/j.1469-185X.2011.00175.x. 10.1111/j.1469-185X.2011.00175.x. [DOI] [PubMed] [Google Scholar]
- 51.Simmons LW. Sperm Competition and Its Evolutionary Consequences in the Insects. Princeton, New Jersey: Princeton University Press; 2001. p. 434. [Google Scholar]
- 52.Cornwallis CK, O'Connor EA. Sperm: Seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc Biol Sci. 2009;276:3467–3475. doi: 10.1098/rspb.2009.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry JC, Rowe L. Condition-dependent ejaculate size and composition in a ladybird beetle. Proc Biol Sci. 2010;277:3639–3647. doi: 10.1098/rspb.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravi Ram K, Sirot LK, Wolfner MF. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:18674–18679. doi: 10.1073/pnas.0606228103. [DOI] [PMC free article] [PubMed] [Google Scholar]