Abstract
The effects of sleep deprivation on dopaminergic systems remain elusive, in part due to the lack of selective ligands for dopamine receptor subtypes. We examined D1, D2 and D3 receptor density in the mouse brain after sleep deprivation by receptor autoradiography using [3H]SCH 23390 for D1R, [3H]raclopride for D2R, and [3H]WC-10 for D3R (a novel D3R-selective compound developed in our laboratory, not previously reported in mouse). Sleep-deprived mice showed a significant decrease in D1R, no change in D2R, and a significant increase in D3R binding in striatum. This pattern of dopamine receptor changes was not seen in mice subjected to restraint stress, suggesting specificity to sleep. These data provide evidence that brain dopaminergic circuits are remodeled after sleep deprivation.
Keywords: Dopamine; Sleep Deprivation; Receptors, Dopamine D1; Receptors, Dopamine D2; Receptors, Dopamine D3; Autoradiography
INTRODUCTION
Multiple neurotransmitters are implicated in the sleep-wake cycle. There is recent evidence for the role of dopamine in the regulation of sleep and wakefulness. Microdialysis studies in rat have found that dopamine levels in the prefrontal cortex and nucleus accumbens exhibit a diurnal rhythm [1]. Mice lacking the dopamine transporter gene show increased wakefulness, while mice lacking the dopamine D2 receptor show significantly decreased wakefulness [2,3]. Medications used to maintain wakefulness increase dopaminergic activity by primarily targeting the dopamine transporter reuptake mechanism [4]. In addition, patients with Parkinsons Disease, who show neurodegeneration of the striatonigral dopaminergic system, experience sleep disturbances including excessive daytime sleepiness [5]. Taken together, these studies together suggest a probable role for dopamine in the maintenance of wakefulness.
The effects of sleep deprivation on dopamine activity have recently been studied in the human brain using PET radiotracers. Healthy human subjects underwent one night of sleep deprivation and were imaged with [11C]raclopride, which targets primarily D2 receptors with less binding to D3 receptors [6]. [11C]raclopride binding was significantly reduced in the striatum and thalamus. Reduced binding suggests greater endogenous dopamine binding to D2/D3 receptors after sleep deprivation, possibly as a countermeasure to promote wakefulness during sleep deprivation [7]. However, the specific contribution of D3R to sleep deprivation remains to be determined. Until this point it has been difficult to examine D3R due to lack of selective pharmacologic tools.
Recently, a novel D3R selective radioligand was developed in our laboratory, [3H]WC-10, or [3H]4-(Dimethylamino)-N-[4-(4-(2-Methoxyphenyl)Piperazin-1-yl)Butyl]Benzamide, a N-phenyl piperazine analog which displays high affinity and selectivity for D3R [8–10]. The first quantitative autoradiographic analysis of the binding of [3H]WC-10 was performed in rat and rhesus monkey brain confirming localization of D3R to the striatum, among other brain regions [9].
The aim of this study is to characterize the effects of sleep deprivation upon D1R, D2R, and D3R binding in mouse striatum. As a comparison to sleep deprivation, we also examined the effects of restraint stress upon D1R, D2R, and D3R binding in mouse striatum. This is the first report of the use of the [3H]WC-10 to localize D3R binding in mouse.
METHODS
Animals
All experimental procedures involving animals were performed in accordance with guidelines established by the Animal Studies Committee at Washington University in St. Louis. All experiments were performed in a 12 hour dark and 12 hour light controlled room (lights on at 7:00 AM and lights off at 7:00 PM). Animals had access to food and water ad lib throughout the experiments.
Sleep deprivation
C57BL6 female mice at 2 months of age (n = 5) were sleep-deprived for 72 hours using the platform-over-water method as previously described [11]. Briefly, mice were placed on platforms consisting of a closed PVC pipe measuring 3.4 cm in diameter which was affixed to the bottom of a regular sized shoebox cage, with water filling the cage to 1 cm below the surface of the PVC pipe. This method has been validated by us and other laboratories with EEG/EMG electrode recording to substantially reduce both REM and non-REM sleep [11]. Age-and sex-matched control mice (n = 4) remained isolated in their home cages during the 72 hours. The 72 hour time point was chosen based on previous methodology using the platform-over-water method to induce chronic sleep deprivation [12]. Mice were then sacrificed and their brains immediately removed and fresh-frozen on powdered dry ice. Brains were sectioned at 30 μm on a cryostat, thaw-mounted onto Fisher Superfrost Plus slides, with 10 sets taken from the rostral through caudal striatum. Slides were stored at −80 °C until processing for receptor autoradiography.
Restraint stress
Age-matched two month old C57BL6 mice were randomly assigned to restraint stress or control groups (n=6 per group). Mice were subjected to restraint stress in a 50-mL conical tube for 2 h daily for 7 days in total as previously described [13,14], with the time point chosen to represent chronic stress. Control mice remained in their home cages. On the 7th day, all mice were sacrificed. Brains were removed, snap-frozen on powdered dry ice, and sectioned using a cryostat as described above.
Precursor synthesis and radiolabeling
[3H]WC-10 was synthesized by American Radiolabeled Chemicals (St. Louis, MO) by alkylation of the des-methyl precursor with [3H]methyl iodide. The specific activity of the radioligand was 80 Ci/mmol. The detailed synthesis scheme for [3H]WC-10 has been previously described [10].
Quantitative receptor autoradiography
All sections were pre-incubated for 20 min in buffer (50 mM Tris buffer, pH 7.4, 25C, containing 120 mM NaCl, 5 mM KCl) to remove endogenous dopamine binding. Following incubation with the respective tracer or non-specific binding buffers, slides were then rinsed five times at 1 min intervals with ice-cold buffer. Slides were incubated in an open staining jar as previously described, with the free radioligand concentration loss was less than 5% after ligands bound to brain sections, as previously described [9,10].
D1 receptor binding
D1 receptors were labeled with [3H]SCH 23390 using the procedure for rat brain described by Savasta et al. [15] with minor modifications. Specifically, brain sections were incubated for 15 min in a 7.4 pH buffer solution containing 50 mM Tris–HCl, 120 mM NaCl, 5 mM KCl, and 1 mM MgCl2 at room temperature. Sections were then incubated for 60 min at room temperature in a similar buffer solution with the addition of 1.5 nM [3H]SCH 23390 (PerkinElmer) and 30 nM ketanserin tartrate (Tocris) to block 5-HT2 receptors. Non-specific binding was determined from the slides in the presence of 1 μM (+)-butaclamol, as described previously [9,16].
D2 receptor binding
D2 receptors were labeled with [3H]raclopride using the previously described procedure for rat and monkey tissue [9]. Brain sections were incubated for 60 min in buffer solution at room temperature with the addition of 4 nM [3H]raclopride (PerkinElmer). Non-specific binding was determined from the slides in the presence of 1 μM s(−)-eticlopride, as before [9].
D3 receptor binding
D3 receptors were labeled with [3H]WC-10 using the previously described procedure for rat and monkey tissue [9]. Brain sections were incubated for 60 min in buffer solution at room temperature with the addition of 4 nM [3H]WC-10. Non-specific binding was determined from the slides in the presence of 1 μM s(−)-eticlopride, as before [9].
Quantification of total radioactivity
Slides were air-dried and made conductive by coating the free side with a copper foil tape. Slides were then placed in the gas chamber with a mixture of argon and triethylamine (Sigma-Aldrich, USA) of a gaseous detector system, the Beta Imager 2000Z Digital Beta Imaging System (Biospace, France). After the gas was well mixed and a homogenous state was reached, further exposure for 24 hours yielded high-quality images. A [3H]Microscale (American Radiolabeled Chemicals, St. Louis, MO) was counted simultaneously as a reference for total radioactivity quantitative analysis. Quantitative analysis was performed with the program Beta-Vision Plus (BioSpace, France) for the anatomical region of interest.
A total of 7 to 8 brain sections were chosen for each animal. Using known neuroanatomical markers, bilateral regions of interest were drawn freehand along the border of the entire striatum of serial brain sections from each individual mouse brain to define the representative binding density for the striatum (see Figure 1A for representations of the coronal sections chosen). Data were linearly fitted to a standard curve which was then used for calibration with a coefficient (R) greater than 0.99, thereby converting cpm/mm into nCi/mg tissue. Subsequently, the receptor-bound radioligand densities were calculated utilizing the specific activity of each radioligand as previously described [9]. The experimenter was blinded to all conditions during the analysis.
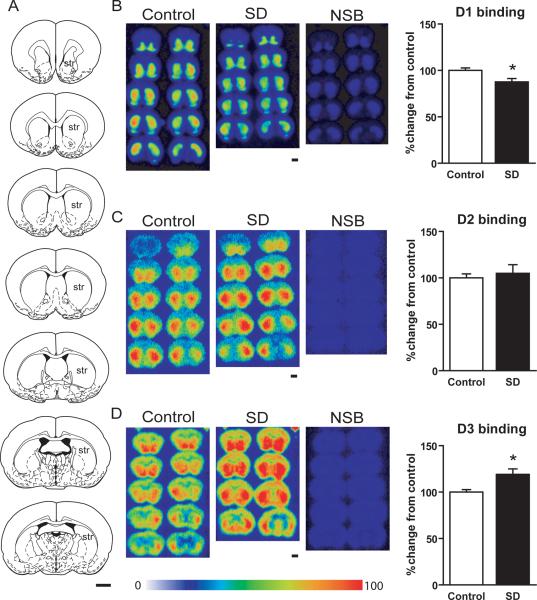
Figure 1.
Changes in D1, D2 and D3 receptor binding in mouse striatum after sleep deprivation. (A) Schematic mouse brain sections showing the rostral to caudal extent of the striatum, the region of interest in which D1R, D2R and D3R were quantified, across a total of 7 sections (modified from [25]). (B) D1 receptor autoradiography on multiple brain sections through the striatum shown in pseudocolor image intensity. D1R binding was significantly decreased after sleep deprivation compared to controls (* p<0.05, two-tailed t-test). (C) D2 receptor autoradiography on multiple brain sections through the striatum shown in pseudocolor image intensity. D2R binding did not significantly change after sleep deprivation (p>0.05, two-tailed t-test). (D) D3 receptor autoradiography on multiple brain sections through the striatum shown in pseudocolor image intensity. D3R binding significantly increased after sleep deprivation compared to controls (* p<0.05, two-tailed t-test). Non-specific binding was negligible in all three dopamine receptor binding assays.
str = striatum; SD = sleep deprivation; NSB = non-specific binding.
Scale bars = 1 mm. Pseudocolor scale = 0 to 100% relative intensity.
RESULTS
Sleep Deprivation
Quantitative receptor autoradiography showed that [3H]SCH 23390, [3H]raclopride, and [3H]WC-10 labeled respective D1R, D2R, and D3R throughout the rostral to caudal regions of the mouse striatum (Figure 1A–D).
Compared to age-matched controls, sleep-deprived mice showed an approximate 15% significant decrease in D1 receptor density in the striatum (87 +/− 3 percent change relative to controls, per fmol/mg tissue, mean value +/− SEM, p < 0.05, t-test) (Figure 1B). There was no significant change in D2 receptor density in the striatum (105 +/− 4 percent change relative to controls, per fmol/mg tissue, mean value +/− SEM, p > 0.05, t-test) (Figure 1C). In contrast, there was nearly a 20% significant increase in D3 receptor density in the striatum (119 +/− 6 percent change relative to controls, per fmol/mg tissue, mean value +/− SEM, p < 0.05, t-test) (Figure 1D).
Restraint stress
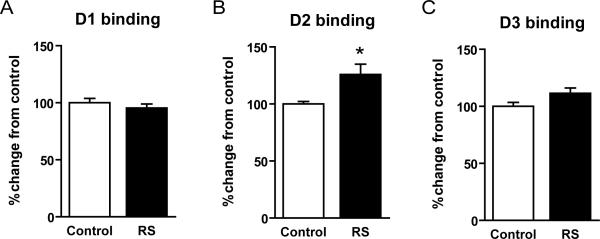
Compared to age-matched controls, mice that underwent restraint stress showed no significant difference in D1 receptor density in the striatum (95 +/− 3 percent change relative to controls, per fmol/mg tissue, mean value +/− SEM, p > 0.05, t-test) (Figure 2A). In contrast, there was a significant increase of approximately 25% in D2 receptor density in the striatum (126 +/− 9 percent change relative to controls, per fmol/mg tissue, mean value +/− SEM, p < 0.05, t-test) (Figure 2B). Lastly, there was no significant difference in D3 receptor density in the striatum (111 +/− 5 percent change relative to controls, per fmol/mg tissue, mean value +/− SEM, p > 0.05, t-test) (Figure 2C).
Figure 2.
Changes in D1, D2 and D3 receptor binding in mouse striatum after restraint stress. (A) D1R binding was not significantly changed after restraint stress compared to controls (p>0.05, two-tailed t-test). (B) D2R binding significantly increased after restraint stress compared to controls (* p<0.05, two-tailed t-test). (C) D3R binding was not significantly changed after restraint stress compared to controls (p>0.05, two-tailed t-test).
It should be noted that this pattern of dopamine receptor changes markedly differs from that seen after sleep deprivation. This suggests that dopamine receptor changes may be specific to sleep deprivation and not merely a byproduct of stress.
DISCUSSION
Until this point, it has been a challenge to elucidate the specific role of D3R due to lack of selective radioligands available for receptor autoradiography. Our data are the first to show the full complement of D1R, D2R, and D3R changes that occur together in the same animal after chronic sleep deprivation. This is also the first report of the localization of [3H]WC-10, the selective D3R radioligand, in mouse striatum.
We show that total sleep deprivation for 72 hours decreases D1R and increases D3R density in the striatum, but has no significant effect on D2R binding. Only one previous report has examined dopamine receptor changes after sleep deprivation, and this focused on D1 and D2 receptor binding after 96 hours of selective REM-sleep deprivation in rat [17].
One could argue that sleep deprivation is inherently stressful, and stress has well-established effects on dopamine receptor dynamics. However, mice that were subjected to chronic restraint stress did not show the same pattern of dopamine receptor changes that were seen after sleep deprivation. In contrast, stressed mice showed no such changes in D1R or D3R densities, but rather, they showed a significant increase in D2R binding. This finding in stressed mice is consistent with previously published reports showing that both restraint stress and social stress significantly increase D2R binding, but do not change in D1R binding in rats [18]. Chronic stress has been reported to enhance dopamine uptake in the striatum, along with alterations in the dopamine transporter (DAT) activity [19]. Changes in DAT and D2R density may represent homeostatic scaling mechanisms for the modulation of dopaminergic activity following periods of stress.
Dopamine receptors are a family of seven-transmembrane domain, G-protein coupled receptors. D1-like dopamine receptors (D1R and D5R) are positive regulators of cAMP levels [20]. D1R are thought to be exclusively post-synaptic in location. In contrast, D2-like dopamine receptors (D2R, D3R, and D4R) inhibit adenylyl cyclase activity in general. D2R and D3R are found both pre- and post-synaptically [21]. D1, D2 and D3 receptors are extensively co-expressed in the striatum. It has been reported that D1 and D3 receptors may heterodimerize in the striatum, despite having opposing second messenger signaling cascades [22]. In the current manuscript, we observed that D1 and D3 receptors changed in opposite directions after sleep deprivation, whereas D2 receptors remained unchanged. This finding invites renewed efforts in elucidating the functional roles of the different dopamine receptor subtypes in the brain.
Volkow et al. recently reported decreased [11C]raclopride binding in humans after a single night of total sleep deprivation [7]. It would be interesting to image these subjects again after 72 hours to see if receptor changes persist, as we found for D1R and D3R density in mice. Our study results are not inconsistent with their finding of decreased [11C]raclopride binding in humans; [11C]raclopride was not used as a quantitative measure of D2R density, but rather as an indirect measure of endogenous dopamine release [7]. Total sleep deprivation is thought to increase dopamine release in the striatum, whether as a consequence of, or perhaps even to help promote, wakefulness. Indeed, other studies have found that human sleep deprivation increases fMRI activation in the nucleus accumbens and is associated with risky decision making, which would be consistent with increased dopamine release [23].
The functions of the dopamine D3R subtype in the brain are still being elucidated. Recent evidence uncovered a link between D3R and a mouse model of restless legs syndrome, a sleep disorder with a circadian dysregulation of sensory and motor gating which is treated with dopaminergic agonist medications [24]. Other potential functions for D3R include motor learning, motivation/reward, cognition, and locomotor response [22]. Given our results, future studies should be performed to better elucidate the specific role of D3R and sleep.
SHORT CONCLUSION
Taken together, our data show that sleep deprivation differentially affects D1, D2, and D3 receptor density in the striatum in a pattern that appears to be specific to sleep deprivation as opposed to generalized stress. In particular, D3R changes may point to a novel role for D3R in sleep behavior. These data provide further evidence that brain dopaminergic circuits may undergo remodeling during sleep deprivation.
ACKNOWLEDGEMENTS
The authors would like to thank support from the following sources: NIH Grant MH081281 to RHM, NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University, and Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Alzheimer's Disease Research Center and Cure Alzheimer's Fund to DMH.
Support: NIH Grant MH081281 to RHM, NIH Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University, and Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Alzheimer's Disease Research Center and Cure Alzheimer's Fund to DMH.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep--wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–899. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- [2].Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci. 2010;30:4382–4389. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- [5].Arnulf I, Konofal E, Merino-Andreu M, Houeto JL, Mesnage V, Welter ML, et al. Parkinson's disease and sleepiness: an integral part of PD. Neurology. 2002;58:1019–1024. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- [6].Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- [7].Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, et al. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorg Med Chem. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- [9].Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, et al. [3H]4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl) butyl)benzamide: a selective radioligand for dopamine D(3) receptors. II. Quantitative analysis of dopamine D(3) and D(2) receptor density ratio in the caudate-putamen. Synapse. 2010;64:449–459. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, et al. [(3)H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin- 1-yl)butyl]benzamide, a selective radioligand for dopamine D(3) receptors. I. In vitro characterization. Synapse. 2009;63:717–728. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci USA. 2006;103:19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zeng C, Pan F, Jones LA, Lim MM, Griffin EA, Sheline YI, et al. Evaluation of 5-ethynyl-2'-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010;1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- [16].Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of dopamine D1 receptors in Wistar-Kyoto (WKY) and Wistar rats. Life Sci. 2008;83:74–78. doi: 10.1016/j.lfs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nunes GP, Junior, Tufik S, Nobrega JN. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull. 1994;34:453–456. doi: 10.1016/0361-9230(94)90018-3. [DOI] [PubMed] [Google Scholar]
- [18].Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108–115. doi: 10.1016/j.brainres.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Copeland BJ, Neff NH, Hadjiconstantinou M. Enhanced dopamine uptake in the striatum following repeated restraint stress. Synapse. 2005;57:167–174. doi: 10.1002/syn.20169. [DOI] [PubMed] [Google Scholar]
- [20].Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- [21].Deransart C, Landwehrmeyer GB, Feuerstein TJ, Lucking CH. Up-regulation of D3 dopaminergic receptor mRNA in the core of the nucleus accumbens accompanies the development of seizures in a genetic model of absence-epilepsy in the rat. Brain Res Mol Brain Res. 2001;94:166–177. doi: 10.1016/s0169-328x(01)00240-6. [DOI] [PubMed] [Google Scholar]
- [22].Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- [23].Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- [24].Dowling P, Klinker F, Stadelmann C, Hasan K, Paulus W, Liebetanz D. Dopamine D3 receptor specifically modulates motor and sensory symptoms in iron-deficient mice. J Neurosci. 31:70–77. doi: 10.1523/JNEUROSCI.0959-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. Gulf Professional Publishing; 2004. [Google Scholar]