Abstract
Object
Complete resection of skull base meningiomas provides patients with the best chance for a cure; however, surgery is frequently difficult given the proximity of lesions to vital structures, such as cranial nerves, major vessels, and venous sinuses. Accurate discrimination between tumor and normal tissue is crucial for optimal tumor resection. Qualitative assessment of protoporphyrin IX (PpIX) fluorescence following the exogenous administration of 5-aminolevulinic acid (ALA) has demonstrated utility in malignant glioma resection but limited use in meningiomas. Here the authors demonstrate the use of ALA-induced PpIX fluorescence guidance in resecting a skull base meningioma and elaborate on the advantages and disadvantages provided by both quantitative and qualitative fluorescence methodologies in skull base meningioma resection.
Methods
A 52-year-old patient with a sphenoid wing WHO Grade I meningioma underwent tumor resection as part of an institutional review board–approved prospective study of fluorescence-guided resection. A surgical microscope modified for fluorescence imaging was used for the qualitative assessment of visible fluorescence, and an intraoperative probe for in situ fluorescence detection was utilized for quantitative measurements of PpIX. The authors assessed the detection capabilities of both the qualitative and quantitative fluorescence approaches.
Results
The patient harboring a sphenoid wing meningioma with intraorbital extension underwent radical resection of the tumor with both visibly and nonvisibly fluorescent regions. The patient underwent a complete resection without any complications. Some areas of the tumor demonstrated visible fluorescence. The quantitative probe detected neoplastic tissue better than the qualitative modified surgical microscope. The intraoperative probe was particularly useful in areas that did not reveal visible fluorescence, and tissue from these areas was confirmed as tumor following histopathological analysis.
Conclusions
Fluorescence-guided resection may be a useful adjunct in the resection of skull base meningiomas. The use of a quantitative intraoperative probe to detect PpIX concentration allows more accurate determination of neoplastic tissue in meningiomas than visible fluorescence and is readily applicable in areas, such as the skull base, where complete resection is critical but difficult because of the vital structures surrounding the pathology.
Keywords: skull base meningioma, fluorescence-guided resection, protoporphyrin IX, 5-aminolevulinic acid, optical spectroscopy, biophotonics
Skull base meningiomas remain some of the most challenging tumors to treat given their proximity to the origin of major vessels supplying the cerebrum and several cranial nerves crucial to everyday function.5,21 The involvement of normal structures can render resection difficult, making the need for highlighting the neoplastic tissue imperative.21 Neuronavigation has significantly improved the precision of dissections but remains impractical for the sharp differentiation of structures, especially when confounded by intraoperative soft tissue shifts.21 The use of tumor-specific biomarkers for intraoperative guidance appears to be a promising adjuvant therapeutic tool to better address this problem.
In fact, several clinical trials have shown that high-grade gliomas specifically accumulate the endogenous fluorescent biomarker, PpIX, and in concentrations sufficient for visual detection during surgery under blue light exposure following the exogenous administration of ALA.11,19,20,22,23,25,26,28 Currently, there is little experience and no consensus on the use of this technique for meningiomas.7,12,17,24 Further, the recent development of quantitative probes14,26 that can detect and quantify fluorescence invisible to the naked eye is opening new frontiers for the application of this technique in lower-grade tumors, metastases, and meningiomas. In the current study, we present our experience using both state-of-the art qualitative visible fluorescence imaging and a novel quantitative (detected with a spectroscopic probe) fluorescence detection technology for the resection of a skull base meningioma.14,26
Methods
Patient and Study Characteristics
A 52-year-old woman presented with a 2.1-cm right sphenoid wing meningioma that had a small amount of intraorbital extension in the area of the anterior clinoid process. It was incidentally discovered on MR imaging performed for depression. The patient's medical history was otherwise unremarkable.
She was enrolled in an investigational study of fluorescence-guided tumor resection. The study protocol was approved by the institutional review board of the Dartmouth-Hitchcock Medical Center, controlling the participation of human subjects in research, and the patient participated under informed consent. Inclusion criteria for the study have been reviewed in earlier publications.20 Our patient received an oral dose (20 mg/kg body weight) of ALA (DUSA Pharmaceuticals) dissolved in 100 ml of water approximately 3 hours prior to the induction of anesthesia. Preoperative axial high-resolution, contrast-enhanced T1-weighted MR images were acquired and used for image-guided neuronavigation.
Surgical Procedure
The patient was supine with her head in 3-point fixation and turned to the right side. The head was registered with a StealthStation Treon image-guidance system (Medtronic). A Zeiss OPMI Pentero surgical microscope (Carl Zeis Surgical GmbH) was modified for fluorescence guidance with a 400-nm wavelength source for excitation and a 620- to 710-nm bandpass filter to record fluorescence emissions. The microscope's field of view was coregistered with the surgical field.
The patient underwent a frontotemporal craniotomy for resection of the tumor. To facilitate the approach an anterior clinoidectomy was performed using the ultrasonic bone aspirator. At several points during the operation, the surgeon alternated from white to blue light exposure to reveal fluorescence. Biopsy specimens were collected at several stages of the resection, in both fluorescing and nonfluorescing regions within the preoperatively planned resection volume.
Immediately before obtaining a biopsy specimen, the surgeon placed the probe on the region of the tumor to be biopsied and recorded spectroscopic fluorescence data using the intraoperative probe. Control data were also acquired, in each case consisting of spectroscopic measurements in normal brain (of indiscriminate subtypes) or normal dura mater. Each site was assigned a fluorescence score from 0 to 4 (no [0], minimal [1], moderate [2], high [3], and very high [4] fluorescence) based on the impression of the surgeon, who was blinded to the quantitative results, before biopsy acquisition.
Intraoperative Probe
We used a handheld probe to quantify PpIX concentrations in vivo based on in situ spectroscopic fluorescence measurements.26 Four optical fibers with a 200-μm-diameter core linearly spaced 260 μm apart were bundled into a stainless steel shaft (1.10-mm-diameter end), and the fibers were connected through a 3-m cable to a data acquisition system. During each measurement, sequential white light (wavelength range 450–720 nm) and fluorescence excitation light (405-nm exposure) through the fiberoptics were used to collect reflectance and fluorescence emission spectra. White light reflectance data provided the necessary information to compute the non-fluorescent optical properties of tested tissue, namely the intrinsic absorption and scattering coefficients. Based on these computed values, light-transport modeling was applied to correct for the distorting effects of intrinsic tissue optical properties on the raw fluorescence spectra and to quantify the absolute PpIX concentrations in tissue to an accuracy of about ± 10%.14,26
Pathological Analysis
Neuropathological analysis was done on formalin-fixed, paraffin-embedded biopsy tissue specimens processed for H & E staining. A neuropathologist blinded to the final pathological diagnosis assessed the specimens based on WHO histopathological criteria.16
Statistical Analysis
Data processing was performed using MATLAB software (Version R2009b, The Mathworks, Inc.). Wilcoxon rank-sum (Mann-Whitney) tests were used to assess a difference between groups. Two-sided p values < 0.05 were described as statistically significant.
Results
Tumor Fluorescence
Both qualitative visible fluorescence imaging and the quantitative intraoperative probe were used during resection in our patient (Fig. 1). The intraoperative probe detected varying PpIX concentrations in tissue (normal tissue, below the level of quantitation; tumor tissue, mean 4.81 μg/ml, range 0.11–19.15 μg/ml; Fig. 2). Further, the intraoperative probe was able to detect neoplastic tissue more accurately than the state-of-the-art qualitative visible fluorescence approach. In some instances, histologically confirmed tumor tissue showed no visible levels of fluorescence but did accumulate significant PpIX concentrations (> 0.10 μg/ml) above the previously reported optimal cutoff value for meningiomas (< 0.01 μg/ml).26 For sampled areas of the tumor, visible fluorescence demonstrated 80% sensitivity in detecting pathology, whereas the intraoperative probe was associated with 100% sensitivity. The spatial resolution of the probe was approximately 1 mm2 (that is, the probe tip that detects the fluorescence of tissue was approximately 1 mm in diameter). This device can pick up small areas of uncertainty, which can be particularly useful in regions such as the skull base in which the tumors are close to vital neurovascular structures. Complete tumor resection was achieved without any complications or injuries to nearby normal structures. Histological analysis of the tumor revealed a WHO Grade I meningioma.
Fig. 1.
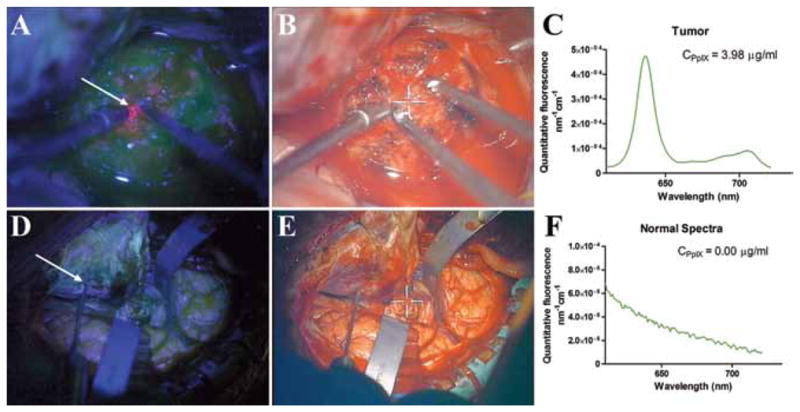
Qualitative and quantitative ALA-induced PpIX fluorescence–guided resection images. Fluorescence-guided resection of a skull base meningioma showed varying levels of qualitative and quantitative fluorescence. A histologically confirmed tumor region with large amounts of accumulated PpIX showing high levels of visible fluorescence under blue light excitation (A) with the corresponding white light image (B), and the quantitative fluorescence spectrum (C) showing the distinctive PpIX spectrum. A region of normal dura showed no visible fluorescence under blue light excitation (D) with the corresponding white light image (E), and the quantitative fluorescence spectrum (F) showed no PpIX peaks, only a distinctive autofluorescence spectrum.
Fig. 2.
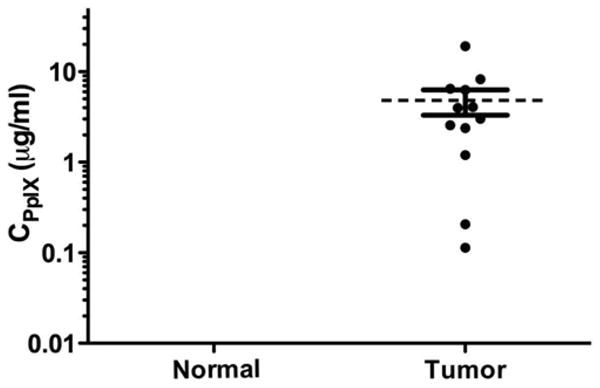
Protoporphyrin IX concentrations in skull base meningioma. Scatter plot of PpIX concentrations in normal and tumor tissue (mean 4.81 ± 1.49 μg/ml, range 0.11–19.15 μg/ml). Tumor tissue accumulated significantly more PpIX than normal tissue. Concentrations in normal dura were all below the level of quantification and outside the axis limits.
Discussion
Microsurgical approaches and navigational technology have significantly evolved.5,21 However, skull base surgery for meningiomas is lacking intraoperative markers for surgical guidance to allow adequate tumor localization and resection, minimize collateral damage, and prevent disruption of normal anatomy. In the current study we demonstrated the combination of qualitative visual fluorescence and quantitative in vivo fluorescence measurements (using an intraoperative probe) based on a light-transport modeling approach, which corrects for the marked distorting effects of variations in tissue optical properties on the fluorescence emission spectrum and intensity and substantially improves the performance of PpIX as an intraoperative imaging tool for a skull base meningioma.
5-Aminolevulinic acid is a natural precursor in the heme biosynthetic pathway. Exogenous administration of ALA leads to significant accumulation of the fluorescent compound PpIX one step prior to the conversion of PpIX to heme by the enzyme ferrochelatase.7,8 Protoporphyrin IX selectively accumulates in malignant cells as a result of several proposed (and not fully understood) mechanisms, such as reduced activity of ferrochelatase, elevated intracellular ALA uptake, and delayed PpIX secretion from the cell.6,19 Similar mechanisms have been speculated to be the cause of the increased fluorescence observed in patients with meningiomas.7
Previous studies have shown that PpIX accumulates with high specificity and in sufficient concentrations in high-grade glioma to allow visual fluorescence detection of tumor tissue.19,20,22,23,25,28 Stummer et al.22,23 showed that the use of ALA approximately doubles the number of patients without residual tumor on postoperative MR imaging and increases 6-month progression-free survival.
Authors of several small series have underscored the utility of ALA during meningioma surgery.7,12,17,27 Note that in these studies no relationship was found between the degree of malignancy and tumor fluorescence. Kajimoto et al.12 reported that the sensitivity and specificity of PpIX fluorescence of the main tumor mass overall were 83% and 100%, respectively. They describe how the information provided by fluorescence led to a modification in their technique and more complete resections. Morofuji et al.17 demonstrated the use of visible fluorescence to define the areas of bone invasion in a convexity meningioma and successfully correlated the data with histological findings postoperatively. Likewise, Coluccia et al.7 reported that the overwhelming majority of patients (94%) with meningiomas in their series demonstrated visible fluorescence. All of these authors used a modified surgical microscope to visualize fluorescence as an intraoperative tool to assist skull base and convexity meningioma resection.
In many cases, the use of fluorescence in resecting well-circumscribed tumors, such as meningiomas, is not necessary. The need for a highly sensitive intraoperative tool arises in cases in which the surgeon must identify small areas of invasion and differentiate from normal neurovascular structures in vital areas such as the skull base.21 In such instances an intraoperative tool, such as fluorescence guidance, would aid the neurosurgeon in identifying tumor tissue. However, as demonstrated in the current study and in accordance with the literature,7,12,17 ALA-induced PpIX fluorescence does not always display visible levels of fluorescence in meningiomas. We have previously shown the increased diagnostic accuracy of an intraoperative probe in a range of different histologies during intracranial tumor resection. This spectroscopic intraoperative probe takes into account the distortive effects of tissue optical properties on the emitted ALA-induced PpIX fluorescence spectra and quantifies the absolute PpIX concentrations in tissue.14,26 In the current study, we presented our experience using qualitative and quantitative ALA-induced PpIX fluorescence guidance in the resection of a skull base meningioma.
The use of fluorescence and especially the more elaborate results associated with the application of an intraoperative probe appear to be of increased significance in the treatment of skull base meningiomas. Complete resection decreases the recurrence rate and is related to higher rates of progression-free survival. With the involvement of cranial nerves and blood vessels in the skull base, the chances for total removal decrease and the rates of recurrence and surgical morbidity increase.1,9 In addition to involving critical functional anatomy, meningioma resection can be complicated by dural involvement, bone involvement, or peritumoral edema.10,17 Attachments to gliotic brain, major sinuses, and the anterior visual pathways are often left behind and provide foci for recurrence.1,9,18 Even in patients in whom a Simpson Grade I meningioma resection has been achieved, an 8% 5-year recurrence rate has been reported2—and that number only increases with skull base resections.3,18 Aggressive resection of adjacent dura and gliotic brain has been advocated, with margins of 2–4 cm being proposed in the literature.4,15,18 Despite the aggressive approach taken in several studies, most of the dural tail apparent on MR imaging has proven to be venous congestion.13 Morofuji et al.17 and Kajimoto et al.12 have shown, with the use of histological analysis, that visible fluorescence can assist in identifying the diseased segment. Quantification of fluorescence with an intraoperative probe could more elaborately map the problematic area and tailor the extent of resection to the true extent of the disease. This strategy can prevent unnecessary morbidity and CSF leaks associated with extensive resections in the skull base. In our patient, complete resection of the intracranial and intraorbital portion of the tumor was achieved, avoiding injury to the adjacent optic nerve and artery.
Although rarely observed, the use of ALA is associated with some adverse effects, which include photosensitivity (primarily in the first 48 hours), transient liver dysfunction, nausea, and vomiting. To manage these effects, we perform serial liver function tests and keep patients in a dark room for 48 hours. Throughout the duration of our prospective study we have not observed any serious adverse effects associated with ALA administration.
Conclusions
Skull base meningiomas are difficult to treat because they involve or are close to the cranial nerves, major blood vessels, and air sinuses. Radical resection of these lesions is imperative to their cure. The use of ALA-induced PpIX fluorescence appears to be a promising tool that can delineate abnormal pathology, demonstrate invasion, and enable tailoring the extent of tumor resection to avoid destruction of normal surrounding tissue. The quantitative approach of intraoperative in vivo measurement of PpIX concentrations opens the door to real-time delineation of these pathologies with much greater accuracy than visible fluorescence. We report on the application of this approach for a skull base meningioma. The intraoperative fluorescence probe could be used as an adjunct to standard white light and qualitative fluorescence image-guided resection, maximizing the therapeutic effect and minimizing complications in the treatment of skull base meningiomas.
Acknowledgments
This work was supported in part by a National Institutes of Health Grant No. R01NS052274-04 (D.W.R.) awarded by the National Institute of Neurological Disorders and Stroke and Grant No. K25CA138578 (F.L.) awarded by the National Cancer Institute. Carl Zeiss (Carl Zeiss Surgical GmbH) and Medtronic Navigation (Medtronic) provided the fluorescence-enabled OPMI Pentero operating microscope and the StealthStation Treon navigation system, respectively. DUSA Pharmaceuticals supplied the ALA. Drs. Kim and Wilson have a provisional patent (61,297,969) for the intraoperative probe device.
Abbreviations used in this paper
- ALA
5-aminolevulinic acid
- PpIX
protoporphyrin IX
Footnotes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Roberts, Valdes, Kim, Wilson, Paulsen. Acquisition of data: Bekelis, Valdes, Erkmen, Harris. Analysis and interpretation of data: all authors. Drafting the article: Bekelis. Critically revising the article: Roberts, Bekelis, Valdes, Erkmen, Leblond, Kim, Wilson, Harris, Paulsen. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Valdes. Study supervision: Roberts.
References
- 1.Al-Mefty O, Kadri PA, Pravdenkova S, Sawyer JR, Stangeby C, Husain M. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101:210–218. doi: 10.3171/jns.2004.101.2.0210. [DOI] [PubMed] [Google Scholar]
- 2.Ayerbe J, Lobato RD, de la Cruz J, Alday R, Rivas JJ, Gómez PA, et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien) 1999;141:921–932. doi: 10.1007/s007010050398. [DOI] [PubMed] [Google Scholar]
- 3.Bloss HG, Proescholdt MA, Mayer C, Schreyer AG, Brawanski A. Growth pattern analysis of sphenoid wing meningiomas. Acta Neurochir (Wien) 2010;152:99–103. doi: 10.1007/s00701-009-0556-2. [DOI] [PubMed] [Google Scholar]
- 4.Borovich B, Doron Y. Recurrence of intracranial meningiomas: the role played by regional multicentricity. J Neurosurg. 1986;64:58–63. doi: 10.3171/jns.1986.64.1.0058. [DOI] [PubMed] [Google Scholar]
- 5.Bulsara KR, Al-Mefty O. Skull base surgery for benign skull base tumors. J Neurooncol. 2004;69:181–189. doi: 10.1023/b:neon.0000041881.59775.d5. [DOI] [PubMed] [Google Scholar]
- 6.Collaud S, Juzeniene A, Moan J, Lange N. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Curr Med Chem Anticancer Agents. 2004;4:301–316. doi: 10.2174/1568011043352984. [DOI] [PubMed] [Google Scholar]
- 7.Coluccia D, Fandino J, Fujioka M, Cordovi S, Muroi C, Landolt H. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir (Wien) 2010;152:1711–1719. doi: 10.1007/s00701-010-0708-4. [DOI] [PubMed] [Google Scholar]
- 8.Fotinos N, Campo MA, Popowycz F, Gurny R, Lange N. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem Photobiol. 2006;82:994–1015. doi: 10.1562/2006-02-03-IR-794. [DOI] [PubMed] [Google Scholar]
- 9.Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis Surg Neurol. 1986;26:461–469. doi: 10.1016/0090-3019(86)90259-4. [DOI] [PubMed] [Google Scholar]
- 10.Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233–242. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 11.Johansson A, Palte G, Schnell O, Tonn JC, Herms J, Stepp H. 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem Photobiol. 2010;86:1373–1378. doi: 10.1111/j.1751-1097.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- 12.Kajimoto Y, Kuroiwa T, Miyatake S, Ichioka T, Miyashita M, Tanaka H, et al. Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. Case report. J Neurosurg. 2007;106:1070–1074. doi: 10.3171/jns.2007.106.6.1070. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara Y, Niiro M, Yokoyama S, Kuratsu J. Dural congestion accompanying meningioma invasion into vessels: the dural tail sign. Neuroradiology. 2001;43:462–465. doi: 10.1007/s002340000524. [DOI] [PubMed] [Google Scholar]
- 14.Kim A, Khurana M, Moriyama Y, Wilson BC. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J Biomed Opt. 2010;15:067006. doi: 10.1117/1.3523616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinjo T, al-Mefty O, Kanaan I. Grade zero removal of supratentorial convexity meningiomas. Neurosurgery. 1993;33:394–399. doi: 10.1227/00006123-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I. Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: technical case report. Neurosurgery. 2008;62(3 Suppl 1):102–104. doi: 10.1227/01.neu.0000317378.22820.46. [DOI] [PubMed] [Google Scholar]
- 18.Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999;90:455–462. doi: 10.3171/jns.1999.90.3.0455. [DOI] [PubMed] [Google Scholar]
- 19.Pogue BW, Gibbs-Strauss S, Valdés PA, Samkoe K, Roberts DW, Paulsen KD. Review of Neurosurgical Fluorescence Imaging Methodologies. IEEE J Sel Top Quantum Electron. 2010;16:493–505. doi: 10.1109/JSTQE.2009.2034541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts DW, Valdés PA, Harris BT, Fontaine KM, Hartov A, Fan X, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between δ-aminolevulinic acid–induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg. 2011;114:595–603. doi: 10.3171/2010.2.JNS091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samii M, Gerganov VM. Surgery of extra-axial tumors of the cerebral base. Neurosurgery. 2008;62(6 Suppl 3):1153–1168. doi: 10.1227/01.neu.0000333782.19682.76. [DOI] [PubMed] [Google Scholar]
- 22.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 23.Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 24.Tsai JC, Hsiao YY, Teng LJ, Chen CT, Kao MC. Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Lasers Surg Med. 1999;24:296–305. doi: 10.1002/(sici)1096-9101(1999)24:4<296::aid-lsm7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Valdés PA, Fan X, Ji S, Harris BT, Paulsen KD, Roberts DW. Estimation of brain deformation for volumetric image updating in protoporphyrin IX fluorescence-guided resection. Stereotat Funct Neurosurg. 2010;88:1–10. doi: 10.1159/000258143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdés PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. Clinical article. J Neurosurg. 2011 Mar 25; doi: 10.3171/2011.2.JNS101451. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitson WJ, Valdes PA, Harris BT, Paulsen KD, Roberts DW. Confocal microscopy for the histologic fluorescence pattern of a recurrent atypical meningioma. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e318217163c. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widhalm G, Wolfsberger S, Minchev G, Woehrer A, Krssak M, Czech T, et al. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer. 2010;116:1545–1552. doi: 10.1002/cncr.24903. [DOI] [PubMed] [Google Scholar]


