SUMMARY
Wnt canonical signaling is critical for normal development as well as homeostasis of several epithelial tissues, and constitutive activation of this pathway is commonly observed in carcinomas. We show here that 50% of human sarcomas (n=45) and 70% of sarcoma cell lines (n=23) of diverse histological subtypes exhibit upregulated autocrine canonical Wnt signaling. Further, we identify alterations including overexpression or gene amplification of Wnt ligands and/or LRP5/6 co-receptors, epigenetic silencing of different cell surface Wnt antagonists in autocrine and mutations in adenomatous polyposis coli (APC) gene in two non-autocrine Wnt positive sarcoma cell lines. Finally, downregulation of the activated Wnt pathway inhibited sarcoma cell proliferation both in vitro and in vivo by a mechanism involving the downregulation of CDC25A.
SIGNIFICANCE
Sarcomas comprise the most common malignancy of childhood and afflict adults as well. Canonical Wnt signaling influences the maintenance of mesenchymal stem cells, and our findings indicate that sarcomas commonly select for upregulation of Wnt autocrine signaling, which acts to increase their proliferation through the functions of a TCF/β-catenin target gene, CDC25A, a major regulator of cell cycle progression. The high frequency at which the Wnt pathway is activated in diverse human sarcomas identifies Wnt signaling as a potential target for therapies that could decrease morbidity and mortality from this disease.
INTRODUCTION
The Wnt family of secreted proteins serve evolutionarily conserved functions in normal development and adult tissue homeostasis (Clevers, 2006). Wnt signaling through its receptors activates intracellular signaling networks for at least three distinct pathways, including canonical Wnt/β-catenin, noncanonical planar cell polarity and Wnt/Ca2++ pathways (MacDonald et al., 2009; van Amerongen and Nusse, 2009). Canonical Wnts activate a cell surface receptor complex consisting low-density lipoprotein receptor-related proteins (LRP) 5 and 6 and Frizzled (Fz) leading to inactivation of a β-catenin degradation complex containing Adenomatous Polyposis Coli (APC), Axin and glycogen synthase kinase 3 (GSK3) β, which otherwise targets β-catenin for proteosomal degradation (Clevers, 2006). β-catenin then accumulates in the cytoplasm, translocates to the nucleus and binds to T-cell-specific transcription factor/lymphoid enhancer binding factor (TCF/LEF) complex to modulate TCF dependent transcription of target genes. Certain TCF/β-catenin target genes including AXIN2, c-MYC, and LEF1 appear to be transcriptionally upregulated in a tissue independent manner while other target genes may be tissue or context specific (He et al., 1997; Hovanes et al., 2001; Jho et al., 2002; Leung et al., 2002).
Wnt signaling is kept in check at multiple levels including receptor downregulation (Khan et al., 2007) and feedback negative regulators such as AXIN2 and DKK1, (Bafico et al., 2001; Jho et al., 2002; Wu et al., 2000) a member of one class of Wnt cell surface antagonists. DKKs bind to LRP5/6 and inhibit Wnt canonical signaling (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001; Tamai et al., 2000). Another class of antagonists, FRPs, bind to and sequester Wnts, blocking both canonical and noncanonical signaling (Bafico et al., 1999; Rattner et al., 1997). Wnt/β-catenin signaling is involved in the maintenance of normal tissue stem/progenitors of epithelial tissues including the gastrointestinal tract, skin, mammary gland, and lung (Radtke and Clevers, 2005; Reya and Clevers, 2005). Carcinomas that arise in these same tissues often exhibit aberrant Wnt pathway activation by mechanisms such as mutations of APC, CTNNB1 or AXIN (Polakis, 2000) and more recently through autocrine Wnt activation (Akiri et al., 2009; Bafico et al., 2004; Giles et al., 2003).
Wnt signaling is also among the developmental pathways that regulate the self-renewal and differentiation of mesenchymal stem cells (MSCs) (Hartmann, 2006; Jaiswal et al., 2000; Siddappa et al., 2008; Tezuka et al., 2002). MSCs isolated from bone marrow can differentiate along osteogenic, chondrogenic, adipogenic, connective tissue, as well as myogenic lineages (Pittenger et al., 1999). Sarcomas, which account for the majority of pediatric malignancies (Jemal et al., 2008), and occur in adults as well, involve mesenchymal tissues and comprise a diverse array of histological subtypes (Skubitz and D’Adamo, 2007). Mesenchymal stem cells (MSCs) have been identified as the targets of “first-hit” in Ewing’s sarcoma and malignant fibrous histiocytoma (MFH) (Matushansky et al., 2007; Miyagawa et al., 2008; Tirode et al., 2007). We recently showed that human MSCs exhibit low levels of endogenous Wnt signaling and that high levels of Wnt signaling promoted human MSC self-renewal and inhibited differentiation along osteogenic or adipogenic lineages (Liu et al., 2009). Based on these findings, the major goal of the present study was to investigate the role of canonical Wnt pathway in human sarcomagenesis.
RESULTS
Detection of increased Wnt activity in a high fraction of human sarcomas of diverse histological types
To investigate a possible role of Wnt pathway activation in sarcomas, we screened human tumor lines representing major sarcoma subtypes for canonical Wnt activity. We identified upregulated Wnt signaling relative to that observed in hMSCs as evidenced by increased levels of uncomplexed β-catenin (Fig. 1A and Fig. S1) and increased TCF reporter activity (Fig. 1B) in 17 of 23 tumor lines analyzed including those derived from Ewing’s’, fibro-, leiomyo-, lipo-, osteo-, rhabdomyo- and synovial sarcoma (Fig. S1). We next screened both soft-tissue and bone sarcomas for uncomplexed β-catenin levels. Fig. 1C shows a representative blot for distinct sarcoma subtypes showing high levels of uncomplexed β-catenin, which were similar to those found in 53S, a previously reported autocrine Wnt activated tumor line (Bafico et al., 2004). Among a total of 45 primary sarcomas analyzed, Wnt activation was observed in 25, representing 12 different histological subtypes (Fig. 1D). These results demonstrate that the Wnt canonical pathway is upregulated in a large fraction of human sarcoma cell lines and sarcomas of diverse subtypes.
Figure 1. High frequency of upregulated canonical Wnt activity in human sarcomas and sarcoma lines of multiple histological subtypes.

(A) Total cell lysates were subjected to precipitation with a glutathione S-transferase (GST)-E cadherin fusion protein (Bafico et al., 1998) followed by immunoblot analysis with mAb directed against β-catenin. α-tubulin was used as a loading control.
(B) TCF luciferase reporter activity in human sarcoma cells. Results are depicted as the ratio of TOP/FOP luciferase activity at 72 hours after transduction. Error bars indicate SD of mean values obtained from triplicates and are representative of two independent experiments.
(C) Protein extracts from frozen sections of primary sarcoma tissues were subjected to analysis of uncomplexed β-catenin levels, total β-catenin, and β-actin as in (A). 53S and MDAMB361 cell lysates served as positive and negative controls (Bafico et al., 2004). A representative blot is shown.
(D) Frequency of elevated uncomplexed β-catenin levels in primary sarcomas. Results reflect at least two independent analyses for each tumor specimen. LMS:Leiomyo-, FS:Fibro-, SS: Synovial-, ES or EW: Ewing’s-, OS:Osteosarcoma, GS:Glio-, PS:Pleo-, LS:Lipo-, GIST:Gastrointestinal stromal tumor, RMS:Rhabdomyo-. See also Figure S1.
Wnt activation in human sarcomas involves multiple mechanisms
We initially surveyed each Wnt positive tumor line for evidence of β-catenin activating lesions, a commonly observed mechanism for Wnt activation in tumors (Polakis, 1999), but found none among 11 lines tested. To test whether constitutive Wnt pathway activation in these sarcoma lines was due to an autocrine loop, we treated sarcoma cells with either DKK1 or FRP. Many showed pathway inhibition in response to DKK1 and/or FRP (Fig. 2A and 2B), supporting an autocrine mode of Wnt pathway activation in these sarcoma lines. Neither treatment inhibited the upregulated Wnt pathway in two cell lines, A2984 and SK-UT-1 (Fig. 2B and Fig. S1). Thus, we analyzed these lines for APC alterations by Western blotting and detected truncated APC proteins in both cell lines (Fig. S2D). We then searched the Sanger Institute database on somatic mutations in cell lines and found that SK-UT-1 had been identified as having heterozygous APC mutations, consistent with our results of immunoblot analysis. We confirmed these mutations by sequence analysis (data not shown) and identified an insertion of “G” base at 4790_4791, resulting in an amino acid substitution of Alanine to Glycine and the generation of a premature stop codon in A2984 sarcoma line (Fig. S2D).
Figure 2. Wnt autocrine activation in human sarcomas occurs commonly and is associated with several pathway aberrations.
(A) Effects of DKK1 on Wnt/β-catenin signaling in human sarcoma cells. Cells were treated with DKK1 for 3 hrs followed by analysis of uncomplexed β-catenin levels as described in Fig. 1A.
(B) Wnt activated sarcoma lines resistant to DKK1 inhibition were stably infected with human FRP followed by DKK1 treatment and immunoblot analysis as described in A.
(C) Real-time PCR analyses for various canonical Wnt ligands were conducted on total RNAs. Normalized values are represented relative to those in hMSC.
(D) Protein extracts from whole cell lysates of different human sarcoma lines and hMSCs were subjected to immunoblot analysis.
(E) Real-time PCR was performed on genomic DNAs extracted from the indicated sarcoma lines to analyze LRP5 gene copy number. SUV420H1 and GAL are two flanking genes 5′ and 3′ of LRP5, respectively. Albumin was used as a standard for diploid gene copy number. Normalized values are represented relative to those in normal human epithelial cells.
Error bars indicate SD of mean values obtained from triplicates and are representative of two independent experiments. See also Figure S2.
To further investigate the mechanisms involved in autocrine Wnt activation, we analyzed expression levels of various canonical Wnt ligands and antagonists. The relative mRNA levels of Wnt ligands analyzed varied among sarcoma cell lines, but each line exhibited much higher levels of at least some Wnts capable of triggering canonical signaling when compared to hMSCs (Fig. 2C). Additionally, some sarcoma lines showed much higher levels of LRP5 and/or LRP6 protein expression compared to hMSCs (Fig. 2D). HOS and G-292 cells showed markedly increased LRP5 mRNA (Fig. S2A) and protein levels (Fig. 2D), and also exhibited increased LRP5 gene copy number (Fig. 2E) when compared to flanking genes. In other sarcoma lines with increased LRP5/6 protein levels, there was no significant increase in the transcript level of either gene, implying that increased protein stabilization was responsible. In a subset of the sarcoma cell lines in our series, reduced expression of one or more Wnt antagonists correlated with the presence of promoter methylation (Fig. S2B). Fig. S2C summarizes expression levels of both positive and negative cell surface regulators of the Wnt pathway relative to hMSCs in the sarcoma lines identified with Wnt autocrine or yet to be unidentified activation mechanisms.
Identification of CDC25A as a TCF/β-catenin target gene in Wnt activated human sarcoma cells
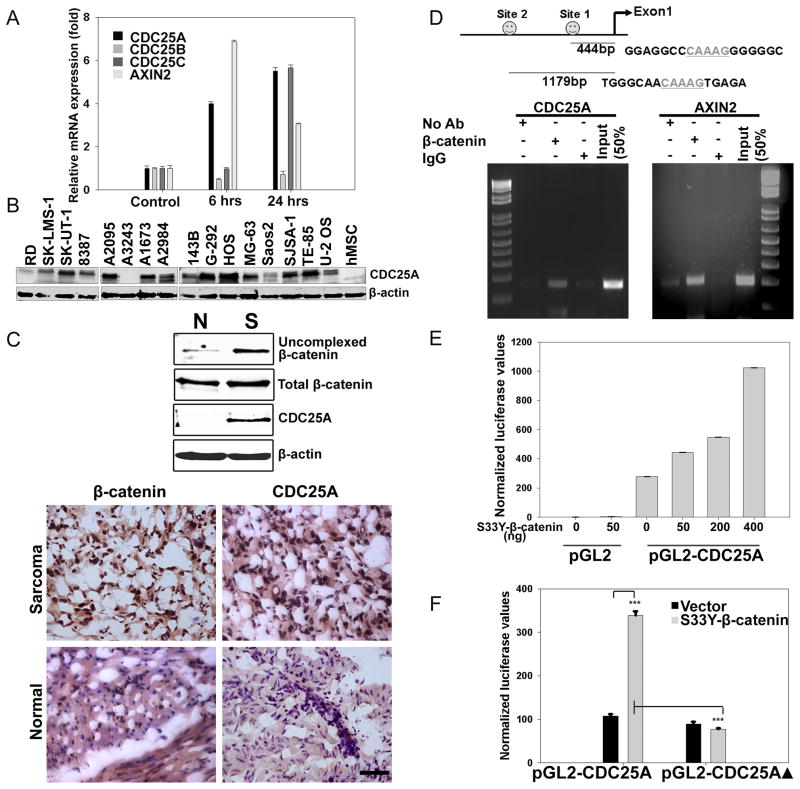
In a microarray experiment conducted to discover potential targets of Wnt signaling in hMSCs, we identified CDC25A, an important regulator of cell cycle (Boutros et al., 2007), to be upregulated markedly in response to WNT3A treatment (Table S1) and validated this result by real-time PCR and Western blotting (Fig. 3A and Fig. S3). As shown in Fig. 3A, the relative expression levels of CDC25A and AXIN2 increased by more than four-fold within 6 hrs of hMSC treatment with WNT3A. Expression levels of the other two CDC25 family members did not increase under these same conditions. Sarcoma lines of diverse subtypes with Wnt activation also showed preferential expression of CDC25A compared to hMSCs (Fig. 3B). Moreover, analysis of a primary human sarcoma, which was positive for nuclear β-catenin by immunostaining, also stained positive for nuclear CDC25A, while the paired normal tissue was negative for both (Fig. 3C). It should be noted that CDC25A protein expression level and the level of Wnt activation did not uniformly correlate among the sarcoma lines analyzed, which may be accounted for by the complexity of CDC25A regulation at posttranslational as well as transcriptional levels (reviewed in (Boutros et al., 2007)).
Figure 3. CDC25A is a direct TCF/β-catenin transcriptional target.
(A) hMSCs were treated with 100ng/ml of WNT3A for either 6 or 24 hrs, and total RNA was extracted. Real-time PCR was conducted to measure the mRNA expression changes for the indicated genes as described in Fig. 2C.
(B) Whole cell lysates of different human sarcoma lines and hMSCs were subjected to immunoblot analysis for expression of CDC25A.
(C) Uncomplexed β-catenin levels in a primary human leiomyosarcoma and corresponding adjacent normal tissue were determined as described in Fig. 1. Protein extracts from tissues were subjected to immunoblot analysis with specific antibodies to β-catenin and CDC25A (Top). Immunostaining for β-catenin or CDC25A was performed on frozen sections (Bottom) from the same leiomyosarcoma and corresponding normal tissue as used for Western blotting above. Scale bar indicates 50μm.
(D) Schematic representation of CDC25A promoter region containing putative TCF binding elements (Top) and ChIP assay in autocrine Wnt activated U-2 OS osteosarcoma cells (Bottom).
(E) Empty PGL2 luciferase reporter vector or a reporter construct containing the 1.3Kb promoter region of CDC25A was transfected into HEK293T cells with or without an expression vector for constitutively active S33Y-β-catenin. Luciferase reporter activities were measured 48 hrs post-transfection.
(F) Site-directed mutagenesis was used to mutate the two putative TCF-binding elements in the 1.3Kb CDC25A promoter region. Wild type or mutant CDC25A reporter constructs (200ng each) were co-transfected with a constitutively active S33Y-β-catenin expression construct (50ng) in HEK293T cells. Luciferase activities were measured 48 hrs post transfection and normalized to Renilla as in (E). ***p<0.001 by Student’s t-test.
Error bars indicate SD of mean values from triplicates and are representative of two independent experiments. See also Figure S3 and Table S1.
To determine whether CDC25A was a direct TCF/β-catenin transcriptional target, we performed an in silico analysis of the CDC25A promoter and identified two putative TCF binding (TCFB) sites within a 1.3kb fragment upstream of the first exon (Fig. 3D). Chromatin immunoprecipitation (ChIP) performed on DNA extracted from Wnt activated U-2 OS cells revealed that β-catenin associated with the two putative TCFB sites in the CDC25A promoter and at a similar site present within the AXIN2 promoter (Fig. 3D). When the CDC25A reporter was co-transfected with a constitutively activated S33Y β-catenin in HEK293T cells, we observed a dose dependent increase in reporter activity (Fig. 3E). Moreover, the observed CDC25A promoter activity was inhibited when the putative TCFB sites (ΔTCFB) in the CDC25A promoter were mutated (Fig. 3F), further establishing CDC25A as a direct transcriptional target of canonical Wnt signaling.
Downregulation of activated Wnt signaling inhibits human sarcoma proliferation in vitro and in vivo
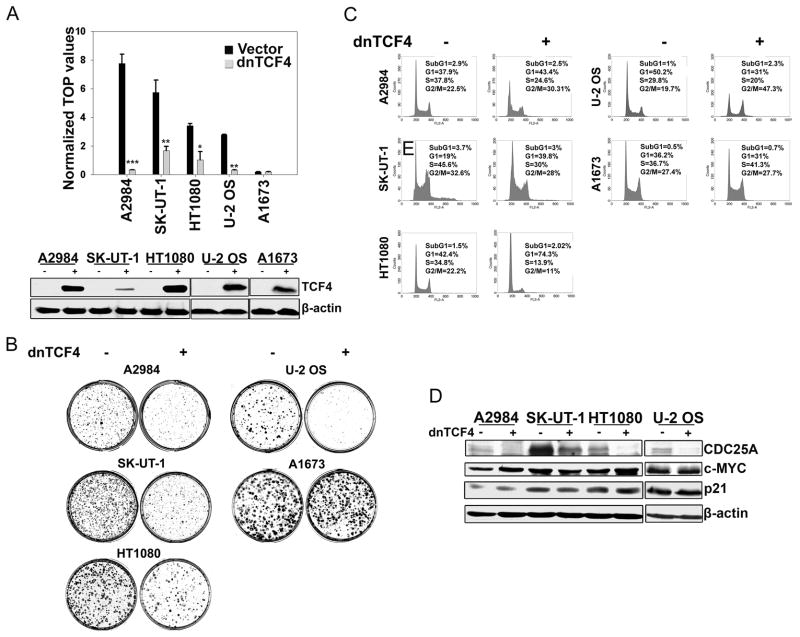
To address the biological significance of upregulated Wnt signaling in human sarcoma cells, we transduced tumor lines representing four distinct sarcoma subtypes exhibiting either autocrine Wnt activation or APC truncation mutations, with a constitutive dnTCF4 lentivirus. Using this strategy we observed greater than 50% inhibition of TCF reporter activity (Fig. 4A) associated with markedly decreased colony forming ability in sarcoma cells having either mechanism of Wnt activation (Fig. 4B). As a specificity control, dnTCF4 expression did not significantly alter colony formation by A1673, a sarcoma line with very low or undetectable levels of uncomplexed β-catenin (Fig. 1A & 4B). Analyses of cell cycle profiles of sarcoma lines expressing dnTCF4 indicated that growth inhibition was achieved either via G1 or G2 arrest (Fig. 4C). We also tested the effects of shRNA knockdown of either LRP5 or LRP6 in HOS and A204 sarcoma lines, which exhibited high levels of endogenous LRP5 and LRP6 proteins, respectively. Downregulation of these receptors led to a concomitant decrease in Wnt reporter activity and inhibition of in vitro proliferation (Fig. S4A), implying the involvement of these overexpressed receptors in autocrine Wnt mediated proliferation of these sarcoma lines. In addition, the ectopic expression of a constitutively active β-catenin rescued FRP-mediated inhibitory effects of Wnt downregulation on in vitro proliferation, further confirming the involvement of the canonical Wnt pathway (Fig. S4B).
Figure 4. Downregulation of activated Wnt signaling in human sarcoma cells induces inhibition of proliferation in vitro.
(A) Downregulation of Wnt signaling in sarcoma lines constitutively expressing lentiviral transduced dnTCF4 was assessed by TCF luciferase reporter assay as in Fig.1B. Error bars indicate SD of mean values from triplicates and are representative of two independent experiments (top). Western blot analysis was performed using whole cell lysates from sarcoma lines expressing dnTCF4 (bottom).
(B) Human sarcoma lines were transduced with either vector control or dnTCF4 expressing lentiviruses. In vitro proliferation was assessed by colony forming assays. Results are representative of at least two independent experiments.
(C) Cell cycle profile analysis of sarcoma cells stably expressing dnTCF4 performed by FACS.
(D) Cell lysates from sarcoma lines expressing dnTCF4 were subjected to immunoblot analysis for CDC25A, c-MYC, and p21.
See also Figure S4.
Since CDC25A plays a crucial role in both G1/S and G2/M progression (reviewed in (Boutros et al., 2007)), we further analyzed CDC25A levels in representative Wnt activated sarcoma cell lines. We observed in all cases that CDC25A protein (Fig. 4D) and mRNA (Fig. S4C) levels were significantly reduced when Wnt signaling was inhibited by dnTCF4 as well as by DKK1, FRP, and shRNA directed against LRP5 (Fig. S4D). Each approach downregulated CDC25A transcript levels comparably to AXIN2. In contrast, there were no significant effects on CDC25B and CDC25C mRNA levels under the same conditions. Furthermore, DKK1 inhibition of CDC25A expression was detectable by 36 hours, prior to any observable alteration of cell cycle profile (Fig. S4 E). For comparison, we also measured transcript levels of the three CDC25 genes in HT1080 sarcoma cells synchronized either at G1/S or G2/M phases. Expression levels of all three CDC25 genes showed similar decreases in cells arrested in G1/S (Fig. S4F), whereas Wnt pathway inhibition by dnTCF4 resulted in specific downregulation of CDC25A transcript levels as indicated above. All of these results argue that CDC25A downregulation observed as a result of Wnt pathway inhibition is not due to a cell cycle positional effect and confirm our conclusions that CDC25A is a direct Wnt transcriptional target gene.
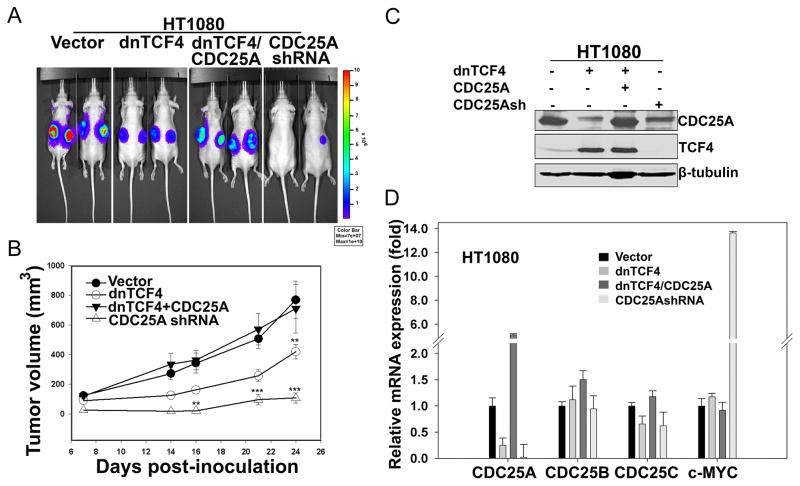
We next compared the effects of downregulating TCF signaling or CDC25A in Wnt autocrine HT1080 cells. Constitutive dnTCF4 expression significantly inhibited tumor formation (Fig. 5A&B), confirming the inhibition of cell proliferation observed in vitro (Fig. 4B). Of note, exogenous CDC25A expression in these same cells rescued in vivo tumor growth (Fig. 5A), while downregulation of CDC25A alone using a specific shRNA (Fig. 5C&D) resulted in decreased HT1080 tumor formation (Fig. 5A&B). Ectopic CDC25A also rescued dnTCF4 mediated inhibition of cell proliferation with several additional Wnt activated sarcoma cell lines analyzed (Fig. S4G). These findings establish that Wnt signaling through the TCF/β-catenin target gene, CDC25A, contributed importantly to tumor formation of this Wnt activated sarcoma.
Figure 5. Downregulation of activated Wnt signaling in HT1080, a human fibrosarcoma cell line, induces inhibition of proliferation in vivo.
(A) HT1080 cells stably expressing firefly luciferase were transduced with lentiviruses expressing the indicated genes and selected in the presence of puromycin for 3 days. 1 × 106 cells were injected sub-cutaneously in nude mice (n=4), and whole animal imaging was performed 6 weeks post inoculation.
(B). Graph showing tumor growth over time measured 1by calipers. Each data point represents a mean of 8 values (n=4 mice/group), and error bars indicate SD of mean values. **p<0.01, ***p<0.001 by two-way ANOVA.
(C). Lysates from HT1080 cells expressing the indicated constructs prior to inoculation in mice were subjected to immunoblot analysis.
(D). Real-time PCR was conducted to measure the mRNA expression changes for the specified genes as described in Fig. 2C. Error bars indicate SD of mean values from triplicates.
Comparative analysis of CDC25A and c-MYC expression identifies CDC25A as a major mediator of β-catenin driven sarcoma cell proliferation
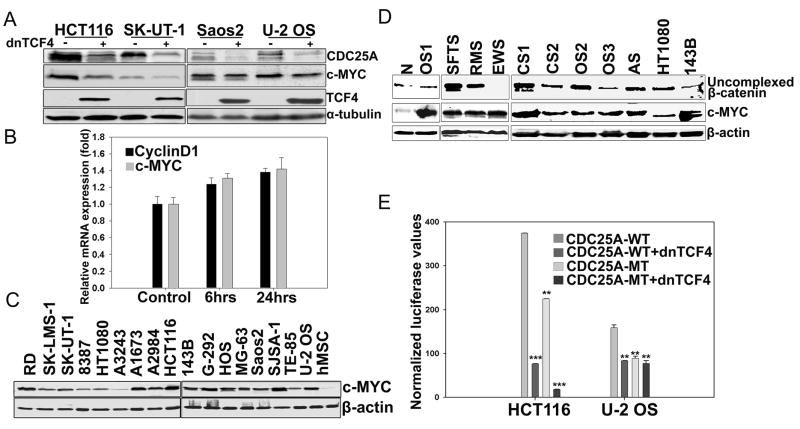
c-MYC is a direct Wnt transcriptional target in colon and other carcinoma cells (Finch et al., 2009; He et al., 1998; Sansom et al., 2007; van de Wetering et al., 2002) As shown in Fig. 6, dnTCF4 expression reduced c-MYC levels in HCT116, a β-catenin mutated colon cancer line reported to have high Wnt activity (He et al., 1998; Morin et al., 1997; Suzuki et al., 2004) (Fig. 6A, lanes 1–2). In contrast, downregulation of Wnt signaling in several sarcoma lines failed to significantly affect c-MYC levels although CDC25A levels were reduced in each case (Fig. 4D and Fig. 6A, lanes 3–8). The CDK inhibitor, p21, is a known c-MYC repression target and is upregulated in response to c-MYC downregulation in colon (van de Wetering et al., 2002) and other Wnt activated carcinomas (Akiri et al., 2009; Bafico et al., 2004; van de Wetering et al., 2002). However, Wnt signaling downregulation had no effect on p21 levels in the sarcoma lines analyzed (Fig. 4D). Whereas WNT3A significantly induced CDC25A expression in hMSCs (Fig. 3A), WNT3A treatment of these cells did not significantly alter mRNA levels of c-MYC or CyclinD1 (Fig. 6B), another reported Wnt target gene (Tetsu and McCormick, 1999). These results indicate that neither is a direct transcriptional target of Wnt signaling in hMSCs. All of these findings argue that CDC25A, but not c-MYC, is a TCF/β-catenin transcriptional target in hMSCs or sarcoma cells.
Figure 6. Lack of correlation between Wnt signaling and c-MYC levels in human sarcomas.
(A) Human sarcoma lines and HCT116 cells were stably transduced with dnTCF4 and proteins were analyzed by Western blotting.
(B) Real-time PCR analysis of c-MYC and CyclinD1 in hMSCs treated with Wnt3A as described in Figure 3A. The values were normalized to TBP and represented relative to those in untreated cultures (Control), which were set at 1. Error bars indicate SD of mean values from triplicates and are representative of two independent experiments.
(C) Western blot analysis of human sarcoma cell lines for c-MYC protein.
(D) C-MYC protein expression in protein extracts from frozen sections of primary sarcoma tissues. N:Normal tissue; CS: Chondrosarcoma; AS:Angiosarcoma. All other abbreviations for sarcomas are described in Figure 1. HT1080 and 143B lines were used as positive and negative controls for Wnt activated sarcomas.
(E) HCT116 or U-2 OS cells were co-transfected with vector control, CDC25A-WT, or CDC25A-MT (see Fig. 3F) luciferase reporter constructs (200ng) and a dnTCF4 expression construct (400ug). Luciferase values were measured 48 hrs post-transfection and normalized to Renilla. Error bars indicate SD of mean values from triplicates and are representative of two independent experiments. *p<0.1, **p<0.01, ***p<0.001 by student’s t test.
See also Figure S5.
When we analyzed c-MYC protein levels in human sarcoma lines, strikingly, most exhibited upregulated c-MYC expression as compared to hMSCs (Fig. 6C), and primary sarcomas exhibited increased c-MYC protein levels compared to their normal tissue counterparts (Fig. 6D). These results imply that a Wnt independent mechanism involving increased protein stabilization frequently increases c-MYC protein expression in sarcomas. Moreover, both c-MYC and CDC25A specific shRNAs inhibited in vitro proliferation of A2984 and HT1080 sarcoma as well as HCT116 carcinoma cells, implying that both genes contribute importantly to proliferation of these tumor cells (Fig. S5). CDC25A is also known to be a c-MYC transcriptional target gene in mammalian cells (Galaktionov et al., 1996). To test whether CDC25A was also a direct TCF/β-catenin target in these carcinoma cells, we transfected HCT116 and Wnt autocrine U2-OS cells with the CDC25A reporter and compared relative activities of both wild and mutant reporter constructs. In both tumor lines, the wild type CDC25A reporter exhibited activity which was inhibited in the presence of dnTCF4 (Fig. 6E). When U-2 OS and HCT116 cells were co-transfected with the mutant (ΔTCFB)-CDC25A reporter in the presence or absence of dnTCF4, its activity was further decreased in HCT116 but not in U-2 OS cells presumably due to reduced c-MYC transcriptional function specific to the carcinoma cells (Fig. 6E). Thus, it is likely that CDC25A is both a direct and indirect TCF/β-catenin target in Wnt activated carcinoma cells.
Wnt activity and in vitro differentiation potential of human sarcoma cell lines
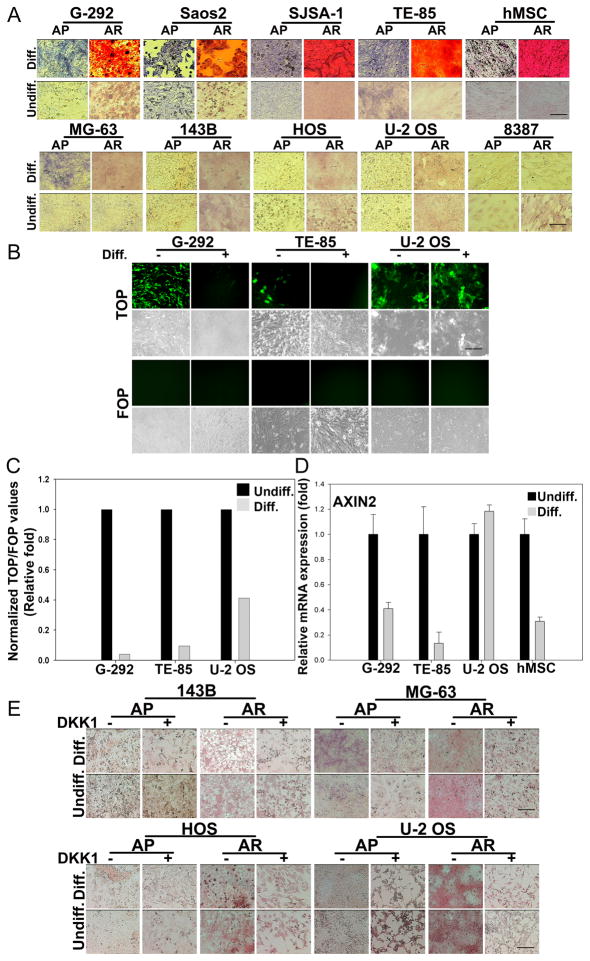
High levels of Wnt signaling inhibit differentiation of hMSCs along osteogenic or adipogenic lineages in vitro and in vivo (Liu et al., 2009). Osteosarcoma cell lines, G-292, Saos2, SJSA-1 and TE-85, exhibited the ability to differentiate along the osteogenic lineage, as detected by staining for two markers of osteogenic differentiation, alkaline phosphatase (ALPL) and alizarin redS (AR) (Fig. 7A). Wnt signaling is downregulated in hMSCs during later stages of osteogenic differentiation, allowing complete maturation of osteoblasts to osteocytes (Li et al., 2005). We observed a similar suppression of Wnt activity in G-292 and TE-85 cell lines in which Wnt signaling was downregulated by more than 80% under osteogenic induction conditions (Fig. 7B and Fig. 7C). In U-2 OS, which failed to differentiate in vitro, Wnt signaling was downregulated less efficiently under osteogenic conditions when compared to either G-292 or TE-85 cells (Fig. 7B and Fig. 7C). Expression of AXIN2, which has been previously shown to be downregulated during hMSC osteogenic and adipogenic differentiation (Liu et al., 2009), decreased during osteogenic differentiation in G-292 and TE-85 cells, but was unaffected in U-2 OS cells (Fig. 7D). Together, these results suggest that under differentiation inducing conditions, high Wnt activity in osteosarcoma cells with osteogenic differentiation ability is downregulated by an intrinsic mechanism.
Figure 7. In vitro differentiation properties of Wnt activated human sarcoma cells.
(A) Sarcoma lines were plated at 10,000 cells/well in 12-well plates and exposed to osteogenic differentiation media (Diff.) for two weeks followed by fixation and staining for either alkaline phosphatase (AP, blue) or Alizarin Red S (AR, red). Control cells were maintained in basal medium (undiff.).
(B) Sarcoma lines stably expressing TOP- or FOP-GFP reporters were cultured as described in (A) and images were captured with a fluorescence microscope or by phase contrast.
(C) TOP-luciferase reporter activity in sarcoma cells cultured in basal or osteogenic differentiation media. Values are normalized to Renilla. Normalized values obtained for cells cultured in basal media (undiff.) were set at 1.
(D) Real-time PCR measurement of AXIN2 using total RNA extracted from sarcoma cells or hMSCs cultured in the presence or absence of osteogenic medium for two weeks as described in Fig. 2C. Normalized values obtained for cells cultured in basal media (undiff.) were set at 1. Error bars indicate SD of mean values from triplicates and are representative of two independent experiments.
(E) Sarcoma lines resistant to osteogenic differentiation were cultured in the presence or absence of osteogenic medium along with DKK1 (100ng/ml) added every other day for two weeks and stained for AP and AR.
Scale bars indicate 100μm. See also Figure S6.
None of the Wnt activated sarcoma lines analyzed showed evidence of multi-lineage differentiation capacity when exposed to osteogenic (Fig. 7E) or adipogenic (Fig. S6A) differentiation conditions in the absence or presence of Wnt downregulation. These results suggested that Wnt-independent aberrations might be responsible for the differentiation block. Frequent genetic aberrations observed in sarcomas include highly specific chromosomal translocations as in the case of Ewing’s-, synovial- and alveolar rhabdomyosarcomas, while osteosarcomas, commonly exhibit Rb and p53 loss of function (reviewed in (Helman and Meltzer, 2003; Skubitz and D’Adamo, 2007)). Thus, we sought to model genetic aberrations observed in osteosarcomas by ectopic expression of Cyclin dependent kinase 4 (CDK4) or dominant negative p53 (dnp53), and oncogenic rasG12D either alone or in combination in hMSCs. The expression of any one of the three genes in addition to hTERT, which did not itself block hMSC osteogenic differentiation, was sufficient to partially inhibit ALPL expression without exhibiting an obvious effect on mineralization as detected by AR staining (Fig. S6B). However, the severity of osteogenic differentiation inhibition based on both reduced ALPL expression and mineralization, increased with the number of oncogenes added. These findings argue that genetic alterations independent of Wnt activation may mask the ability to correlate Wnt activity with stemness in sarcomas.
DISCUSSION
Mesenchymal stem cells can differentiate along osteogenic, adipogenic, chondrogenic, myogenic and other connective tissue lineages in response to specific differentiation media (Pittenger et al., 1999). hMSCs have been shown to exhibit a low level of endogenous Wnt signaling, which decreases in response to differentiation stimuli (Li et al., 2005). Moreover, evidence that exogenous Wnt stimulation specifically increased hMSC proliferation and inhibited differentiation (Liu et al., 2009), properties commonly associated with tumor cells (Hanahan and Weinberg, 2000; Liu et al., 2009; van de Wetering et al., 2002) led us to explore whether Wnt upregulation might contribute to sarcomagenesis. Our present studies establish constitutive Wnt activation relative to levels observed in hMSCs in a diverse array of human sarcomas. In contrast to colon carcinomas, in which Wnt activation involves genetic aberrations in intracellular components, we established that Wnt activation in sarcomas commonly involves an autocrine mechanism as shown by the ability of antagonists, which act at the cell surface to block Wnt ligand/receptor interactions, to downregulate this signaling. β-catenin mutations were absent in any of the Wnt activated sarcoma cell lines screened. However, we identified APC truncation mutations in two sarcoma lines which lacked an autocrine activation mechanism. Of note, A2984 and SK-UT-1, like representative Wnt autocrine sarcoma lines were growth inhibited in response to dnTCF4, which had no effects on non-Wnt activated sarcoma cells. These results establish the Wnt specificity of this inhibition and the essential role of Wnt signaling in driving the proliferation of sarcoma cells in which the pathway is upregulated. Further, Wnt autocrine activation contributes as importantly to the Wnt proliferative drive as an APC mutation, previously established as the initiating step in colon tumorigenesis (Bienz and Clevers, 2000; Korinek et al., 1997; Morin et al., 1997; Polakis, 1997; Roose et al., 1999).
The frequency of Wnt activation was greater than 90% in osteosarcomas and was at least 50% in synovial, Ewing’s, rhabdomyo-, fibro- and leiomyosarcoma cell lines. Constitutive Wnt activation was also identified in primary human sarcomas, which exhibited elevated uncomplexed β-catenin levels in more than 50% of samples analyzed from a wide array of sarcoma subtypes. There are reports implicating the hMSC as the cell of origin for Ewing’s (Miyagawa et al., 2008; Tirode et al., 2007) and MFH/pleomorphic sarcomas (Matushansky et al., 2007), and we detected Wnt activation in 50% of Ewing’s sarcoma cell lines. Of note, hMSC adipogenic differentiation is particularly sensitive to Wnt inhibition (Ross et al., 2000), and the lowest frequency of Wnt activation was observed in sarcomas classified histopathologically as liposarcomas. All of these findings are consistent with a model, in which sarcomas commonly initiate from upregulation of autocrine Wnt signaling, and less frequently from mutations in intracellular components, in a mesenchymal stem cell associated with its clonal expansion and accumulation of additional alterations that contribute to malignant transformation. However, our findings do not exclude the possibility that Wnt pathway activation occurs at some later stage of neoplastic progression and/or involves a committed progenitor rather than a stem cell.
We observed that the sarcoma lines analyzed lacked multi-lineage differentiation capacity even under the conditions in which Wnt signaling downregulation resulted in profound inhibition of proliferation. Thus, if the target for Wnt pathway activation were a mesenchymal stem/progenitor cell, Wnt independent alterations must override the differentiation potential of such cells. In fact, we found that oncogenic alterations of Rb, p53 or Ras pathways, observed in osteosarcomas, (Kansara and Thomas, 2007) alone or in combination resulted in progressive inhibition of the ability of hMSCs to undergo osteogenic differentiation. These findings argue strongly that oncogenic alterations independent of Wnt pathway activation can impair the ability of a mesenchymal stem cell to undergo lineage differentiation.
Mechanisms involved in activation of an autocrine Wnt loop in human sarcomas
There are reports in the literature of nuclear accumulation of β-catenin in a significant fraction (28–57%) of synovial and uterine soft-tissue sarcomas without exploration of possible mechanisms (Jung et al., 2008; Kildal et al., 2009; Ng et al., 2005). Our present findings of Wnt autocrine activation establish the molecular basis for nuclear accumulation of β-catenin in such tumors. In a mouse model generated by loss of function of WIF1, a Wnt antagonist, there was an increased incidence of radiation induced sarcomas (Kansara et al., 2009), and these same authors identified epigenetic silencing of WIF1 in some human osteosarcomas associated with upregulation of Wnt signaling (Kansara et al., 2009). In contrast, (Cai et al., 2010) recently reported the inability to detect canonical Wnt activity in osteosarcomas analyzed. Our present findings demonstrate activation of this pathway by several different approaches in osteosarcoma lines studied by Cai et al. Thus, the failure of Cai et al to detect Wnt pathway activation in human osteosarcomas may involve differences in assay sensitivity.
Our findings that sarcomas of diverse types exhibit autocrine canonical Wnt activation adds this tumor to those including breast, ovarian and lung carcinoma exhibiting autocrine Wnt activation at high frequencies (Akiri et al., 2009; Bafico et al., 2004). We observed that relative to hMSCs, sarcomas often exhibited upregulated expression at the RNA level of canonical Wnt ligands, most commonly Wnt7b and Wnt10b. Sarcoma lines also frequently overexpressed LRP5, LRP6 or both receptors with evidence of LRP5 gene amplification in some. Epigenetic silencing of Wnt antagonists including FRP1, FRP2, FRP4, FRP5, DKK1 and DKK2 was identified in some sarcoma lines as well. Thus, there appear to be several mechanisms by which autocrine Wnt signaling can be upregulated in human sarcomas as demonstrated by the specific growth inhibition associated with LRP5 and LRP6 downregulation in HOS and A204 sarcoma lines respectively. Our findings of more than one alteration in Wnt cell surface components in some autocrine tumor lines could imply continued selection for Wnt pathway activation by this mechanism.
Cell context differences in the role of Wnt target genes in tumor cell proliferation
Wnt signaling exerts its oncogenic functions via upregulation of genes involved in cell cycle, exemplified by the positive TCF/β-catenin transcriptional effects on c-MYC expression in carcinomas (Akiri et al., 2009; Bafico et al., 2004; van de Wetering et al., 2002). c-MYC is required for efficient activation of all cyclin/CDK complexes and, thus, is a vital player acting at multiple phases of the cell cycle (Mateyak et al., 1999). We observed that unlike HCT116 colon carcinoma cells in which c-MYC is responsible for Wnt induced proliferation (He et al., 1998; van de Wetering et al., 2002) c-MYC was not transcriptionally regulated in a Wnt dependent manner in Wnt activated sarcomas. Instead, we identified CDC25A as a Wnt transcriptional target as demonstrated by ChIP assay and β-catenin dependent activation of CDC25A reporter in sarcoma cells. Furthermore, we showed that CDC25A was an important mediator of Wnt induced sarcoma cell proliferation both in vitro and in vivo. While Wnt pathway downregulation in each sarcoma line analyzed led to reduced CDC25A transcript and protein levels, the level of CDC25A protein expression did not correlate perfectly with Wnt activation among the sarcoma lines analyzed, likely due to differences among tumor lines in CDC25A posttranslational regulation (Boutros et al., 2007). Recently, it was reported that GSK3β, an effector kinase inhibited by Wnt ligand induced stimulation, regulates
CDC25A protein levels by proteasome mediated degradation (Kang et al., 2008). Thus, GSK3β inhibition by autocrine Wnt activation could in theory lead to increased CDC25A protein stabilization. However, our results provide no evidence for Wnt dependent regulation of CDC25A expression at the protein level by such a mechanism. CDC25A is overexpressed in multiple cancers, including breast, ovarian, lung, colorectal, pancreatic and head and neck cancer (reviewed in (Boutros et al., 2007)). It is inferred from animal studies that among the CDC25 family, CDC25A plays a non-redundant role in regulating cell cycle (Chen et al., 2001; Ferguson et al., 2005; Ray et al., 2007b). MMTV-targeted expression of CDC25A cooperated with neu or ras induced mammary tumorigenesis, while mice hemizygous for Cdc25A exhibited prolonged latency of neu or ras induced mammary tumors (Ray et al., 2007a; Ray et al., 2007b). CDC25A is also transcriptionally regulated by c-MYC as well as by E2F1 in mammalian cells (Galaktionov et al., 1996; Vigo et al., 1999). Given that CDC25A is transcriptional target of both TCF/β-catenin as shown here and c-MYC (Galaktionov et al., 1996), the inhibition of proliferation induced by dnTCF4 in colon and other carcinomas may be mediated through CDC25A as well as c-MYC.
In summary, our present studies show that the Wnt canonical upregulation by autocrine or less frequently by other mechanisms is common in human sarcomas of many subtypes. Further, Wnt upregulation by autocrine activation or other mechanisms plays an important causal role in the proliferative drive of Wnt activated sarcomas through a Wnt target gene, CDC25A. Over the past two and a half decades since the discovery of the involvement of activated tyrosine kinase receptor pathways in tumors, agents that specifically target these pathways have provided clinically useful adjuvants to standard chemo-irradiation therapies (reviewed in (Zhang et al., 2009)). The high prevalence of Wnt pathway activation in human sarcomas described here and the ability to identify Wnt pathway activation in primary sarcoma tissues make it reasonable to test whether naturally occurring Wnt antagonists such as DKK1 or FRP or recently reported small molecule Wnt inhibitors (Doghman et al., 2008; Huang et al., 2009) may complement standard agents in the treatment of this most common childhood malignancy.
EXPERIMENTAL PROCEDURES
Uncomplexed β-catenin Analyses
Immunoprecipitation to detect uncomplexed β-catenin was performed as described previously (Bafico et al, 2004). Briefly, 1 mg of protein from whole cell lysate was incubated with GST-E-cadherin/Glutathione-Sepharose beads (Amersham) for 1hr at 4°C. Beads were pelleted and washed thrice in lysis buffer and resuspended in loading buffer for Western blot analysis as described in Supplemental Experimental Procedures.
Human Sarcoma Tissues
Frozen sarcoma tissues were purchased from NCI’s Cooperative Human Tissue Network or obtained through the Tisch Cancer Institute’s Biorepository at Mount Sinai Hospital. Sarcoma tissues acquired from the Mount Sinai Hospital used in this study were de-identified prior to analysis. The use of human tissues was approved by the Institutional Review Board. Frozen tissues were embedded in Optimal Cutting Temperature (O.C.T) and 20 sections of 20μm each were used. Tumor tissues were analyzed histologically to confirm the presence of at least 70% tumor cells. Excess OCT was removed, and tissues were rinsed thrice in cold PBS. Buffer containing 1% NP-40, 150mM sodium chloride and protease inhibitors was used for protein solubilization..
Microarray
Total RNA extracted from hMSCs was used for gene expression analysis on the GeneChip Human Genome U133A 2.0 Array (Affymetrix). Hybridization and data acquisition were conducted at Mount Sinai’s Microarray Shared Research Facility according to the manufacturer’s protocol. Data were analyzed using GeneChip Operating Software (Affymetrix) and Ingenuity’s Pathway Analysis software.
In Vivo Tumorigenicity Assay
HT1080 cells stably expressing firefly luciferase were transduced with vector control, dnTCF4 or CDC25A shRNA and selected as described for colony forming assay. Firefly luciferase was cloned in a lentiviral vector carrying a neomycin marker gene. Standard procedures were used to clone the full length CDC25A cDNA into a PGK promoter driven lentiviral construct containing a blasticidine marker gene. Cells expressing both dnTCF4 and CDC25A were selected in medium containing 1ug/ml puromycin and 5ug/ml blasticidine (Invitrogen). Around 1 × 106 cells were resuspended in PBS and mixed with 50% matrigel (BD Biosciences) prior to subcutaneous inoculation at two sites in six weeks old athymic nude mice (NCI). All animal experiments were approved and performed according to the relevant regulatory standards set by Mount Sinai’s Animal Care and Use Committee. Mice were anaesthetized using isoflurane, and in vivo imaging was performed using the Xenogen IVIS-200 imaging system at Mount Sinai’s shared in vivo imaging facility.
Osteogenic and Adipogenic Differentiation Assay
Cells were transferred to 24-well plates and cultured in either basal medium (DMEM, 10% FBS, 100U/m P/S) or osteogenic medium consisting of DMEM, 10% FBS, 100U/m P/S supplemented with 50μg/ml Ascorbic acid (Biochemika), 10mM β-Glycerol-2-phosphate (Sigma) and 1μM Dexamethasone (Sigma). Cells were subjected to differentiation conditions for around two weeks and processed for staining as described previously (Liu et al., 2009). Adipogenic differentiation was performed as previously described (Liu et al., 2009).
Statistical Analyses
Statistical significance was determined with Student’s t-test or one way analysis of variance (ANOVA) in the case of more than two comparisons (calculated with GraphPad Prism 5 software).
Accession Number
Microarray data generated in this study have been deposited at the NCBI Gene Expression Omnibus (http://ncbi.nlm.nih.gov/geo/) with the accession number GSE27313.
Supplementary Material
Acknowledgments
The authors thank HuiFang Qiao and RuiFang Qiao for technical support in processing human sarcoma samples. This work was supported by grants from State of New York, Department of Health (Contract#C024313) and National Cancer Institute (5 R01 CA071672-13) to SAA. SV is recipient of a post-doctoral fellowship award from American Urological Association. GL is supported by a Young Investigator grant from Breast Cancer Alliance, Inc. We appreciate helpful suggestions provided by Romi Biswas and Stefania Asciutti at various stages of this study, and also acknowledge contributions by Robert A. Chong.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009:28. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, Miura K, Harris CC. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003;63:728–734. [PubMed] [Google Scholar]
- Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–16187. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nature Reviews Cancer. 2007;7:495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- Chen MS, Hurov J, White LS, Woodford-Thomas T, Piwnica-Worms H. Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol Cell Biol. 2001;21:3853–3861. doi: 10.1128/MCB.21.12.3853-3861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Doghman M, Cazareth J, Lalli E. The T cell factor/beta-catenin antagonist PKF115–584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab. 2008;93:3222–3225. doi: 10.1210/jc.2008-0247. [DOI] [PubMed] [Google Scholar]
- Ferguson AM, White LS, Donovan PJ, Piwnica-Worms H. Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol Cell Biol. 2005;25:2853–2860. doi: 10.1128/MCB.25.7.2853-2860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AJ, Soucek L, Junttila MR, Swigart LB, Evan GI. Acute overexpression of Myc in intestinal epithelium recapitulates some but not all the changes elicited by Wnt/beta-catenin pathway activation. Mol Cell Biol. 2009;29:5306–5315. doi: 10.1128/MCB.01745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway [see comments] Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- Huang SMA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CK, Jung JH, Lee A, Lee YS, Choi YJ, Yoon SK, Lee KY. Diagnostic use of nuclear [beta]-catenin expression for the assessment of endometrial stromal tumors. Mod Pathol. 2008;21:756–763. doi: 10.1038/modpathol.2008.53. [DOI] [PubMed] [Google Scholar]
- Kang T, Wei Y, Honaker Y, Yamaguchi H, Appella E, Hung MC, Piwnica-Worms H. GSK-3 beta targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell. 2008;13:36–47. doi: 10.1016/j.ccr.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26:1–18. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice.[see comment] Journal of Clinical Investigation. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z, Vijayakumar S, de la Torre TV, Rotolo S, Bafico A. Analysis of endogenous LRP6 function reveals a novel feedback mechanism by which Wnt negatively regulates its receptor. Mol Cell Biol. 2007;27:7291–7301. doi: 10.1128/MCB.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kildal W, Pradhan M, Abeler VM, Kristensen GB, Danielsen HE. [beta]-Catenin expression in uterine sarcomas and its relation to clinicopathological parameters. European Journal of Cancer. 2009;45:2412–2417. doi: 10.1016/j.ejca.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma [see comments] Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nature Genetics. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- Liu G, Vijayakumar S, Grumolato L, Arroyave R, Qiao H, Akiri G, Aaronson SA. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol. 2009;185:67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mateyak MK, Obaya AJ, Sedivy JM. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, Singer S, Maki RG, Cordon-Cardo C. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest. 2007;117:3248–3257. doi: 10.1172/JCI31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa Y, Okita H, Nakaijima H, Horiuchi Y, Sato B, Taguchi T, Toyoda M, Katagiri YU, Fujimoto J, Hata J, et al. Inducible expression of chimeric EWS/ETS proteins confers Ewing’s family tumor-like phenotypes to human mesenchymal progenitor cells. Mol Cell Biol. 2008;28:2125–2137. doi: 10.1128/MCB.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC [see comments] Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Terao Y, Fuhrken PG, Ma ZQ, DeMayo FJ, Christov K, Heerema NA, Franks R, Tsai SY, Papoutsakis ET, Kiyokawa H. Deregulated CDC25A expression promotes mammary tumorigenesis with genomic instability. Cancer Res. 2007a;67:984–991. doi: 10.1158/0008-5472.CAN-06-3927. [DOI] [PubMed] [Google Scholar]
- Ray D, Terao Y, Nimbalkar D, Hirai H, Osmundson EC, Zou X, Franks R, Christov K, Kiyokawa H. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res. 2007b;67:6605–6611. doi: 10.1158/0008-5472.CAN-06-4815. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, van Rijn L, Gaspar C, Fodde R, Janssen F, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105:7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz KM, D’Adamo DR. Sarcoma. Mayo Clin Proc. 2007;82:1409–1432. doi: 10.4065/82.11.1409. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Dong Chen W, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tezuka K, Yasuda M, Watanabe N, Morimura N, Kuroda K, Miyatani S, Hozumi N. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res. 2002;17:231–239. doi: 10.1359/jbmr.2002.17.2.231. [DOI] [PubMed] [Google Scholar]
- Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11:421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D, Holstege FC, Brummelkamp TR, Agami R, Clevers H. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–615. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC, Helin K. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol. 1999;19:6379–6395. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr Biol. 2000;10:1611–1614. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.