Abstract
To understand the mechanisms and epidemiology of antimicrobial resistance (AR), the genetic elements responsible must be identified. Due to the myriad of possible genes, a high-density genotyping technique is needed for initial screening. To achieve this, AR genes in the National Center for Biotechnology Information GenBank database were identified by their annotations and compiled into a nonredundant list of 775 genes. A DNA microarray was constructed of 70mer oligonucelotide probes designed to detect these genes encoding resistances to aminoglycosides, β-lactams, chloramphenicols, glycopeptides, heavy metals, lincosamides, macrolides, metronidazoles, polyketides, quaternary ammonium compounds, streptogramins, sulfonamides, tetracyclines, and trimethoprims as well as resistance transfer genes. The microarray was validated with two fully sequenced control strains of Salmonella enterica: Typhimurium LT2 (sensitive) and Typhi CT18 (multidrug resistance [MDR]). All resistance genes encoded on the MDR plasmid, pHCM1, harbored by CT18 were detected in that strain, whereas no resistance genes were detected in LT2. The microarray was also tested with a variety of bacteria, including MDR Salmonella enterica serovars, Escherichia coli, Campylobacter spp., Enterococcus spp., methicillin-resistant Staphylococcus aureus, Listeria spp., and Clostridium difficile. The results presented here demonstrate that a microarray can be designed to detect virtually all AR genes found in the National Center for Biotechnology Information database, thus reducing the subsequent assays necessary to identify specific resistance gene alleles.
Introduction
Antimicrobial resistance (AR) in bacteria is an ongoing problem in human and animal health. Virtually from the inception of antimicrobial chemotherapies to treat bacterial infections, resistance was found and began to expand.1 Despite regulations and controls of antimicrobial use designed to reduce its development and spread, bacterial resistance to antimicrobials continues to increase.1 To help understand the development of AR and its spread, the genetic mechanisms must be identified. These studies often require assaying for dozens or hundreds of possible resistance-encoding genes to investigate the underlying genetics behind the phenotypic resistance observed in bacteria.12 This problem has been compounded by the growth of multidrug resistance (MDR) in important pathogenic bacteria, opportunistic pathogens, commensal bacteria, and environmental bacteria. To address this issue, many researches have turned to high-density gene detection techniques, primarily DNA microarrays.2,5,7,9,10,13,16,24,26,37,46
Several studies have demonstrated that DNA microarrays can be used to detect resistance genes as effectively as standard techniques such as polymerase chain reaction, sequencing, conjugation, and Southern hybridization.2,5,7,9,10,13,16,24,26,37,46 Most of these microarrays rely on short to medium-length (20mer–80mer) oligonucleotides as detection probes because they are easily synthesized without the requirement of template DNA and can be cheaply made and arrayed onto a variety of substrates such as glass slides.13,34 Modified microscope glass slides have become the most widespread format for custom microarrays, with most universities and research institutes having access to printing robots and scanners designed to manufacture and analyze these arrays. With the advent of this technology, it should be theoretically possible to design microarrays for the detection of all known sequenced AR genes available in the public domain and cheaply construct them in many research facilities world wide.13,23 It is important to note that this microarray is not intended to replace phenotypic testing in diagnostic and clinical settings, although there has been considerable progress in the development of diagnostic microarrays.2,4,15,31 Microarray data are difficult to interpret in a clinical setting because the detection of a gene is a potentially nebulous result. Indeed, genes detected may not be functional or expressed, and negative hybridization results are even more difficult to interpret, as previously uncharacterized resistance mechanisms could lead to failure of a selected treatment. In the clinical setting, the goal is to select a successful treatment regimen, thus making phenotypic testing more informative than gene detection. However, when the goal is to study the molecular epidemiology of AR, DNA microarrays are an exceptional tool for detecting multiple AR genes in a single assay.
In the present study, a DNA microarray was designed to detect as many resistance genes as possible for use as a screening technique in studies of prevalence, epidemiology, and spread of resistance genes. The National Center for Biotechnology Information (NCBI) databases and simple bioinformatics processes were used to build a local database of target gene sequences. Unique oligonucleotide probes were designed for the detection of each AR-associated gene in this database.13,43 Probes 70 bases in length were chosen because of their ability to tolerate possible mismatches within the probe regions and to detect as many alleles of resistance genes as possible. These sequences were used to construct the microarray utilizing the most widely available microarray format, glass slides, which was then tested on control strains and a variety of MDR bacteria. The results demonstrate that these simple and relatively inexpensive techniques yielded a highly useful research tool to study the epidemiology of AR genes in a wide range of important bacteria. This report supplies researchers in the field with information they can use to build their own arrays and improve upon this technique.
Materials and Methods
Identification and selection of target genes
Previous work demonstrated that oligonucleotide microarrays can be used to detect resistance genes in a wide variety of bacteria.13,25,26,37,41 The major short coming of these microarrays as well as our previous array (described in Frye et al.13) was the limited number of genes they were designed to detect and thus the limited number of bacteria they could be used to investigate. To design a microarray representing the most comprehensive set of genes associated with AR, sequences available in the NCBI GenBank database were identified by employing several database search strategies. A query designed to yield the maximum number of nonredundant genes annotated as “bacteria & antibiotic & resist*” yielded 3,391 genes from the nonredundant DNA sequence database as well as 1,115 genes from the translated protein database. All of these sequences were sorted by nucleotide coding sequence enabling the identification and elimination of identical duplicate genes, leaving 3,751 genes. These sequences were downloaded into a local database, and 70mer oligonucleotides were designed as described below.35,43 All probe sequences likely to detect a homologous gene in the probe set (>90% identity over the length of the 70mer) were eliminated from the list for a final total of 1,224 gene probes. Probe sequences that would detect any of the 94 genes from our previous work (Frye et al.13) were excluded, as those previously designed probes were included on the new microarray and used as working control probes.13 Next, the annotation of each gene was examined, and genes not likely to be related to AR or AR gene transfer were deleted leaving 681 genes. The sequences of these probes as well as the probes from the previous array (total n = 775) are included in Supplemental Table S1 (available online at www.liebertonline.com/mdr). Finally, the probe sequences were BLAST-Like Alignment Tool (BLAT) searched against the entire NCBI database to identify all close homologs to which they would likely hybridize.18 This yielded 24,489 genes in the complete NCBI database that were likely to be detected by the microarray at the time of the query (data not shown).
Oligonucleotide probe design and microarray construction
Sequences for the 681 genes were used to design an optimized unique 70mer oligonucleotide probe for each gene using the program OligoWiz 2.0 following methods and settings recommended by the authors (probe sequences are presented in Supplemental Table S1).35,43 Oligonucleotide probes of this length were selected, as previous work had demonstrated that 70mers have good specificity, can tolerate several nucleotide mismatches in the target sequence, require no chemical modification to adhere to the slide surface, and give good results without the need for problematic labeling or amplification of DNA samples.11,13,19,23 These probes were synthesized (Operon, Huntsville, AL), diluted in printing buffer (35 μM in 50% dimethyl sulfoxide [DMSO]), and arrayed in triplicate onto UltraGaps amino silane–coated slides (Corning, Life Sciences, Acton, MA) with an Omnigrid robot (Genemachines, San Carlos, CA), and postprocessed as previously described.13,38 As stated above, the 94 probes from our test microarray were added for a total of 775 unique AR-associated genes.13 Additional controls included 3 positive control probes designed to detect bacterial 16s rDNA sequences,22 2 Cy3-labeled and 1 Cy5-labeled Lambda DNA controls, 12 buffer (50% DMSO) only spots, and 130 empty (background) spots. Twelve probes were also synthesized in duplicate or triplicate as controls (indicated in Table 1 and Supplemental Table S1 with an “*”).
Table 1.
Summary of Gene Probes with Positive Hybridizations to the Control Strains and Test Isolates
| Aminoglycoside | Beta-lactam | Chloramphenicol | Efflux | Glycopeptide | Heavy metal | Macrolide | Metronidazole | Poly-ketide | Quaternary ammonium | Sulfonamides | Tetracycline | Transfer associated | Trimethoprim | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LT2 | aadA1 | acrR, emrR, marA, marB | nitroimidazole resistance | |||||||||||
| CT18 | aac(3), aadA, strA,strB | bla, blaPER2, blaTEM-1, tem | cat, cat4, catIII | acrR, corA, emrR, marA, marB, msrA | ble | merACDRT | sulII | tet(A), tet(A)(B), tet(R) | htdAFT, InsA, IntI, parB, putative transposase, repC, repE, stbA, tnpA, tnpAIS26, tnpR, traI, trhBCFNUV | dfrA, dfrV, dfrV, dfrB | ||||
| SE Tc JF201 | aac(3), aadA, aadA, aadAb, aadA2*, aadA2, aadB, aadE, acc(6)-Ib*, aph(3)-I, aph2, aphA7, aphAI, ksgA, ntpII, strA*, strB, str3K | ampC, ampR, blaL2, bla, PER2, cmy-2*, sme-1*, tem | cat, cmlA5, flo* | acrR, corA, EmrA, emrR, marA, marB, msrA, ABC transporter, ybit | ble | merACFT | nonR | qac, qacEdelta | sul, sulII | tet(A), tet(A)(B), tet(C), tet(D), tet(R) | InsA, IntI, putative transposase, tnpA, tnpAIS26, tnpM, tnpR | dfr, dfr6, dfrA | ||
| SE Hd JF210 | aac(3), aadA, aadA, aadAb, aadA2*, aadA2, aadA7, aadB, acc(6)-Ib*, aph(3)-I, aphA7, aphAI, ksgA, ntpII, orfE, SAT-2, strA*, strB, str3K | ampC, bla, blaL2, blaTEM-1, cmy-2*, sme-1*, tem | cat, cmlA5, flo* | acrR, corA, emrR, marA, marB, msrA, ABC transporter, ybit | ble | merACDFPRRT, orf5 | nonR | qac, qacEdelta | sul, sulII | tet(A), tet(A)(B), tet(C), tet(D), tet(R) | InsA, IntI, mobA, putative transposase, tnpA, tnpAIS26, tnpM, tnpR,trhCF | dfr, dfr6, dfrA | ||
| EC JF220 | aac(3), aac(6), aadA, aadAb, aadA2*, aadE, aph(3)-I, aphA7, aphAI, ksgA, strA*, strB, str3K | ampC, ampR, bla, blaL2, blaTEM-1, cfxA, cmy-2*, penA, sme-1*, tem | flo* | acrR, corA, EmrA, emrR,marA, marB, msrA, potassium-transporting ATPase, ABC transporter, ybit | ble, vanH | merACDFPRRT, orf5 | nonR | qac, qacEdelta | sul, sulII | tet(A), tet(C), tet(D), tet(R) | InsA, IntI, putative transposase, tnpA, tnpAIS26, tnpM, tnpR | dfrA, dhf | ||
| EC JF227 | aacC3, aadA, aadAb, aadA2*, aadE, aph(3)-I, aphA7, aphAI, ksgA, strA*, strB, str3K | ampC, ampR, bla, blaL2, blaTEM-1, cfxA, cmy-2*, sme-1*, tem | cat, flo* | acrR, corA, EmrA, emrR, marA, marB, msrA, potassium-transporting ATPase, ABC transporter, ybit | ble, vanH | merADFRRT, orf5 | nonR | qac, qacEdelta | sul, sulII | tet(A), tet(A)(B), tet(C), tet(D), tet(R) | InsA, IntI, putative transposase, tnpA, tnpAIS26, tnpM | dfrA, dhf | ||
| CC 14-22 | aad9, aphA-3 | cam, cfiA | cmeBC, hsdR, marR | tet(O) | btgA, trfA2, trhU | |||||||||
| CC 141-27 | aphA-3 | blaL2, cam | cmeBC, hsdR, potassium-transporting ATPase | tet(O) | htdT | |||||||||
| ECF ATCC25788 | vanC* | merT | tnpA, trans | |||||||||||
| EGM ATCC49573 | blaL2, MecR | acrR, EmrA, potassium-transporting ATPase, ABC transporter, ybit | ble, vanC* | tet(M), tet(O), tet(S) | putative transposase, tnpA, tnpR, trans | |||||||||
| LI03 | msrC, potassium-transporting ATPase, ybit | ble | tet(M), tet(O), tet(S) | putative transposase, recP | ||||||||||
| LI04 | blaTEM-1 | msrC, potassium-transporting ATPase, ybit | ble | nitroimidazole resistance | tet(M), tet(O) | putative transposase | ||||||||
| MRSA G10-C87-2 | blaI, mecA, mecI, mecR, MecR, pre, penicillinase repressor, spc | marR, potassium-transporting ATPase | ble | ermA | putative transposase, tnpA, tnpAIS26, tnpB, tnpC, transposase B | |||||||||
| MRSA DN | aadE, aphA-3, sat4 | blaI, mecA, mecR, MecR, pre, penicillinase repressor, spc | cat | marR, potassium-transporting ATPase | ble | ermA | putative transposase, tnpA, tnpB, tnpC, transposase B | |||||||
| MRSA 36-1 | aadE, aphA-3, sat4 | blaI, mecA, MecR, pre | ermC | putative transposase, repC | ||||||||||
| MRSA 59 | aadE, aphA-3, sat4 | mecA, MecR | putative transposase, repC | |||||||||||
| CD 70 | ||||||||||||||
| CD 98 | orfE | |||||||||||||
| CD 112 | blaL2 | tet(C) | parB, tnpB, tnpR, trhC | |||||||||||
| CD 113 |
Complete data in Supplemental Table S1 (available online at www.liebertonline.com/mdr).
Indicates multiple identical probes for this gene. Genes with more than one different probe are listed for each positive hybridization detected.
Isolates: SE, Salmonella enterica; Hd, Heidelberg; Tc, serovar Typhimurium variant Copenhagen; EC, Escherichia coli; CC, Campylobacter coli; ECF, Enterococcus casseliflavus; EGM, Enterococcus gallinarum; LI, Listeria Innocua; MRSA, Methicillin Resistant Staphylococcus aureus; CD, Clostridium difficile.
Strains, growth conditions, and antimicrobial susceptibility
The fully sequenced control strains were Salmonella enterica serovar Typhimurium LT228 and Salmonella enterica serovar Typhi CT18 (S. Typhi CT18).36 Enterococcus control strains obtained from the American Type Culture Collection (ATCC, Manassas, VA) are indicated by their ATCC numbers in Tables 1 and 2. Test isolates of Salmonella serovars, Escherichia coli, Enterococcus spp., Campylobacter coli, Listeria innocua, methicillin-resistant Staphylococcus aureus (MRSA), and Clostridium difficile were obtained from the National Antimicrobial Health Monitoring System (NARMS) bacteria collection. Phenotypic analyses were conducted as previously described (www.cdc.gov/narms/). Bacteria were grown from frozen stock cultures stored at −70°C by standard methods with appropriate media. Salmonella and E. coli were grown in the Luria Bertani (LB) medium, on LB agar, or blood agar plates (BAP) at 37°C as indicated. C. coli were grown on Campy-cefex plates and incubated at 42°C for 48 hours under microaerobic conditions (5% O2, 10% CO2, and 85% N2) in zip-top storage bags. Enterococcus spp., MRSA, and L. innocua were grown in LB, in brain heart infusion, or on BAP at 37°C with standard methods. C. difficile was grown on BAP at 37°C in an anaerobic atmosphere generated by AnaeroGen gas pack system (Oxoid Unipath, Basingstoke, United Kingdom).
Table 2.
Antimicrobial Susceptibility Phenotypes of Test Isolates Used in This Study
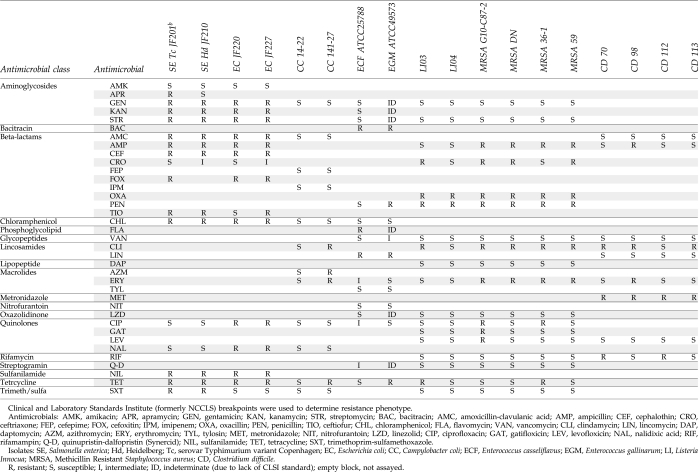
Susceptibility testing for Salmonella, E. coli, Enterococcus spp., L. innocua, and MRSA were performed using custom-made broth microdilution plates for the Sensititer™ System (TREK Diagnostic Systems, Westlake, OH). Clinical and Laboratory Standards Institute (CLSI, formerly National Committee for Clinical Laboratory Standards) guidelines for interpretation and recommended quality control organisms were used when available.32,33 C. coli isolates were tested following CLSI guidelines using the E-test (AB-Biodisk, Piscataway, NJ) or Sensititer.32,33 Susceptibility of C. difficile was performed by E-test (AB-Biodisk) following CLSI guidelines.32,33
DNA extraction and labeling
Genomic DNA from Salmonella, E. coli, Enterococcus spp., L. innocua, and MRSA isolates was extracted from 5 ml of overnight cultures grown in LB (Gram-negative bacteria) or brain heart infusion (Gram-positive bacteria) broth using the GenElute Bacterial Genomic DNA kit (Sigma, St. Louis, MO) as described previously.13 C. coli genomic DNA was isolated from colonies collected from Campy-cefex plates using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN) according to manufacturer's directions. C. difficile DNA was extracted from colonies collected from BAP using the UltraClean Microbial DNA Isolation Kit (MoBio Lab, Carlsbad, CA), following manufacturer's instructions for Gram-positive DNA isolation. DNA (1.5 μg) was labeled with either Cy-5 dye– or Cy-3 dye–labeled dCTP (Amersham, Piscataway, NJ) via random priming and extension with Klenow fragment (New England Biolabs, Beverly, MA), followed by purification with a Qiagen PCR cleanup kit (Qiagen, Valencia, CA) as previously described.39
Hybridization and scanning
Dye-labeled DNA (1.5 μg) was dried and resuspended in 80 μl of hybridization buffer (25% formamide, 5 × sodium chloride sodium citrate [SSC], 0.1% sodium dodecyl sulfate [SDS], and 1% bovine serum albumin), boiled 5 minutes, and applied to the microarray under a LifterSlip (Erie Scientific, Portsmouth, NH). Hybridizations were performed overnight in a hybridization chamber (Corning) submerged in a 42°C water bath in the dark. Protocols suggested by the manufacturer for hybridizations in formamide buffer were used for prehybridization, hybridization, and posthybridization wash processing (step 1: 2 × SSC, 0.1% SDS, 5 minutes at 42°C; step 2: 0.1 × SSC, 0.1% SDS, 10 minutes at room temperature; step 3: 4 × 0.1 × SSC, 1 minute at room temperature). Microarrays were scanned with a ScanArray Lite Laser scanner (PerkinElmer Life and Analytical Sciences, Waltham, MA) using ScanArray Express 3.0 software or with a GenePix 4100A (Molecular Devices, Sunnyvale, CA) and GenePix Pro software.
Data analysis
Images were analyzed and quantified using ScanArray Express 3.0 software (PerkinElmer). Hybridization signal intensities were measured in arbitrary intensity units (IU) by adaptive quantification mode followed by local background subtraction, and the median of the triplicate gene probes was recorded. The control strain hybridizations were used to evaluate methods for interpreting the quantitative data. Optimum calls were achieved by setting a cutoff at two times the median of intensities for all probes for each hybridization. Since the great majority of probes should not hybridize to a sample, this was an accurate measure of background (no hybridization) or cross hybridization (hybridization to nonhomologous DNA sequences) by a sample lacking a particular gene for all probes on the array. Therefore, all genes with a median intensity of its triplicate probes greater than two times the median intensity of all probes on the chip were scored as present. Additionally, hybridizations with no positive controls detected (less than one out of three 16s rDNA gene probes), too many negative controls detected (greater than two out of twelve 50% DMSO spots), or with obvious hybridization anomalies (streaks, spots, blotches, etc.) were considered as failed and were repeated.
Results
Validation of the AR gene microarray construction
The new AR gene probes were arrayed along with the 94 probes from our previous study that demonstrated the detection of resistance genes with 70mer oligonucleotides.13 This approach allowed the 671 new probes to be tested and compared directly to the previously verified oligonucleotide probes. After printing and postprocessing, the arrays were evaluated by DNA staining.3 Scanning and image analysis demonstrated proper quantities of oligonucleotides in spots, good spot morphology, detection of positive controls and fluorescence dye controls, and negative controls below detection limits (data not shown).
Hybridization of control strains to the AR gene microarray
Initial test hybridizations were performed using two S. enterica strains with published complete genome sequences: serovar Typhimurium strain LT2 (antimicrobial sensitive) and serovar Typhi strain CT18 (harboring the pHCM1 MDR plasmid).28,36 Labeled LT2 DNA hybridized strongly to the three 16s rDNA positive control spots but not to any of the buffer-only or negative control spots on the microarray (background hybridization controls). Ten gene probes also had positive hybridizations (Supplemental Table S1 and summarized in Table 1), and all but one of these probes were found to be 100% (70/70 bp) identical to genes in the LT2 genome using a low stringency basic local alignment search tool (BLAST) of all probe sequences against the LT2 genome sequence (settings: discontinuous megablast, short queries, word size 11 bp, match/mismatch scores 2, gap costs −3, and template length 16 bp). Probe AR2-1-0032 also showed a positive hybridization, but a BLAST search failed to find any homologous sequence in LT2. This probe was designed to detect the marC gene (a homolog of the family of multiple antibiotic resistance proteins) from E. coli (gi|1170657:c1234-569). Conversely, nine other gene probes were found to have similarity to sequences within the LT2 complete genome, but did not hybridize above the cutoff. These probes had identity to LT2 sequences ranging from 73% to 91%. Alignments of the probe sequences to the LT2 genes in most cases revealed nucleotide mismatches along the length of the alignments that could lead to a lack of hybridization (data not shown). Five of these genes were also homologous to members of the marBARC multiple antibiotic resistance operon.20
Hybridizations of labeled S. Typhi CT18 DNA to the microarray resulted in positive hybridizations to all three 16s rDNA probes and to 95 gene probes; 92 of those were found to be homologous to genes in the CT18 genome (n = 14) or encoded by the pHCM1 MDR plasmid carried by CT18 (n = 78) (Table 1 and Supplemental Table S1). One hundred percent identity to CT18 or pHCM1 genes was found for 78 out of the 92, while 14 had identity ranging from 77% to 99%. Three gene probes with positive hybridizations were not found to be homologous to the CT18 or pHCM1 genome by BLAST, and five gene probes were found to have homologous sequences in CT18 by BLAST, but no hybridization was detected. Included in the positive hybridizations were the same 12 probes with positive hybridizations to CT18 from previous microarray experiments.13 These were probes for the genes aph6 (strB), dfrA1, aph3″ (strA)*, tnpA, blaTEM, intI1*, tnpM, aph3″ (strA)*, aadA1, sulII, intI1*, and cat4 (* indicates probes synthesized in duplicate). New microarray probes with positive hybridizations included tet(A) and homologs of dfr1B, tnpR, strA, strB, dfrV, intI1, sulII, catIII, blaTEM-1, acc(3), bla, blaPER3, blaTEM-13, tnpA, tet(B), cat, and several pHCM1 genes, including conjugation genes (n = 15), heavy metal resistance genes (n = 6), and plasmid replication genes (n = 3) (Supplemental Table S1 and summarized in Table 1). Overall, the new array detected all resistance genes in S. Typhi CT18 and also detected genes indicating the presence of an MDR plasmid (pHCM1).36
AR gene microarray analysis of test strains
To assess the ability of the microarray to detect AR genes in unsequenced bacteria, several of the test strains used in the previous work were re-evaluated with the new microarray as well as additional new isolates (Table 1). Hybridization results for all strains are summarized in Table 1, and complete hybridization results are available in Supplemental Table S1. Two Salmonella MDR strains (Salmonella Typhimurium JF201 and Salmonella Heidelberg JF210) were analyzed. In addition to strong hybridization signals to the 16s rDNA probes, many gene probes had positive hybridizations to these MDR strains with 150 IU above the cutoff for JF201 and 170 IU above the cutoff for JF210. Positive hybridizations for JF201 included 16 out of the 17 genes detected by the previous array; the aacC1 gene probe (AR1-0025) was not above the cutoff. An additional 137 probes had positive hybridizations. These included probes designed to detect resistance genes for aminoglycosides (n = 31), β-lactams (n = 12), chloramphenicol (n = 6), quaternary ammonium (n = 3), sulfanilamide (n = 5), tetracycline (n = 10), trimethoprim (n = 4), and heavy metals (n = 6). Twenty genes potentially involved in horizontal gene transfer such as integrons, transposons, and plasmid functions also had positive hybridization signals as well as 40 genes with no known function but associated with AR by their NCBI annotation. Hybridization of JF210 to the array yielded similar results. All but 1 probe of the 19 probes from the previous array (the vanR gene, AR1-0028) had positive hybridizations along with an additional 152 probes of the new array. Those include probes for genes encoding resistance to aminoglycosides (n = 38), β-lactams (n = 12), chloramphenicol (n = 8), efflux pumps (n = 11), quaternary ammonium (n = 5), sulfanilamide (n = 5), tetracycline (n = 11), trimethoprim (n = 4), and heavy metals (n = 14), as well as genes related to integrons (n = 4), transposons (n = 19), plasmid transfer (n = 3), and 35 other genes annotated as associated with AR.
E. coli isolates were analyzed with the array, and hybridizations were very strong to the 16s rDNA–positive control probes, indicating excellent labeling and hybridization efficiency of E. coli DNA samples. Isolate JF220 had positive hybridizations to genes for resistance to aminoglycosides (n = 28), β-lactams (n = 18), chloramphenicol (n = 15), efflux pumps (n = 15), quaternary ammonium (n = 3), sulfanilamide (n = 5), tetracycline (n = 8), trimethoprim (n = 2), and heavy metals (n = 13), and genes associated with integrons (n = 7), transposons (n = 15), as well as 45 other genes annotated as AR related. JF227 presented similar results with probes detecting resistance genes for aminoglycosides (n = 24), β-lactams (n = 16), chloramphenicol (n = 6), efflux pumps (n = 16), quaternary ammonium (n = 3), sulfanilamide (n = 5), tetracycline (n = 6), trimethoprim (n = 2) and heavy metals (n = 8), integrons (n = 8), transposons (n = 8), as well as 38 other genes annotated as associated with AR.
C. coli isolates assayed with the microarray hybridized above the cutoff to only two of the positive control 16s rDNA probes (16S-1333_1402 and 16S-507_576). BLAST analysis of the third 16s probe (16S-93_162) revealed only very short regions of identity to the published C. coli 16s rDNA gene, explaining hybridization below the cutoff (data not shown). Eighteen gene probes with hybridizations above the cutoff for C. coli isolate 14–22 included aminoglycoside resistance (aphA-3 and aad9), β-lactam resistance (cam1 and cifA), tetracycline resistance (tet0), efflux pumps (cmeB, cmeC, hdsR, and marR), and plasmid transfer (btgA and thrU). C. coli isolate 141–27 showed positive hybridizations for aphA-3, aph, cam1, blaL2 cmeB, cmeC, tetO, htdT (plasmid transfer), and hsdR. These positive hybridizations are also very similar to the results of C. jejuni hybridizations obtained with the previous AR microarray where aph, cam1, cmeB, cmeC, and tetO gene probes were positive.13
E. casseliflavus ATCC25788 and E. gallinarum ATCC49573 were analyzed. Both strains had hybridization signals well above the cutoffs for the positive control 16s rDNA probes. Both strains, used as positive controls for vancomycin resistance, hybridized to probes for the vanC gene. Each strain hybridized to several probes for transposons (trans and tnpA in ATCC25788; trans, tnpA, and tnpR in ATCC49753) and AR-associated gene sequences (hur and murT in ATCC25788; tetO, tetM, tetS, and blaL2 in ATCC49753).
L. innocua isolates were also tested. These strains hybridized to all three 16s rDNA gene probes as well as to several other probes. Strain LI03 and LI04 hybridized to tetracycline resistance probes tetO and tetM, efflux gene probes (marC and ybiT), several resistance transfer genes (IS1542, recP, p9123, and Tn5), six Listeria-specific genes, as well as several genes annotated as being resistance associated. LI03 also hybridized to a β-lactamase gene (blaTEM-1).
MRSA isolates were tested with the microarray. Only two 16s rDNA gene probes were detected with a very strong hybridization. All four MRSA isolates had hybridizations to as many as 51 Staphylococcal methicillin resistance chromosomal cassettes (mec) gene probes. MRSA isolate G10-C87-2 had 51 probes with positive hybridizations, 42 of which were mec gene probes. These included probes for genes involved in resistance to β-lactams (mecA, mecI, and mecR1) and erythromycin (ermA). MRSA isolate DN had 51 positive hybridizations, 42 of those were mec gene probes. These included β-lactam resistance (mecR1, mecA, spe, pre, and blaI), erythromycin resistance (ermA), and aminoglycoside resistance (aphA-3 and homologs). MRSA isolate 36-1 had 33 positive hybridizations, including 5 mecA β-lactamase genes as well as several MRSA-associated genes (mecR1, pre, ccrA, and ccrB), 7 aminoglycoside resistance genes related to ahpA-3, macrolide resistance gene ermC, 13 resistance-associated genes, and 3 transposase-associated genes. MRSA isolate 59 presented similar results with 30 positive hybridizations, 23 to mec genes including β-lactam resistance and aminoglycoside resistance; however, there were no erm genes detected in MRSA 59.
C. difficile isolates 70, 98, 112, and 113 were tested with the microarray. All three 16s rDNA probes were detected in each isolate; however, only two resistance genes were detected. In C. difficile isolate 98 orfE, a gene annotated as plasmid encoded and in isolate 112, blaL2 was detected, both of which showed homology to β-lactamase genes. Probes for several other genes annotated as related to resistance also had positive hybridizations for these isolates, including tet(C) in isolate 112.
Antimicrobial susceptibility of isolates tested
All of the test strains used to evaluate the microarray were analyzed for susceptibility to various antimicrobial compounds (Table 2). Although phenotypic testing is not necessarily expected to correlate well with gene detection, agreement between the two techniques was surprisingly high. Out of 266 phenotypes tested, 203 (76.3%) were in agreement with genes detected. This included 71 (26.7%) where resistant phenotypes agree with one or more gene probe hybridizations, and 132 (49.6%) where sensitive phenotypes had no genes detected. Nonconcordant results were found for 63 (23.7%) detected phenotypes as compared to the microarray data. These fell into two categories, with 26(9.8%) having positive hybridizations to a resistance gene but no corresponding phenotype was detected, and 37(13.9%) where an isolate was resistant but no genes explaining the phenotype were detected.
Discussion
In this study, standard techniques were used to build a glass slide microarray for the detection of a large representation of all sequenced AR genes. Previous work by our group and many others has established oligonucleotide microarrays as an easy approach for high-density screening for AR genes.2,13,24,26,30,37,42,44 Therefore, the primary challenge of the current study was to identify the resistance genes to be detected by this assay. This project used simple key word searches to compile a list of genes associated with AR based upon their NCBI annotations. This list was pared down by deletion of identical genes from different organisms, deletion of genes unlikely to be associated with resistance based upon their annotation, and deletion of probes for these genes with greater than 90% homology. This approach led us to the construction of a microarray with probes for the detection of 775 AR or resistance-associated genes.
Control and experimental hybridizations demonstrated that the array could detect a wide variety of resistance genes in diverse Gram-positive and Gram-negative bacteria. Phenotypic data correlated with the array data over 76% of the time, which was an interesting result considering the differences between the two assays, the diversity of the bacteria tested, and the variety of antimicrobial compounds tested. It is not surprising that these assays sometimes yield different results. Phenotypic testing does not include an induction step and could suffer from false-negatives when a bacterium's resistance genes are tightly regulated. The microarray assay developed in this study also lacks probes for some resistance genes and can also yield false-negative results. This occurred if a gene was absent from the NCBI database, if gene was incorrectly annotated, if the gene was erroneously deleted during review of the annotations, or if the resistance was due to a point mutation of an endogenous gene (e.g., most quinolone resistances). For example, we failed to identify resistance genes in several of the Gram-positive bacterial species (e.g., Listeria, Clostridium, and Enterococcus).8,37,41 This probably reflects that resistance mechanisms in some of these bacteria have not been well studied, so few resistance genes attributed to them have been added to the database or properly annotated. We have also discovered that our array design missed several resistance genes identified by other researchers for macrolide, lincosamide, and streptogramin resistance.8 A comparison of their list of genes and our list showed that our array lacked 14 out of the 23 genes identified in their study.8 By reviewing the files generated during our design process, we found that probes for those genes had been discarded during inspection of the annotations because the sequences were annotated as RNA-methylases and not as macrolide resistance RNA-methylases. These problems will be remedied by periodic searches of the NCBI database to identify re-annotated and newly discovered genes as they are submitted to NCBI. This is easily facilitated by setting up an automatic e-mail notification from NCBI. Probes for these and other omitted genes will be added to future versions of the microarray as well as probes published by other research groups.
This microarray is designed to detect as many AR-associated genes as possible. This is illustrated by some of the results of the test strains where multiple gene probes for a single class of resistances have positive hybridizations. For example, strain JF201 had positive hybridizations to 31 aminoglycoside resistance gene probes. However, these genes are fairly homologous, and it is expected that only one or a few of these genes are present in this strain.17 In cases where the research project must identify specific alleles, data from the microrarray can be used to determine a set of polymerase chain reaction primers necessary to identify an allele by amplification and sequencing.
The current array was not designed to detect single-nucleotide polymorphisms (SNPs) that are responsible for specific alleles or for specific phenotypic resistances. For example, resistances due to mutations of endogenous genes such as the quinolone resistance determining region of gyrA leading to nalidixic acid or fluoroquinolone resistance would require detection of SNPs.44,45 Similarly, SNP detection would be necessary to detect changes resulting in macrolide resistance in Campylobacter spp. due to mutations in ribosomal protein genes or 23S rDNA.6,21,27 Another example would be β-lactam resistance, which is complex because alleles differing by only one of a few SNPs can result in different phenotypes. Thus, a β-lactamase gene (e.g., blaTEM-1) encoding resistance to ampicillin and an extended spectrum β-lactamase gene encoding resistance to ampicillin and also to third- and fourth-generation cephalosporins (e.g., blaTEM-37) differ only by an SNP. Several groups have presented microarrays capable of better discriminating specific alleles and even SNPs.14,26,31,40 However, these arrays exhibit some limitations. Short oligonucleotide arrays printed on glass slides are more specific, but require the additional expense of modified oligonucleotides, and samples may require amplification with specific primers during labeling that would be problematic for an array designed to detect nearly 1,000 genes. Tiling arrays (e.g., Affymetrix, Inc., San Francisco, CA, and Roche Nimblegen, Inc., Madison, WI) with perfect match/mismatch probes have shown the most promise, but are cost prohibitive for most laboratories as design costs and specialized hybridization and scanning equipment are not as widely available as microscope slide arrays and scanners. The ArrayTube (Clondiag GmbH, Jena, Germany) format is also promising and could potentially be developed for diagnostic assays within the limitations of the utility of gene detection versus phenotypic testing data in the clinical setting.2,31 However, it also requires specialized equipment and exclusive contracts with the company, and is limited to 196 probes per assay. Our design and construction process uses the most freely available databases, bioinformatics, techniques, and equipment to produce an array with the widest application at the lowest possible cost to researchers.
High-throughput sequencing may soon offer an alternative to microarray techniques, but that technology will continue to be out of reach for most researchers in the near term. Currently, microarray detection of AR genes presents the most comprehensive tool for studying the genetic causes of resistance. Even with the limitations of allele discrimination and SNP detection, it is a very powerful tool and has begun to demonstrate its utility in a variety of research studies.2,5,7,9,10,13,24,29,37,46 In addition, the high density of data provided by microarrays also allows analysis of gene prevalence by new bioinformatic tools such as cluster analysis and linkage disequilibrium, which may be helpful in identifying factors leading to development and spread of AR.29,46 This type of data will be necessary in the development of possible strategies to prevent increasing AR and its spread to human infections. It is our hope that other researchers will find these methods useful in their studies, and that they will continue to improve upon the technique and expand the catalog of resistance genes detectable by microarray analysis.
Disclaimer Statement
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Supplementary Material
Acknowledgments
The authors thank Mike Asher, LaShanda Glenn, Russ Turpin, Jonathan Cudnik, Jovita Haro, Lari Hiott, and Takiyah Ball for technical assistance. M.M. was funded by NIH Grants R01AI34829 and R21AI057733 and by the generosity of Sidney Kimmel. The authors would also like to thank the NCBI helpdesk for assistance in searching the nonredundant sequence database.
Disclosure Statement
All authors state that no competing financial interests exist.
References
- 1.Alanis A.J. Resistance to antibiotics: are we in the post-antibiotic era? Arch. Med. Res. 2005;36:697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Batchelor M. Hopkins K.L. Liebana E. Slickers P. Ehricht R. Mafura M. Aarestrup F. Mevius D. Clifton-Hadley F.A. Woodward M.J. Davies R.H. Threlfall E.J. Anjum M.F. Development of a miniaturised microarray-based assay for the rapid identification of antimicrobial resistance genes in Gram-negative bacteria. Int. J. Antimicrob. Agents. 2008;31:440–451. doi: 10.1016/j.ijantimicag.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia C. Salani G. Consolandi C. Bernardi L.R. De Bellis G. Analysis of DNA microarrays by non-destructive fluorescent staining using SYBR green II. Biotechniques. 2000;29:78–81. doi: 10.2144/00291st01. [DOI] [PubMed] [Google Scholar]
- 4.Boissinot M. Bergeron M.G. Toward rapid real-time molecular diagnostic to guide smart use of antimicrobials. Curr. Opin. Microbiol. 2002;5:478–482. doi: 10.1016/s1369-5274(02)00362-4. [DOI] [PubMed] [Google Scholar]
- 5.Bruant G. Maynard C. Bekal S. Gaucher I. Masson L. Brousseau R. Harel J. Development and validation of an oligonucleotide microarray for detection of multiple virulence and antimicrobial resistance genes in Escherichia coli. Appl. Environ. Microbiol. 2006;72:3780–3784. doi: 10.1128/AEM.72.5.3780-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell D.B. Wang Y. Lin J. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 2008;52:3947–3954. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Call D.R. Bakko M.K. Krug M.J. Roberts M.C. Identifying antimicrobial resistance genes with DNA microarrays. Antimicrob. Agents Chemother. 2003;47:3290–3295. doi: 10.1128/AAC.47.10.3290-3295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone M. D'Andrea M.M. Iannelli F. Oggioni M.R. Rossolini G.M. Pozzi G. DNA microarray for detection of macrolide resistance genes. Antimicrob. Agents Chemother. 2006;50:2038–2041. doi: 10.1128/AAC.01574-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassone M. Giordano A. Pozzi G. Bacterial DNA microarrays for clinical microbiology: the early logarithmic phase. Front Biosci. 2007;12:2658–2669. doi: 10.2741/2262. [DOI] [PubMed] [Google Scholar]
- 10.Chen S. Zhao S. McDermott P.F. Schroeder C.M. White D.G. Meng J. A DNA microarray for identification of virulence and antimicrobial resistance genes in Salmonella serovars and Escherichia coli. Mol. Cell. Probes. 2005;19:195–201. doi: 10.1016/j.mcp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Flibotte S. Moerman D.G. Experimental analysis of oligonucleotide microarray design criteria to detect deletions by comparative genomic hybridization. BMC Genomics. 2008;9:497–508. doi: 10.1186/1471-2164-9-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fluit A.C. Visser M.R. Schmitz F.J. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 2001;14:836–871. doi: 10.1128/CMR.14.4.836-871.2001. , table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frye J.G. Jesse T. Long F. Rondeau G. Porwollik S. McClelland M. Jackson C.R. Englen M. Fedorka-Cray P.J. DNA microarray detection of antimicrobial resistance genes in diverse bacteria. Int. J. Antimicrob. Agents. 2006;27:138–151. doi: 10.1016/j.ijantimicag.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Harrington C.R. Lucchini S. Ridgway K.P. Wegmann U. Eaton T.J. Hinton J.C. Gasson M.J. Narbad A. A short-oligonucleotide microarray that allows improved detection of gastrointestinal tract microbial communities. BMC Microbiol. 2008;8:195–214. doi: 10.1186/1471-2180-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland C.A. Kiechle F.L. Point-of-care molecular diagnostic systems—past, present and future. Curr. Opin. Microbiol. 2005;8:504–509. doi: 10.1016/j.mib.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins K.L. Batchelor M.J. Anjum M. Davies R.H. Threlfall E.J. Comparison of antimicrobial resistance genes in nontyphoidal salmonellae of serotypes enteritidis, hadar, and virchow from humans and food-producing animals in England and Wales. Microb. Drug Resist. 2007;13:281–288. doi: 10.1089/mdr.2007.779. [DOI] [PubMed] [Google Scholar]
- 17.Jana S. Deb J.K. Molecular understanding of aminoglycoside action and resistance. Appl. Microbiol. Biotechnol. 2006;70:140–150. doi: 10.1007/s00253-005-0279-0. [DOI] [PubMed] [Google Scholar]
- 18.Kent W.J. BLAT—the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreil D.P. Russell R.R. Russell S. Microarray oligonucleotide probes. Methods Enzymol. 2006;410:73–98. doi: 10.1016/S0076-6879(06)10004-X. [DOI] [PubMed] [Google Scholar]
- 20.Kunonga N.I. Sobieski R.J. Crupper S.S. Prevalence of the multiple antibiotic resistance operon (marRAB) in the genus Salmonella. FEMS Microbiol. Lett. 2000;187:155–160. doi: 10.1111/j.1574-6968.2000.tb09153.x. [DOI] [PubMed] [Google Scholar]
- 21.Ladely S.R. Stock R.A. Klopfenstein T.J. Sindt M.H. High-lysine corn as a source of protein and energy for finishing calves. J. Anim. Sci. 1995;73:228–235. doi: 10.2527/1995.731228x. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y. Han J.X. Huang H.Y. Zhu B. Development and evaluation of 16S rDNA microarray for detecting bacterial pathogens in cerebrospinal fluid. Exp. Biol. Med.(Maywood.) 2005;230:587–591. doi: 10.1177/153537020523000810. [DOI] [PubMed] [Google Scholar]
- 23.Lyons P. Advances in spotted microarray resources for expression profiling. Brief. Funct. Genomic. Proteomic. 2003;2:21–30. doi: 10.1093/bfgp/2.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Ma M. Wang H. Yu Y. Zhang D. Liu S. Detection of antimicrobial resistance genes of pathogenic Salmonella from swine with DNA microarray. J. Vet. Diagn. Invest. 2007;19:161–167. doi: 10.1177/104063870701900204. [DOI] [PubMed] [Google Scholar]
- 25.Majtan T. Majtanova L. Timko J. Majtan V. Oligonucleotide microarray for molecular characterization and genotyping of Salmonella spp. strains. J. Antimicrob. Chemother. 2007;60:937–946. doi: 10.1093/jac/dkm326. [DOI] [PubMed] [Google Scholar]
- 26.Malorny B. Bunge C. Guerra B. Prietz S. Helmuth R. Molecular characterisation of Salmonella strains by an oligonucleotide multiprobe microarray. Mol. Cell. Probes. 2007;21:56–65. doi: 10.1016/j.mcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Mamelli L. Prouzet-Mauleon V. Pages J.M. Megraud F. Bolla J.M. Molecular basis of macrolide resistance in Campylobacter: role of efflux pumps and target mutations. J. Antimicrob. Chemother. 2005;56:491–497. doi: 10.1093/jac/dki253. [DOI] [PubMed] [Google Scholar]
- 28.McClelland M. Sanderson K.E. Spieth J. Clifton S.W. Latreille P. Courtney L. Porwollik S. Ali J. Dante M. Du F. Hou S. Layman D. Leonard S. Nguyen C. Scott K. Holmes A. Grewal N. Mulvaney E. Ryan E. Sun H. Florea L. Miller W. Stoneking T. Nhan M. Waterston R. Wilson R.K. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 29.McGowan-Spicer L.L. Fedorka-Cray P.J. Frye J.G. Meinersmann R.J. Barrett J.B. Jackson C.R. Antimicrobial resistance and virulence of Enterococcus faecalis isolated from retail food. J. Food Prot. 2008;71:760–769. doi: 10.4315/0362-028x-71.4.760. [DOI] [PubMed] [Google Scholar]
- 30.Mitterer G. Huber M. Leidinger E. Kirisits C. Lubitz W. Mueller M.W. Schmidt W.M. Microarray-based identification of bacteria in clinical samples by solid-phase PCR amplification of 23S ribosomal DNA sequences. J. Clin. Microbiol. 2004;42:1048–1057. doi: 10.1128/JCM.42.3.1048-1057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monecke S. Jatzwauk L. Weber S. Slickers P. Ehricht R. DNA microarray-based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin. Microbiol. Infect. 2008;14:534–545. doi: 10.1111/j.1469-0691.2008.01986.x. [DOI] [PubMed] [Google Scholar]
- 32.[NCCLS] National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Wayne, PA: 2002. [Google Scholar]
- 33.[NCCLS] National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne, PA: 2003. [Google Scholar]
- 34.Nickelsen P.A. Sparling P.F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen H.B. Wernersson R. Knudsen S. Design of oligonucleotides for microarrays and perspectives for design of multi-transcriptome arrays. Nucleic Acids Res. 2003;31:3491–3496. doi: 10.1093/nar/gkg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkhill J. Dougan G. James K.D. Thomson N.R. Pickard D. Wain J. Churcher C. Mungall K.L. Bentley S.D. Holden M.T. Sebaihia M. Baker S. Basham D. Brooks K. Chillingworth T. Connerton P. Cronin A. Davis P. Davies R.M. Dowd L. White N. Farrar J. Feltwell T. Hamlin N. Haque A. Hien T.T. Holroyd S. Jagels K. Krogh A. Larsen T.S. Leather S. Moule S. O'Gaora P. Parry C. Quail M. Rutherford K. Simmonds M. Skelton J. Stevens K. Whitehead S. Barrell B.G. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 37.Perreten V. Vorlet-Fawer L. Slickers P. Ehricht R. Kuhnert P. Frey J. Microarray-based detection of 90 antibiotic resistance genes of gram-positive bacteria. J. Clin. Microbiol. 2005;43:2291–2302. doi: 10.1128/JCM.43.5.2291-2302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porwollik S. Frye J. Florea L.D. Blackmer F. McClelland M. A non-redundant microarray of genes for two related bacteria. Nucleic Acids Res. 2003;31:1869–1876. doi: 10.1093/nar/gkg298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porwollik S. Wong R.M. Sims S.H. Schaaper R.M. DeMarini D.M. McClelland M. The DeltauvrB mutations in the Ames strains of Salmonella span 15 to 119 genes. Mutat. Res. 2001;483:1–11. doi: 10.1016/s0027-5107(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 40.Sachse K. Laroucau K. Hotzel H. Schubert E. Ehricht R. Slickers P. Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiol. 2008;8:63–74. doi: 10.1186/1471-2180-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volokhov D. Chizhikov V. Chumakov K. Rasooly A. Microarray analysis of erythromycin resistance determinants. J. Appl. Microbiol. 2003;95:787–798. doi: 10.1046/j.1365-2672.2003.02046.x. [DOI] [PubMed] [Google Scholar]
- 42.Vora G.J. Meador C.E. Bird M.M. Bopp C.A. Andreadis J.D. Stenger D.A. Microarray-based detection of genetic heterogeneity, antimicrobial resistance, and the viable but nonculturable state in human pathogenic Vibrio spp. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19109–19114. doi: 10.1073/pnas.0505033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wernersson R. Nielsen H.B. OligoWiz 2.0—integrating sequence feature annotation into the design of microarray probes. Nucleic Acids Res. 2005;33:W611–W615. doi: 10.1093/nar/gki399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X. Susa M. Knabbe C. Schmid R.D. Bachmann T.T. Development and validation of a diagnostic DNA microarray to detect quinolone-resistant Escherichia coli among clinical isolates. J. Clin. Microbiol. 2004;42:4083–4091. doi: 10.1128/JCM.42.9.4083-4091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X. Susa M. Weile J. Knabbe C. Schmid R.D. Bachmann T.T. Rapid and sensitive detection of fluoroquinolone-resistant Escherichia coli from urine samples using a genotyping DNA microarray. Int. J. Med. Microbiol. 2007;297:417–429. doi: 10.1016/j.ijmm.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Zou W. Frye J.G. Chang C.W. Liu J. Cerniglia C.E. Nayak R. Microarray analysis of antimicrobial resistance genes in Salmonella enterica from preharvest poultry environment. J. Appl. Microbiol. 2009;107:906–914. doi: 10.1111/j.1365-2672.2009.04270.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.