Abstract
Background
Many infectious diseases that cause significant morbidity and mortality, especially in the developing world, could be preventable through vaccination. The effort to produce safe, thermally stable, and needle-free mucosal vaccines has become increasingly important for global health considerations. We have previously demonstrated that a thermally stable nanoemulsion, a mucosal adjuvant for needle-free nasal immunization, is safe and induces protective immunity with a variety of antigens, including recombinant protein. The successful use of nanoemulsion-based vaccines, however, poses numerous challenges. Among the challenges is optimization of the formulation to maintain thermal stability and potency and another is accuracy and efficiency of dispensing the vaccines to the nasal mucosa in the anterior and turbinate region of the nasal cavity or potentially to the nasopharynx-associated lymphoid tissue.
Methods
We have examined the effects of different diluents [phosphate-buffered saline (PBS) and 0.9% NaCl] on the stability and potency of nanoemulsion-based vaccines. In addition, we have determined the efficiency of delivering them using commercially available nasal spray devices (Pfeiffer SAP-62602 multidose pump and the BD Hypak SCF 0.5 ml unit dose AccusprayTM).
Results
We report the stability and potency of PBS–diluted ovalbumin–nanomeulsion mixtures for up to 8 months and NaCl-diluted mixtures up to 6 months when stored at room temperature. Significant differences in spray characteristics including droplet size, spray angle, plume width, and ovality ratios were observed between the two pumps. Further, we have demonstrated that the nanoemulsion-based vaccines are not physically or chemically altered and retain potency following actuation with nasal spray devices. Using either device, the measured spray characteristics suggest deposition of nanoemulsion-based vaccines in inductive tissues located in the anterior region of the nasal cavity.
Conclusions
The results of this study suggest that nanoemulsion-based vaccines do not require specially engineered delivery devices and support their potential use as nasopharyngeal vaccine adjuvants.
Key words: nasal delivery of vaccines, nasal sprayer devices, mucosal adjuvant, aerosol transport and deposition, nanoemulsion, effects of actuation
Introduction
The development of heat-stable and needle-free vaccines is considered critical to some populations.(1,2) On a global scale, traditional vaccines have successfully reduced the burden of infectious diseases, including tetanus, diphtheria, pertussis, poliomyelitis, rubella embryopathy, and measles.(1,3) Despite these remarkable accomplishments, vaccine preventable diseases continue to cause significant morbidity and mortality, especially in populations of developing countries. Recently, immunization frequency in many developing countries has actually declined for adults and children.(4) The disparity of vaccine coverage in these areas results, in part, from the requirement for sterile needles and the cost and burden associated with noninterrupted cold chain handling. Adding to the problem, the unsafe use of needle injections have been linked to the transmission of life-threatening infections such as hepatitis B and C, HIV, Ebola, Lassa virus infections, and malaria.(5) However, until recently, there have been relatively low levels of interest in developing new generation vaccines designed to safely prevent the predominant diseases in the developing world.(2,6)
Fortunately, a number of noninvasive delivery routes, including nasal, ophthalmic, pulmonary, transdermal, buccal, rectal, and vaginal, are available for which vaccine technologies are under development.(6,7) Among these routes, nasal vaccine delivery seems most promising given the accessibility of the mucosal tissue, the interface of a range of systems, the relative lack of barriers such as the stratum corneum, the proven track record of therapeutic nasal drug delivery technologies, and improved patient compliance.(5) Despite the many attractive features of nasal vaccination,(8–13) only one intranasal vaccine (FluMist®, MedImmune) has been approved for human use.(13) Experimental vaccines, which consist of live attenuated virus reassortment strains, have the potential to provide long-lasting humoral and cell-mediated immunity, but they bear the potential risk of reversion to virulence and carry considerable logistical and bio-safety problems associated with storage.(8) Many experimental vaccines consisting of killed or purified antigens are typically poorly immunogenic and require inflammatory adjuvants.(8,14)
Nanoemulsion (NE) is a promising noninflammatory mucosal adjuvant for nasal immunization. We have previously demonstrated protective immunity in a variety of antigen systems including influenza virus, recombinant anthrax protective antigen, HIV gp120, vaccinia virus, and hepatitis B surface antigen (HBsAg) in animal models, suggesting a possible utility of NE-based vaccines.(15–19) The successful use of NE-based vaccines, however, poses challenges. One such challenge is optimization of the platform for thermal stability and potency. We previously reported that the potency of a prototype mucosal nanoemulsion-based HBsAg in phosphate-buffered saline (PBS) diluent remains effective for at least 6 weeks when stored at 40°C.(19) However, studying the effects of diluent on the stability of NE-based vaccines may be important because of the documented effects of diluent on the potency of alum-based vaccines.(20) Another challenge for successful mucosal vaccines is the ability to accurately and repeatedly dispense the NE-based preparations to the nasal mucosa, potentially targeting nasopharyngeal-associated lymphoid tissues. The delivery performance of any aerosolized droplets introduced via the nasal cavity depends on many factors, such as the design of the pump, the physical properties of the formulation, and the position of administration.(21–25) In this present work, we have examined the effects of PBS versus sodium chloride (NaCl) diluents on stability and potency of NE-based vaccines when stored at room temperature (accelerated conditions). We have also evaluated the ability to deliver NE-based vaccines using standard nasal spray devices.
Materials and Methods
Nanoemulsion, proteins, and general reagents
NE W805EC, provided by the NanoBio® Corporation (Ann Arbor, MI), was manufactured as previously described.(19) The NE is manufactured by homogenization of soybean oil (64%) in water, containing CPC, 1%, Tween 80 (5%), and ethanol (8%), using a high-speed emulsifier resulting in an average droplet size 200–600 nm. All of these components are included in the Untied States' Food and Drug Administration's generally recognized as safe (GRAS) list, and can be economically manufactured under Good Manufacturing Practices (GMP). Model antigens ovalbumin–Grade V (OVA) and porcine intestinal alkaline phosphatase (AlkP) were purchased from Sigma (St. Louis, MO) and dissolved in either sterile-filtered, PBS (Mediatech, Manassas, VA) or sterile-filtered saline (Hospira, Lake Forest, IL). Recombinant adw serotype HBsAg was supplied by Human Biologicals Institute (Indian Immunologics, Ltd., Hyderabad, India). Alkaline phosphatase (AP)-conjugated rabbit antimouse IgG (H&L) antibody was purchased from Rockland Immunochemicals, Inc. (Gilbertsville, PA).
Preparation of OVA–NE, HBsAg–NE, and AlkP–NE mixtures
OVA–NE, AlkP–NE, and HBsAg–NE formulations were prepared by vigorously mixing the protein solution with the concentrated NE. Neat stock of NE (100%) were diluted in water to a 2 × solution and added to an equal volume of protein. The salt concentrations were normalized to either 150 mM PBS or 0.9% saline (pH 7.03). For the physicochemical analysis and nasal spray characterization studies, the OVA–NE was formulated at 3.125 mg/mL OVA in a range of 0.28–40% NE (v/v). For intranasal immunizations, the OVA–NE dose was 3.125 μg/mL OVA in 20% NE. The AlkP–NE was prepared with 16.7 mg/mL AlkP in 20% NE. The HBsAg–NE doses ranged from 0.625 or 2.5 mg/mL HBsAg in 20% NE. For the rheological and spray pump characteristic studies, the HBsAg–NE was prepared with 0.04 mg/mL HBsAg in 20% NE.
Determination of the effects of formulation stability and potency of NE-based vaccines when stored at 25°C
OVA was chosen as a surrogate antigen because it is a well-defined and frequently utilized antigen for immunological and vaccine studies.(26) To determine the effects of the diluent on stability, two formulations were characterized; each consisting of either OVA–NE diluted with 0.9% NaCl or 150 mM PBS. Each preparation was evaluated for long-term (8–10 months) stability and immunogenicity. The OVA–NE mixtures were stored for a period up to 10 months in 2-mL glass vials with phenolic rubber-lined caps (Wheaton Science Products, Millville, NJ) at room temperature (∼25°C) and in standard lighting conditions. The vials were filled with minimal air contained above the OVA–NE mixture. The stability of the NE adjuvant was evaluated visually and by particle size characterization at the following time points: immediately following mixing, weeks 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36, and 40. Particle size was measured using an LS230 particle sizing instrument (Beckmann-Coulter, Fullerton, CA) fitted with a small-volume module. The procedure was conducted in accordance to manufacturer's directions. Particle size distributions were calculated using a Fraunhofer optical model and number weighted averaging over an average of three 60-sec measurement cycles. The data was analyzed using Beckman Coulter LS Particle Characterization Software (version 3.29). Protein stability was determined by SDS-PAGE, immunoblotting, and in vivo potency as described below. The NE mixture was subjectively considered stable if there was no visual evidence of creaming, settling, or phase separation. For visual analysis of stability, the closed vials were inspected against an incandescent backlight at regular intervals for evidence of creaming, settling, or phase separation. The visual appearance of the mixture was scored on a scale ranging from 0 to 6 according to the following criteria: 6 = normal (homogenous) visual appearance, 5 = flocculant gradient without distinct boundaries, 4 = clear distinct boundary redispersible with minimal mechanical disturbance (clear portion <25% total volume), 3 = same as 4 with the clear portion representing 25–50% of the total volume, 2 = same as 4 with the clear portion representing 51–75% of the total volume, 1 = clear distinct boundary redispersible with the clear portion >75% total volume, and 0 = irreversible phase separation. Care was taken not to disturb or shake the mixture during the visual inspection. NE stability was determined objectively if the lipid droplet size remained consistent with freshly mixed product. The stored samples were maintained until either NE instability was evident or degradation of the protein was detected.
Mice, immunization procedures, sample collection, and antibody measurement
Pathogen-free, outbred CD-1 mice (females 6–8 weeks old) were purchased from Charles River Laboratories and housed in SPF conditions with food and water available ad libitum in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care (AAALAC). All mouse procedures performed for this study were conducted with the approval of the University of Michigan University Committee on Use and Care of Animals (UCUCA).
The mice were immunized with either OVA–NE or HBsAg–NE (n = 5 per group) administered nasally once at the initiation of the experiment (prime immunization) and once 6 weeks later, for a total of two immunizations. All intranasal immunizations were conducted in mice anesthetized with isoflurane, using the IMPAC 6® anesthesia delivery system. For vaccination, anesthetized mice were held in a supine position and 8 μL (4 μL/nare) of vaccine solution was administered slowly to the nares using a micropipette tip.
Whole blood samples were obtained from the lateral saphenous vein every 14 days following prime immunization. The serum was separated by centrifugation at 3500 rpm for 15 min after allowing coagulation for 30–60 min at room temperature. The serum samples were stored at −20°C until analyzed.
Anti-OVA- or HBsAg-specific IgG antibodies were determined by ELISA. The ELISA was performed as previously described(15) with some coating modifications. Briefly, Nunc Microtiter Maxisorb™ plates (Roskilde, Denmark) were coated with 5 μg/mL (100 μL) of HBsAg or OVA in a coating buffer (50 mM sodium carbonate, 50 mM sodium bicarbonate, pH 9.6) and incubated overnight at 4°C. To obtain a 1:100 dilution, 2 μL of the serum was added to 398 μL of PBS containing 0.1% bovine serum albumin (BSA). This solution was then serially diluted by a factor of 10. The detection of antibodies was measured at dilutions ranging from 1:100 to 1:10,000,000. Serum antibody concentrations were defined as endpoint titers (the reciprocal of the highest serum dilution producing an OD above cutoff value). The cutoff value was determined as the OD of the corresponding dilution of control sera plus 2 standard deviations.(27,28)
Nasal spray pumps and preparation
The Pfeiffer SAP-62602 Multi-dose Pump (130 μL/actua-tion) (Pfeiffer, Princeton, NJ) and BD Hypak SCF 0.5 mL AccusprayTM (BD, San Jose, CA) systems were kindly provided by Pfeiffer of America and the Becton, Dickinson, and Company (Franklin Lakes, NJ). Prior to analysis, both devices were briefly soaked in 1% liquinox solution in order to remove any residue from manufacturing and thoroughly rinsed with sterile distilled H2O, and then dried at 37°C for 30 min. For sample dispensing, the Pfeiffer pump was secured tightly to the SGD vials containing NE mixtures 24 h prior to testing. Prior to testing, the pump was primed by manual actuation at least five times in agreement with manufacturer's directions. For the BD unit dose pump, a plunger rod and stopper were then placed into the syringe barrel and the chamber was then pressurized according to manufacturer recommendations using a BD Hypak NSCF hand stoppering tool 24 h preceding testing. Each device was oriented in a vertical position for dispensing.
Determination of the effects of pump actuation on protein–NE stability
The consistency of the emulsion oil-phase particle size was used to assess nanoemulsion stability. Dispensed NE (±OVA) was collected from either the Pfeiffer or BD systems using a sterile Fisher Scientific (Pittsburgh, PA) 15-mL polypropylene tube. An aliquot of the collected sample was measured for particle size as described above.
The protein stability in the protein–NE mixtures was analyzed using PAGE and Western blotting techniques. Samples were reduced using DTT and electrophoresed by SDS-PAGE. Protein electrophoresis was conducted using 4–12% Bis-Tris gels and MES running buffer (Invitrogen, Carlsbad, CA). Gels were either stained using an Invitogen SilverQuestTM silver staining kit or transferred to 0.45 micron-sized PVDF Immobilon-P membranes (Millipore, Bedford, MA). Established immunoblotting procedures were followed.(19) Ovalbumin-specific bands were detected using rabbit anti-ovalbumin polyclonal antibody (RDI, Mount Kisco, NY) and mouse antirabbit IgG-alkaline phosphatase conjugated antibody with the chromogenic substrate NBT/BCIP (Pierce, Rockford, IL).
Measurement of alkaline phosphatase enzymatic activity was used as a more sensitive method to assess the effects of pump actuation on protein stability. Pre-dispensed samples were collected and compared to mixtures dispensed through either the Pfeiffer or BD spray pumps. The samples were diluted 1:1000 in distilled H2O to avoid NE turbidity effect on the optical density measurements. Diluted samples were then aliquotted into wells on a 96-well flat-bottom plate to provide a final concentration of 334 ng of AlkP/100 μL. SIGMA FASTTM p-Nitrophenol Phosphate (pNPP) was used as a chromogenic substrate for AlkP. Optical density measurements were done a SpectraMax 340 spectrophotometer at 405 nm. The data was analyzed using SOFTmax Pro© ver. 2.2.1 software.
Determination of the effects of actuation on the potency of NE-based vaccines
To evaluate the effects of dispensing NE-based vaccines through the nasal spray systems, in vivo immunogenicity studies were conducted in mice. HBsAg–NE was dispensed through either the BD or Pfeiffer system, and the dispensed mixture was collected in 15-mL polystyrene vials. Either preactuated or postactuated HBsAg–NE samples were used to vaccinate the mice intranasally, as described above.
Determination of the consistency of dispensing NE using nasal sprays
The consistency of the NE spray volume was evaluated by two methods. First, the intrinsic turbidity of the NE was measured to quantify the spray pump output. To determine an NE dilution concentration that falls within a measurable linear range, a standard NE turbidity curve was generated by 1:10 to 1:1000 dilutions of NE stock. The optical density was measured using a Milton Roy 1001 spectrophotometer at 405 nm (Ivyland, PA). Based on the standard curves, the optimal dilutions for measurement were identified to be >1:100 of the 100% NE stock. For volume comparison studies, both the Pfeiffer and BD (n = 3 devices/manufacturer and six sprays/device) spray pumps were manually actuated (∼130 μL each actuation) such that the entirety of the emitted NE was dispersed into a 15-mL polypropylene vial. Thirteen milliliters of distilled H2O was then added to the vial, and the vial was centrifuged at 1000 r.p.m. for 60 sec to assure that all of the dispensed NE was combined in the solution.
Spray weights were also used to confirm dose consistency. The nasal spray pumps were weighed before and after actuation using a calibrated AB204 Mettler Toledo (Columbus, OH) enclosed gram scale and using three Pfeiffer and three BD spray pumps. Spray weights were averaged based on six actuations per pump.
Determination of physical properties of NE potentially influencing nasal deposition
Viscosity
The rheology of the NE or the HBsAg–NE was measured using a AR-G2 (TA Instruments, New Castle, DE) control stress rheometer. The measurements were performed at room temperature (RT) with a 2°, 6-cm steel cone and plate geometry to simulate shear forces during actuation. Using a pipette, samples of 5, 10, 20, and 40% NE or 0.04 mg/mL HBsAg–20% NE were loaded into the rheometer, and the mixture was allowed to equilibrate for at least 5 min prior to analysis. A solvent trap was used to avoid evaporation. The rheology flow curves were produced using the steady state flow test, whereby the shear rate (γ), was increased gradually from 10−1 (sec−1) up to 103 (sec−1). Hysteresis curves were generated by gradually increasing the shear rate from 10−1 (sec−1) to 102 (sec−1) where shear rate remained constant at 102 (sec−1) for 30 min. The shear rate was then gradually returned to the starting point under the same conditions.
Surface tension
NE–air surface tension was calculated using a surface capillary rise tensiometer method.(29) Microcaps® precision 20-μL capillary tubes (Drummond Scientific Company, Broomail, PA) were carefully inserted into 5, 10, 20, or 40% NE, 0.04 mg/mL HBsAg–20% mixtures just to the point of contact. The mixture was allowed to equilibrate in the capillary tube. Using a precision caliper Model 14-468-17 (Fisher Scientific, Fairlawn, PA), the height of the column in the capillary tube was measured. The contact angle θ (the angle tangent to the surface of the NE makes with the side of the capillary tube) was measured after scanning the tube with a Hewlett Packard 5300c scanner. In all solutions containing NE, θ = 90°. The condition of the equilibrium of a column of height h is approximated by:
 |
where γ1a is the liquid–air surface tension, ρ is the density of the liquid, r is the radius of the capillary tube (0.0223 inches), and g is the gravity acceleration constant.(30) The validity of the experimental design was confirmed by comparing it to the established values for water, glycerol, and ethanol.
Determination of nasal spray characteristics
Droplet size distribution (DSD)
Droplet-size analysis of the HBsAg–NE was conducted by laser diffraction using a Malvern Spraytec with RT Sizer software. An automated actuation platform (NSx, Proveris Scientific, Marlborough, MA) was used to actuate the BD and Pfeiffer pumps, respectively. Actuation parameters specific to each type of pump were used for the spray characterization studies. The formulations were allowed to warm to room temperature prior to analysis. DSD measurements were performed with a 300-msec test duration and the data acquisition rate was set at 1000 Hz by the Malvern Spraytec Software (Worcestershire, UK). The units were automatically actuated in a vertical position using a SprayVIEW NSx-MS (Proveris Scientific). Droplet size was measured at 3 cm from the spray tip to the laser beam. Droplet size measurements were derived from the stable phase of the spray. The droplet size distribution was characterized by the following metrics: volume distribution (Dv10, Dv50, Dv90) and Span and percentage (%) less than 9 μm per the FDA CMC guidance on Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing and Controls Documentation, July 2002.(31) Neither the BD nor Pfeiffer systems required shaking. The Pfeiffer pump was manually primed five times just prior to sample analysis, whereas the BD spray pump did not require priming. Five units each of BD and Pfeiffer pumps were tested.
Spray pattern
Spray pattern tests were performed from the analysis of a two-dimensional image of the emitted plume. Spray-pattern studies of HBsAg–NE formulations were conducted using SprayVIEW NSP (Proveris Scientific), which is a nonimpaction laser sheet-based instrument. As in DSD studies, an automated NSx Actuation Station was used to actuate the BD and Pfeiffer pumps oriented in a vertical position with the same actuation parameters described above. Spray pattern was measured at 3 cm from the spray tip to the laser sheet. Spray pattern was characterized by the following metrics: Dmax, Dmin, ovality ratio, and % area per the FDA CMC guidance(31) and the FDA draft guidance for industry: Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action.(32) Dmax is defined as the longest diameter measured on the resulting spray pattern image. Dmin is the shortest diameter measured on the resulting spray pattern image. The ovality ratio is the ratio of Dmax to Dmin. This ratio provides a quantitative value for the overall shape of the spray. Percent area indicates the percent of the area that the emitted plume filled the established screening area. Five units each of BD and Pfeiffer pumps were tested.
Plume geometry
SprayVIEW NSP was also used to perform plume geometry studies on the HBsAg–NE formulations. All units were actuated with an automated NSx Actuation Station. The same actuation parameters were used for DSD and spray pattern analysis. The plume geometry was characterized by the following metrics: spray angle (the angle of the emitted plume measured from the vertex of the spray cone and spray nozzle) and plume width (the width of the plume at a given distance from the spray nozzle) per FDA Guidance for Industry.(31,32) Spray angle and plume width were measured from an image representing the stable phase of the emitted spray. Five units each of the BD and Pfeiffer pumps were tested.
Statistics
Data were expressed as mean ± standard deviation (SD) and were subjected to statistical analyses of variance (ANOVA) using the Student t and Fisher exact tests. The analyses were done with 95% confidence limits and two-tailed tests. Probability values of <0.05 were considered statistically significant.
Results
Effect of formulation on stability of NE-based vaccines
Regardless of the diluent, mixtures of OVA–NE remained visually homogenous (score 6) at all time points in this study up to 10 months. Lipid particle size did not significantly change (p > 0.05) in all cases throughout the 10-month duration of the study, confirming the visual inspection results (Fig. 1).
FIG. 1.
Effect of diluent on particle size of OVA–NE. Particle size was not significantly different (p > 0.05) for either OVA–NE diluted in PBS or saline in the course of the study.
SDS-PAGE and immunoblotting studies demonstrated absence of OVA degradation. The antigenic epitopes remain intact in the presence of NE for up to 8 months in PBS-diluted samples and 6 months for NaCl-diluted samples (Fig. 2A and B). OVA-specific, higher molecular-weight bands were detected by immunoblotting at all time points. However, the absence of lower molecular-weight bands and the retained intensity of the major OVA band indicate a lack of protein degradation. Significant decreases in the intact OVA protein was observed by 10 months for the PBS-diluted samples and at 8 months for the NaCl-diluted samples (Fig. 2C). However, no lower molecular-weight OVA-specific products were detected in any of the tested samples.
FIG. 2.
The SDS-PAGE (left column) and Western immunoblotting (right column) of fresh and stored OVA–NE at RT. (A) stored for 6 weeks, (B) stored for 6 to 8 months, and (C) stored for 8 to 10 months. Lanes are labeled according to sample storage conditions as follows: lane 1: MW standard, lane 2: OVA standard, lanes 3–4: freshly prepared (Fr) versus stored OVA–NE at saline, lanes 5–6: freshly prepared versus stored OVA–NE at PBS. Each lane contains 0.5 μg of antigen.
In light of these findings in vivo potency was tested in CD-1 mice (n = 5/treatment) using OVA–NE stored for 6 months for NaCl diluent and 8 months for PBS. Immunogenicity of stored vaccines was measured as serum anti-OVA IgG titers and compared to freshly prepared OVA–NE mixtures. Anti-OVA serum IgG responses were determined at 12 weeks after primary vaccination. No significant differences in potency between the freshly prepared versus the stored samples were observed. However, stored OVA–NE with 0.9% NaCl trended toward reduced potency at 6 months (Fig. 3).
FIG. 3.
OVA specific antibody responses to freshly prepared OVA–NE or OVA–NE stored at RT. CD-1 mice were vaccinated (n = 5/treatment) and boosted at 6 weeks with either freshly prepared or stored OVA–NE. Control mice (n = 5) were not vaccinated. Serum anti-OVA IgG antibody concentrations measured at 6 weeks following boost vaccination are presented as a mean of endpoint titers in individual sera ± SD. OVA–NE diluted in PBS and OVA–NE diluted in saline were stored for 8 and 10 months, respectively. No statistical differences (p > 0.05) between freshly mixed and stored formulation IgG titers were observed.
Testing the effect of nasal spray pumps on stability of NE-based vaccines
To ascertain the effect of dispensing NE-based vaccines using nasal spray pumps, the physicochemical stability characteristics of the inoculums were evaluated. Dispensing with either the Pfeiffer of the BD spray pumps did not significantly change the primary particle size (p > 0.05) of NE alone or the OVA–NE (Fig. 4A and B). The integrity of the OVA was not affected by actuation through either system. Silver-stained SDS-PAGE gels containing samples collected from the pre-spray and post-spray samples (Fig. 5A) showed no evidence of protein degradation. We compared the activity of an enzyme (AlkP) in pre- and postspray samples as a slightly more sensitive way of determining the possibility of physical changes in protein integrity associated with spray pump actuation. The AlkP–NE was collected and enzyme activity was measured prior and post-dispensing through either the Pfeiffer or the BD spray pump systems. AlkPhos activity was not affected after dispensing AlkP–NE through either spray pump device (Fig. 5B).
FIG. 4.
Effect of dispensing OVA–NE through commercially available nasal spray pumps on particle size of NE. (A) Particle size characterization of NE and (B) OVA–NE. Note that nasal spray pumps used did not change particle size of NE or OVA–NE (p > 0.05). Pre indicates samples that were measured prior to device actuation.
FIG. 5.
The spraying effect on integrity of proteins used in vaccine preparations. (A) The comparison of OVA–NE mixtures dispensed through either the BD Accuspray (left panel) or Pfeiffer spray pumps (right panel) to freshly prepared (nondispensed) formulations using SDS-PAGE electrophoresis. Lane 1: MW standard; lanes 2–5: pre-dispensed OVA–NE (NE concentrations are listed above the respective lanes); lanes 6–9: post-dispensed OVA–NE; lane 10: OVA (non-dispensed). Each lane contains 0.5 μg of antigen. (B) AlkP enzymatic activity of AlkP–OVA in pre- and post-dispensed mixtures using the BD Accuspray and Pfeiffer spray pumps. Activity in post-dispensed samples was not significantly different (p > 0.05) in comparison to pre-dispensed mixtures for either spray pump system.
To examine whether nasal spray pumps effect immunogenicity of the vaccine, the following study was performed using HBsAg. Mice were vaccinated (n = 5/treatment) with either 20 μg or 5 μg HBsAg + NE to accentuate potential differences at lower concentrations. Intranasal vaccination with either pre- or post-sprayed samples resulted in comparable high levels of anti-HBsAg serum IgG antibodies. Endpoint titers were 10−3 to 10−4 prior to the boost and 10−4 to 10−5 after the boost (Fig. 6A and B). No significant differences in antibody response at any time point in the study between the pre- and post-sprayed samples were observed for either spray pump system (p > 0.05).
FIG. 6.
Humoral response to nonsprayed and sprayed mixtures of OVA–NE. Mice were vaccinated (n = 5/treatment) with 20% NE + 20 μg HBsAg (A) or 20% NE + 5 μg HBsAg (B) and boosted at 6 weeks. Serum anti-OVA IgG antibody concentrations measured at 0, 4, and 8 weeks following prime vaccination are presented as a mean of endpoint titers in individual sera ± SD. No statistical difference (p > 0.05) between sprayed and non-sprayed IgG titers was observed for either spray pump system.
Characterization of nasal spray pumps actuation of NE-based vaccines
The volume consistency, viscosity, surface tension, droplet size, and spray pattern and plume geometry were determined. Spectrophotometric and spray weight studies were used to quantitatively evaluate the consistency of delivery using both the Pfeiffer and BD systems. Using the inherent turbidity of NE, pre- and post-dispensed samples (consisting of a broad range of NE concentrations) were measured for optical density. As shown in Figure 7A, NE is emitted consistently (minimal standard error) with either the BD or Pfeiffer systems. Also, spray weight measurements demonstrated volume consistency with either spray pump system (Fig. 7B).
FIG. 7.
Evaluation of consistency in dispensed NE volume using commercially available nasal spray pump systems. (A) Turbidity at 405 nm and (B) variation from the target spray weight of 0.13 μg as a measure of the volume of NE dispensed in a single spray using either the BD Accuspray or Pfeiffer spray pump systems. Variation in spray volume is presented as a mean of optical density or spray weight ± SD. The difference in measured optical density and spray weight between the Pfeiffer and BD spray pumps is explained by the inherent dead space within the BD spray nozzle and does not reflect a difference in the ability to deliver NE.
To gain an understanding and to explore the relationship between the rheological properties of NEs and their spray characteristics, viscosity experiments were conducted. The shear-rate dependent viscosity is reported in Figure 8A; a NE concentration-dependent increase in viscosity was observed [η = 0.0011 Pa-s (10% NE), = 0.0015 Pa-s (20% NE and 0.04 mg/mL HBsAg + 20% NE), = 0.0037 Pa-s (40% NE)]. There was no change in the viscosity with the addition of protein (p > 0.05). To assess shear-induced degradation, a hysteresis of the rheology curve was investigated wherein the shear rate was slowly ramped up and maintained at a relatively high rate for 30 min prior to ramp down. The process appeared to be reversible with no prominent hysteresis in the viscosity profile, and there was therefore no evidence of shear-induced degradation (Fig. 8B).
FIG. 8.
Rheological evaluation and surface tension characterization of NE and HBsAg–NE. (A) Shear viscosity of solutions of NE at various concentrations and HBsAg–NE. Rheological measurements were performed using an AR-G2 (TA Instruments, Newcastle, DE) control stress rheometer by linearly increasing the shear stress from 10−1 (sec−1) to 103 (sec−1). (B) A hysteresis curve was generated by gradually increasing the shear rate from 10−1 (sec−1) to 102 (sec−1) where shear rate remained constant at 102 (sec−1) for 30 min. The shear rate was then gradually returned to the starting point under the same conditions. Viscosity (η) is reported in Pa-s units. (C) Changes in surface tension at varying NE concentrations calculated using capillary rise tensiometry. Note that as the concentration of NE from 2.5 to 20% (and therefore surfactant concentration) increases, a statistically significant (p < 0.05) decrease in surface tension was observed. However, no significant change (p > 0.05) in surface tension was observed in the HBsAg–NE and 40% NE mixtures compared to the 2.5% NE.
The surface tension of the NE–air interface was experimentally determined using the capillary rise tensiometer method. As expected, a surfactant concentration-dependent decrease in surface tension was observed for NE concentrations ranging from 2.5 to 20% (Fig. 8C). Surface tension significantly increased between 20% NE and 40% NE by 1.7 dynes (p = 6.0 × 10−3). There was not a significantly different (p > 0.05) surface tension between 2.5% NE and 40% NE groups, however. The addition of HBsAg increased the surface tension significantly by 1.3 dynes (p = 1.3 × 10−3).
Differences in HBsAg–NE droplet size profiles were observed between the BD and Pfeiffer units (Table 1). For example, the droplet size distribution of the BD emitted spray ranged in size from 128 and 406.66 μm (Dv10–Dv90) with a Dv50 value of 251.50 μm. The Dv10–Dv90 for the Pfeiffer emission ranged from 14.44 μm to 59.33 μm, with a Dv50 value of 29.43 μm. In addition, 3.11% of the Pfeiffer emission measured less than 9 μm in size, whereas there were no detectable droplets smaller than 9 μm for BD units.
Table 1.
Droplet Size Analysis of Dispensing HBsAg–NE Through BD Accuspray or Pfeiffer spray Pump Systems
|
BD Accuspray | |||||
|---|---|---|---|---|---|
| |
Dv10 |
Dv50 |
Dv90 |
Span |
<9 μm |
| Droplet size (μm) | |||||
| Mean | 128.0 | 251.5 | 404.7 | 1.1 | 0 |
| STD | 21.8 | 31.9 | 21.0 | 0.2 | 0 |
| %CV | 17.0 | 12.7 | 5.2 | 13.3 | 0 |
|
Pfeiffer | |||||
|---|---|---|---|---|---|
| |
Dv10 |
Dv50 |
Dv90 |
Span |
<9 μm |
| Droplet size (μm) | |||||
| Mean | 14.4 | 29.4 | 59.4 | 1.5 | 3.1 |
| STD | 0.2 | 1.2 | 3.9 | 0.1 | 0.4 |
| %CV | 1.5 | 4.2 | 6.6 | 4.2 | 12.5 |
Each data column represents the average ± SD of six actuations. %CV is the coefficient of variance. Five units each of BD and Pfeiffer pumps were tested.
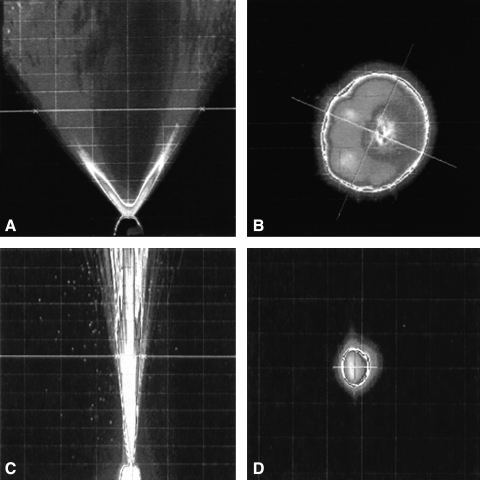
Considerable differences in the spray pattern and plume geometry were also observed between the BD and Pfeiffer units (Tables 2 and 3 and Fig. 9). The BD spray pumps produced a narrow plume that also resulted in a smaller spray pattern when compared to the Pfeiffer spray pump. The average spray angles were 9.8 and 74.8° for the BD and Pfeiffer systems, respectively.
Table 2.
Spray Pattern Characterization of Dispensing HBsAg–NE Through Commercial BD Accuspray or Pfeiffer Spray Pump Systems
| |
Dmin |
Dmax |
Ovality ratio |
% Area |
|---|---|---|---|---|
| Spray pattern | Size (mm) | |||
| BD Accuspray | ||||
| Mean | 1.8 | 4.6 | 3.6 | 0.6 |
| STD | 1.4 | 0.6 | 1.9 | 0.5 |
| %CV | 76.8 | 12.5 | 52 | 89.9 |
| Pfeiffer | ||||
| Mean | 39.5 | 45.4 | 1.2 | 16.2 |
| STD | 0.9 | 2.4 | 0.1 | 1 |
| %CV | 2.2 | 5.2 | 4.0 | 6.3 |
Each data column represents the average ± SD of six actuations. %CV is the coefficient of variance. Five units each of BD and Pfeiffer pumps were tested.
Table 3.
Plume Geometry Characterization of Dispensing HBsAg–NE Through Commercial BD Accuspray or Pfeiffer Spray Pump Systems
| Plume geometry | Spray angle (degrees) | Plume width (mm) |
|---|---|---|
| BD Accuspray | ||
| Mean | 9.8 | 5.1 |
| STD | 3.6 | 1.9 |
| %CV | 36.8 | 36.6 |
| Pfeiffer | ||
| Mean | 74.8 | 46.2 |
| STD | 1.9 | 1.4 |
| %CV | 2.5 | 3.0 |
Each data column represents the average ± SD of six actuations. %CV is the coefficient of variance. Five units each of BD and Pfeiffer pumps were tested.
FIG. 9.
Plume Geometry and Spray Pattern characteristics for nasal spray systems. Influence in pump design on the emission of HBsAg–NE. Plume geometry is represented for (A) Pfeiffer and (C) BD Accuspray devices. Spray pattern is visualized at a distance of 3 cm from the pump orifice for (B) Pfeiffer and (D) BD Accuspray units.
Discussion
NE-based vaccines offer a significant advantage over traditional vaccines, because of their potent adjuvant ability, long shelf life at nonrefrigerated temperatures, and needle-free delivery.(19) Inherent safety profiles associated with recombinant proteins that are often manufactured and diluted in either PBS buffer or nonbuffered NaCl solutions lend attractiveness to their use in NE-based mucosal vaccines.(16,17,19) One of the aims of this study was to characterize the effects of PBS versus nonbuffered saline diluents on the stability and immunogenicity of NE-based vaccines. Our results indicate that antigen integrity was unaltered in the nanoemulsion solution and that its in vivo potency remained intact for at least 8 months when stored at RT and diluted in PBS and at least 6 months when diluted in NaCl (Figs. 2 and 3). The nanoemulsion remained physically stable regardless of the antigen or dilution during all time points during the study. This indicates that aging of nanoemulsion did not affect the particle size regardless of the state of immunogenicity of the antigen. These findings have important implications for assessing the stability of NE-based vaccines, and suggest the need to evaluate the stability of the antigen and the overall immunogenicity of the mixture for potency estimation. The lack of progressive degradation was likely due to the protective effect of the emulsion, which shelters incorporated protein from oxidative processes. The microbiocidal characteristics of the nanoemulsion adjuvant may also prevent destruction of the protein secondary to contamination in accelerated (room temperature) storage conditions. These studies are consistent with previous studies showing HBsAg–NE retained full potency for at least 12 months at 4°C, 6 months at 25°C, and 6 weeks at 40°C. The major factors influencing the thermal stability of HBsAg–NE were entropically driven thermodynamic associations and electrostatic interactions of the HBsAg and the NE.(19) Based on the present results, we propose that the effects of the buffered versus the nonbuffered sodium diluents influence the electrostatic relationship between protein and the NE. The change in protein–NE interaction may result in the difference in thermal stability profiles. Studies designed to further evaluate the thermodynamic and electrostatic relationships of the antigen and the NE are currently underway in our laboratory.
For use in developing populations, NE-based vaccines should be manufactured at low cost and formulated for easy administration, perhaps by nonhighly trained medical personnel using commercially available nasal spray delivery devices. In these studies, we investigated the potential to accurately, efficiently, and reproducibly deliver NE-based vaccines using standard nasal spray devices. We found that either spray pump dispensed NE consistently ± 1% of the target spray weight (Fig. 7).
In light of previously reported findings demonstrating that the act of aerosolizing substances containing proteins alters their biological activity,(33) we undertook pre- and post-actuated in vitro stability and in vivo potency studies to evaluate the ability to nasally deliver NE-based vaccines using standard nasal spray devices. Specifically, we were interested in evaluating the possibility of shear induced protein disaggregation of particulate antigen, damage to eptitopes, or device retention of protein. Several mechanisms for functional changes in protein after aerosolization have been proposed including protein unfolding secondary to shear force,(34) surface denaturation at the hydrophobic air–water interface, or enhanced chemical reaction rates due to the huge increase in total surface area produced.(33,35) Fängmark and Carpin(36) demonstrated that urease is degraded by surface forces during passage through standard nebulizers and not by oxidation. To evaluate the potential for these changes in NE-based vaccines after spraying, we used a monomeric protein (OVA ), a particulate antigen (HBsAg), and a enzyme (AlkP) for evaluation of sensitive epitopes. Shear forces induced during the preparation of the nanoemulsion with the antigen are considered negligible because they are simply mixed together and do not require homogenation. However, the shear rate encountered during actuation through a sprayer device at actual spray conditions can be as high as 105–106 sec−1.(37,38) In our studies, mixing OVA, HBsAg, or AlkP with nanoemulsion and then spraying them through either the BD or the Pfeiffer units did not affect stability or activity. The lack of change in potency would also suggest that a significant portion of the emulsion–antigen complex is not retained in the devices.
The characteristics of nasal spray generation are shown to be dependent on a combination of actuation force, viscosity, rheological properties, surface tension, and pump design.(23,24) A concentration-dependent decrease in surface tension and a respective increase in droplet size are expected to increase with a higher total surfactant.(24) Our data verify this relationship in part in that dilute NEs (with lower concentrations of surfactants) exhibit a higher surface tension compared to less dilute concentrations of NEs (with higher surfactant concentrations) (Fig. 8). With the increased concentration of up to 20% NE we may have observed the predominant effects of the surfactant on the progressive reduction of surface tension. However, we note a small but statistically significant increase in surface tension between the 20 and 40% nanoemulsions. In this case, it is possible at 40% that the effect of lipid content predominates resulting in increased surface tension. Nonetheless, the physical relevance of this finding is doubtful. Further studies evaluating this relationship may clarify our results.
In this study, we have reported that NE behaves as a Newtonian fluid, as evidenced by the constant viscosity at a given temperature regardless of the rate of shear (Fig. 8). Further, as the shear rate was increased, there was no evidence of shear thinning or physical changes in the material over forces well exceeding those encountered during actuation.
Droplets generated by nasal sprays are dynamic and therefore droplet size and spray pattern studies are thought to be important characterization techniques in evaluating product performance. Droplet size may also be useful in predicting nasal deposition.(23,24) With this in mind, we evaluated these characteristics for NE-based vaccines in two commercially available nasal spray devices. Based on the large droplet size of NE-based vaccine produced by either device (128.47–404.66 μm for BD and 14.44–59.33 μm for Pfeiffer), the NE-based vaccines are expected to be deposited mainly in the inductive tissues of the anterior region of the nose with both spray units Tables 1 and 2.(22,39) True nasal deposition patterns of aerosolized substances, however, are more difficult to predict with in vitro modeling and are influenced by a number of pump-related factors, including droplet size, viscosity, plume angle, administration angle, and impaction characteristics.(22,39,40) Unfortunately, there is no convincing evidence that in vitro spray characterization such as spray pattern is predictive for true nasal deposition. This is due in part to the idea that emitted plumes will never have the opportunity to develop in the nasal cavity in the same way as they would by the in vitro shape tests.(41) Because of this, we cannot conclude that the two spray units are inequivalent in the ability to dispense NE-based vaccines. Further experiments are warranted to determine the actual deposition patterns in the human nasal cavity and if those deposition patterns affect clinical outcomes.
In summary, the results of this study support the use of NE as a nasopharyngeal vaccine adjuvant. Our study furthers this goal by demonstrating improved stability and potency by using PBS as a diluent for NE-based vaccine formulations. This may be an antigen-dependent phenomenon, and further detailed investigations with other antigens are needed. We have also demonstrated that NE-based vaccines do not require specially engineered delivery devices, which further promote cost-efficient mucosal vaccines delivery to developing populations. Future studies will assess the actual distribution of NE-based vaccines within the nasal cavity, following intranasal dispensing with spray pumps.
Acknowledgments
We gratefully acknowledge Dr. Joseph Bull, Dr. James Grotberg, Dr. Sherry Bian, and Dr. Mark Sullivan for their assistant with rheological analysis and access to equipment. We wish thank the Bectin, Dickinson, and Company and Pfeiffer of America for supplying the spray pumps. The authors would like to acknowledge the contribution of Cara Waroniki for technical assistance with characterization studies and Pat Gold for her help in editing this manuscript. This project was funded by Bill and Melinda Gates Foundation through Grand Challenges in Global Health Initiative 37868. P. Makidon was supported by T32 RR07008 from National Center Research Resources of NIH.
Author Disclosure Statement
Dr. James R. Baker, Jr. holds an ownership stake in NanoBio, Corp., and is the inventor of technologies that the University of Michigan has licensed to NanoBio Corp. Some of these technologies are involved in this research. NanoBio Corp. had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hauri AM. Armstrong GL. Hutin YJF. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 2.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 3.Heininger U. The success of immunization—shovelling its own grave? Vaccine. 2004;22:2071–2072. doi: 10.1016/j.vaccine.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Miller MA. Sentz JT. Disease, Mortality in Sub-Sahran Africa. 2nd. The World Bank; Washington, DC: 2006. [Google Scholar]
- 5.Simonsen L. Kane A. Llloyd J. Zaffraqn M. Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77:789–800. [PMC free article] [PubMed] [Google Scholar]
- 6.Illum L. Nasal drug delivery—possibilities, problems and solutions. J Controlled Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 7.Almeida AJ. Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Delivy Rev. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S. Mukkur TKS. Benson HAE. Chen Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J Pharmaceut Sci. 2009;98:812–843. doi: 10.1002/jps.21493. [DOI] [PubMed] [Google Scholar]
- 9.Onorato IM. Modlin JF. McBean AM. Thomsm ML. Losonsky GA. Bernier RH. Mucosal immunity induced by enhance-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163:1–6. doi: 10.1093/infdis/163.1.1. [DOI] [PubMed] [Google Scholar]
- 10.McGhee JR. Mestecky J. In defense of mucosal surfaces. Development of novel vaccines for IgA responses protective at the portals of entry of microbial pathogens. Infect Dis Clin North Am. 1990;4:315–341. [PubMed] [Google Scholar]
- 11.Mestecky J. McGhee JR. Prospects for human mucosal vaccines. Adv Exp Med Biol. 1992;327:13–23. doi: 10.1007/978-1-4615-3410-5_3. [DOI] [PubMed] [Google Scholar]
- 12.Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- 13.Slutter B. Hagenaars N. Jiskoot W. Rational design of nasal vaccines. J Drug Targeting. 2008;16:1–17. doi: 10.1080/10611860701637966. [DOI] [PubMed] [Google Scholar]
- 14.Moingeon P. Haensler J. Lindberg A. Towards the rational design of Th1 adjuvants. Vaccine. 2001;19:4363–4372. doi: 10.1016/s0264-410x(01)00193-1. [DOI] [PubMed] [Google Scholar]
- 15.Myc A. Kukowska-Latallo JF. Bielinska AU. Cao P. Myc PP. Janczak K. Sturm TR. Grabinski MS. Landers JJ. Young KS. Chang J. Hamouda T. Olszewski MA. Baker JR., Jr Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine. 2003;21:3801–3814. doi: 10.1016/s0264-410x(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 16.Bielinska AU. Janczak KW. Landers JJ. Makidon P. Sower LE. Peterson JW. Baker JR., Jr Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against bacillus anthracis spore challenge. Infect Immun. 2007;75:4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielinska AU. Janczak KW. Landers JJ. Markovitz DM. Montefiori DC. Baker JR. Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces Th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2008;24:271–281. doi: 10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 18.Bielinska AU. Chepurnov AA. Landers JJ. Janczak KW. Chepurnova TS. Luker GD. Baker JR., Jr A novel, killed-virus nasal vaccinia virus vaccine. Clin Vaccine Immunol. 2008;15:348–358. doi: 10.1128/CVI.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makidon PE. Bielinska AU. Nigavekar SS. Janczak KW. Knowlton J. Scott AJ. Mank N. Cao Z. Rathinavelu S. Beer MR. Wilkinson JE. Blanco LP. Landers JJ. Baker JR., Jr Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS ONE. 2008;3:e2954. doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawtell JA. Ream AJ. Observations on the effect of the diluent used for diluting challenge toxin in the Clostridium botulinum potency Test. Biologicals. 1995;23:249–251. doi: 10.1006/biol.1995.0041. [DOI] [PubMed] [Google Scholar]
- 21.Harris AS. Svensson E. Wagner ZG. Lethagen S. Nilsson IM. Effect of viscosity on particle size, deposition, and clearance of nasal delivery systems containing desmopressin. J Pharm Sci. 1988;77:405–408. doi: 10.1002/jps.2600770510. [DOI] [PubMed] [Google Scholar]
- 22.Cheng YS. Holmes TD. Gao J. Guilmette RA. Li S. Surakitbanharn Y. Rowlings C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J Aerosol Med. 2001;14:267–280. doi: 10.1089/08942680152484199. [DOI] [PubMed] [Google Scholar]
- 23.Suman JD. Laube BL. Dalby R. Validity of in vitro tests on aqueous spray pumps as surrogates for nasal deposition, absorption, and biologic response. J Aerosol Med. 2006;19:510. doi: 10.1089/jam.2006.19.510. –;521. [DOI] [PubMed] [Google Scholar]
- 24.Dayal P. Shaik MS. Singh M. Evaluation of different parameters that affect droplet-size distribution from nasal sprays using the Malvern Spraytec. J Pharmaceut Sci. 2004;93:1725–1742. doi: 10.1002/jps.20090. [DOI] [PubMed] [Google Scholar]
- 25.Guo C. Stine KJ. Kauffman JF. Doub WH. Assessment of the influence factors on in vitro testing of nasal sprays using Box-Behnken experimental design. Eur J Pharmaceut Sci. 2008;35:417–426. doi: 10.1016/j.ejps.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Lillard JW., Jr Boyaka PN. Hedrick JA. Zlotnik A. McGhee JR. Lymphotactin acts as an innate mucosal adjuvant. J Immunol. 1999;162:1959–1965. [PubMed] [Google Scholar]
- 27.Classen DC. Morningstar JM. Shanley JD. Detection of antibody to murine cytomegalovirus by enzyme-linked immunosorbent and indirect immunofluorescence assays. J Clin Microbiol. 1987;25:600–604. doi: 10.1128/jcm.25.4.600-604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey A. Di Canzio J. Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221:35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 29.Batchelor GK. An Introduction to Fluid Dynamics. The Press Syndicate of the University of Cambridge; Cambridge, UK: 2000. [Google Scholar]
- 30.Schramm LL. Green WHF. An absolute differential maximum bubble pressure surface tensiometer employing displaced capillaries. Colloid Polym Sci. 1992;270:694–706. [Google Scholar]
- 31.(CDER) CfDEaR. Guidance for Industry: Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products–Chemistry, Manufacturing, and Controls Documentation. Food and Drug Administration; Rockville, MD: 2002. p. 43. [Google Scholar]
- 32.(CDER) CfDEaR. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. Food and Drug Administration; Rockville, MD: 2003. [Google Scholar]
- 33.Fauml ngmark I. Carpin JC. Protein nebulization. J Aerosol Sci. 1996;27:231. [Google Scholar]
- 34.Tamber H. Johansen P. Merkle HP. Gander B. Formulation aspects of biodegradable polymeric microspheres for antigen delivery. Adv Drug Deliv Rev. 2005;57:357–376. doi: 10.1016/j.addr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Niven RW. Lott FD. Ip AY. Cribbs JM. Pulmonary delivery of powders and solutions containing recombinant human granulocyte colony-stimulating factor (rhG-CSF) to the rabbit. Pharmaceut Res. 1994;11:1101–1109. doi: 10.1023/a:1018924512928. [DOI] [PubMed] [Google Scholar]
- 36.Fängmark I. Carpin JC. Stability of urease during aerosolization: the effect of operating conditions. J Aerosol Sci. 1998;29:279–288. [Google Scholar]
- 37.Barnes HA. A Handbook of Elementary Rheology. The University of Wales Institute of Non-newtonian Fluid Mechanics; Dyfed, Wales: 2000. [Google Scholar]
- 38.Pennington J. Pandey P. Tat H. Willson J. Donovan B. Spray pattern and droplet size analyses for high-shear viscosity determination of aqueous suspension corticosteroid nasal sprays. Drug Dev Industrial Pharmacy. 2008;34:923–929. doi: 10.1080/03639040802149046. [DOI] [PubMed] [Google Scholar]
- 39.Foo MY. Cheng Y-S. Su W-C. Donovan MD. The influence of spray properties on intranasal deposition. J Aerosol Med. 2007;20:495–508. doi: 10.1089/jam.2007.0638. [DOI] [PubMed] [Google Scholar]
- 40.Kublik H. Vidgren MT. Nasal delivery systems and their effect on deposition and absorption. Adv Drug Deliv Rev. 1998;29:157–177. doi: 10.1016/s0169-409x(97)00067-7. [DOI] [PubMed] [Google Scholar]
- 41.Reddy ST. Rehor A. Schmoekel HG. Hubbell JA. Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Controlled Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]