Abstract
The nonreceptor tyrosine kinase Itk plays a key role in TCR-initiated signaling that directly and significantly affects the regulation of PLCγ1 and the consequent mobilization of Ca2+. Itk also participates in the regulation of cytoskeletal reorganization as well as cellular adhesion, which is necessary for a productive T cell response. The functional cellular outcome of these molecular regulations by Itk renders it an important mediator of T cell development and differentiation. This paper encompasses the structure of Itk, the signaling parameters leading to Itk activation, and Itk effects on molecular pathways resulting in functional cellular outcomes. The incorporation of these factors persuades one to believe that Itk serves as a modulator, or rheostat, critically fine-tuning the T cell response.
1. Introduction
Normal T lymphocyte activation occurs through its antigen receptor leading to various signaling cascades and ending in a certain function. These activating signaling cascades are exquisitely balanced, as different signals can lead to many different functional outcomes. Inappropriate signaling or skewed signals can cause many problems for the T cell and for a functional immune system. Aberrant T cell function can induce many devastating diseases such as leukemia and autoimmune disorders.
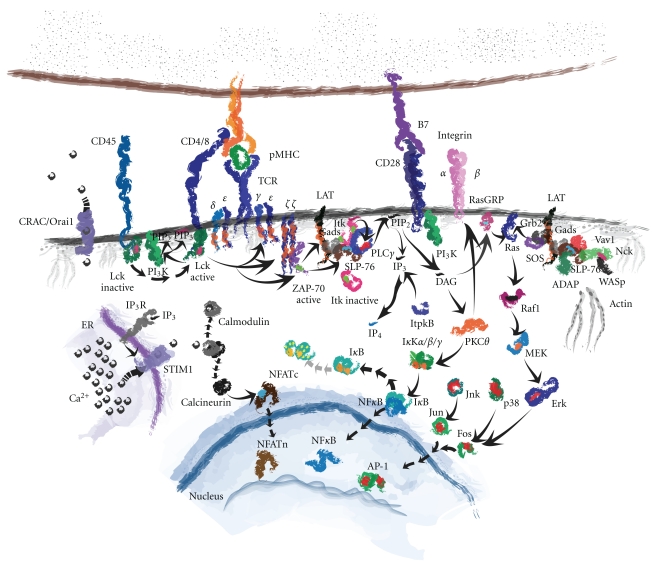
When a T cell encounters an antigen-presenting cell (APC), it must be able to discriminate whether or not the APC is functional. This occurs through physical interaction between surface molecules of the APC and T cell, translating that information into a response. This requires many coordinated molecular interactions from the cell surface, through the cytoplasm, on to the nucleus, and in some instances, back out through the cell surface again [1]. An illustration of this is shown in Figure 1. At the cell surface, interaction between the peptide-loaded major histocompatibility complex (pMHC) of class II or class I on the APC with the T cell receptor (TCR) on the T cell coordinates with coreceptor binding of CD4 on helper T cells or CD8 on cytotoxic T cells, respectively. This interaction causes the dissociation and activation of the tyrosine phosphatase CD45 from CD4/CD8, which dephosphorylates CD4/CD8 bound Src kinase Lck on its inhibitory tyrosine (tyrosine 505). With activated Lck in tow, CD4/CD8 co-receptor pulls Lck into proximity of the TCR/CD3 complex. Lck then phosphorylates the intracellular tyrosine activation motifs (ITAMs) within the CD3 complex. Phosphorylation of the CD3 ITAMs promotes the docking of other molecules to the CD3 complex, namely the zeta-associated protein of 70 kilodaltons (ZAP-70) which is also phosphorylated by Lck for its activation [2, 3]. Activated ZAP-70 then phosphorylates the linker for activated T cells, or LAT, a transmembrane and palmitoylated adaptor that bridges the initial TCR signal to many downstream signaling events [4].
Figure 1.
T cell receptor activated signal transduction pathways. Cartoon diagram of the critical protein interactions necessary for the activation of a T cell when engaging an antigen-presenting cell.
Phosphorylation of LAT is paramount for subsequent signaling due to the critical nature of the proteins binding to it [5]. Another cytoplasmic adaptor molecule, the SH2 domain containing leukocyte phosphoprotein of 76 kilodaltons (SLP-76), binds to LAT through Gads where it becomes phosphorylated by ZAP-70 [6]. SLP-76 binds to and inducibly recruits many other cytoplasmic molecules to the activated LAT complex, including PLCγ1, ADAP, Nck, and Itk, the subject of this paper [7]. LAT-recruited Itk becomes transphosphorylated at the membrane by Lck on its activation tyrosine (tyrosine 511), and then autophosphorylates itself on tyrosine 180 for full activation [8]. Activated Itk phosphorylates PLCγ1 on tyrosines 775 and 783 leading to the activation of this lipase [9–11].
Itk-activated PLCγ1 cleaves membrane phosphatidyl inositol (4,5) bisphosphate (PIP2) into two secondary messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 diffuses into the cytoplasm to bind its cognate IP3 receptors (IP3R) on the endoplasmic reticulum (ER) leading to the release of calcium in the cytoplasm [12]. Cytoplasmic Ca2+ binds calmodulin, causing its inhibitory release from calcineurin, freeing this phosphatase to dephosphorylate cytoplasmic localized nuclear factor of activated T cells (NFAT). NFAT then translocates into the nucleus to perform its transcription factor duties of genetic regulation [13]. The other PLCγ1-generated secondary messenger, DAG, activates protein kinase C theta (PKCθ), which leads to the release of the nuclear factor of kappa light chain enhancer in B cells (NFκB) into the nucleus to perform its transcription factor duties [14, 15]. DAG also promotes the phosphorylation of Ras guanyl releasing protein (RasGRP), a GTP exchange factor (GEF) that activates Ras, leading to the downstream activation of mitogen-activated protein kinase (MAPK) pathways [16], the MAPKs jun-N-terminal kinase (JNK) and p38 phosphorylate Jun and Fos, respectively, prompting them to translocate into the nucleus and combine to form the AP-1 transcription factor important for genetic regulation [17].
The TCR-activated, LAT-bridged, SLP-76 signalosome brings in two more players important for the sustained activation of the T cell necessary for its full response. These two molecules are the adhesion- and degranulation-promoting adaptor protein (ADAP) and noncatalytic region of tyrosine kinase (Nck). ADAP is an adaptor protein that leads to the intracellular activation of extracellular integrin adhesion molecules, in a process termed as “inside-out” signaling [18–20]. Integrin activation helps to maintain T cell contact with the APC for an extended period to allow full T cell response to the presented antigen [21]. Nck is an adaptor that brings the cytoskeleton organizing molecule, Wiskott-Aldrich syndrome protein (WASp), to the proximity of the SLP-76 signalosome [22, 23]. WASp is indirectly activated through the GEF Vav1. Vav1 exchanges GTP for GDP for Rho GTPases, particularly that of Cdc42, which in the GTP-bound conformation binds to and activates WASp. This causes WASp to destabilize its autoinhibitory condition and take on an open conformation allowing the Arp2/3 complex to bind and seed actin polymerization [24]. Therefore, active WASp enables actin to be reorganized at the T cell junction adjacent to the APC, causing the T cell to stay stably in contact with the APC for the duration of its response, a process necessitated by Itk.
The modulator of this TCR-mediated response is Itk, and as such, the focus of this paper will be on Itk, an enigmatic kinase that serves as a rheostat for many of the signaling pathways needed for a full and effective T cell response. Itk is needed for the regulation of Ca2+ flux, actin polymerization, integrin binding, and transcriptional activation. Consequently, it has a profound role in the development of T cells, cytokine expression, and the clearance of pathogens. The mention of other lymphoid signal transduction molecules, particularly that of other Tec family kinases, will only be alluded to as a reference to Itk in order to highlight the importance of Itk [25, 26].
2. Identification of Itk
Interleukin-2 inducible tyrosine kinase was discovered and cloned by three independent research groups. Stephen Desiderio's group was the first to identify the gene and named it Itk (IL-2 Inducible Tyrosine Kinase) [27]. This was quickly followed by Leslie Berg's group (T-cell-specific tyrosine kinase, Tsk) [28] and Toshiaki Kawakami's group (expressed in mast and T cells, emt) [29]. This nearly simultaneous cloning of the gene and subsequent naming by three different groups led to some conflicting nomenclature initially, but the gene name is now accepted as Itk. This kinase is a 72-kDa protein expressed in the thymus, spleen, and lymph nodes. More specifically, Itk is expressed in T cells, natural killer cells, natural killer T cells, and mast cells. It is chromosomally localized to 5q31-32 in humans [30]. This localization is unique to Itk as other Tec family kinases are seemingly duplicated; Tec and resting lymphocyte kinase (Rlk) both are on the 4p12 locus [31], while Bruton's tyrosine kinase (Btk) and bone marrow kinase (Bmx) are both on the X chromosome (albeit separated somewhat, Btk on Xq22 and Bmx on Xp22 [32]). Evolutionarily, the Tec kinases have been found in flies (Drosophila melanogaster), zebrafish (Danio rerio), skates (Raja eglanteria), and in sea urchins (Anthocidaris crassispina) [33]. Two different forms of Itk have been cloned in the mouse, which have six amino acids either included or deleted [34]. The shorter version is exclusively detected in human cells. Itk has substantial effects on T cell development and function, due in large part to its disproportionate expression levels compared to other Tec kinases. As measured by qRT-PCR, naïve mouse T cells contain 100-fold more copies of Itk compared to Tec, and 3-fold more copies than that of Rlk [35]. Upon T cell stimulation, Itk expression is increased, especially in TH2 cells, while Rlk expression is reduced and becomes relegated to TH1 cells [36]. Tec expression in T cells increases only after several days of stimulation [37].
3. Protein Structure of Itk
Itk is a modular protein, consisting of five distinct structural domains [38]. At its amino terminus, Itk contains a pleckstrin homology (PH) domain, which allows the protein to bind phosphorylated lipids on the membrane. Adjacent to this is the Tec homology (TH) domain from which comes Itk's familial association. This domain contains a polyproline rich region (PRR) necessary for binding Src homology 3 (SH3) domains. The adjoining domain is a SH3 domain, which binds to PRR both in cis- (to itself) and in trans- (to other proteins). The following Src homology 2 (SH2) domain enables the protein to bind tyrosine-phosphorylated substrates. At its carboxyl terminus, Itk possesses an Src homology 1 (SH1) or kinase domain, which specifically phosphorylates tyrosine residues, particularly that of PLCγ1 [39]. Itk, as well as the Tec kinases, shares evolutionary similarities to the Src family kinases in structure. However, the Tec kinases lack the N-terminal myristoylation sequence constitutively localizing Src kinases to the membrane and lack the C-terminal negative regulatory tyrosine residue present on Src kinases.
3.1. The Pleckstrin Homology (PH) Domain (Amino Acids 5–112)
PH domains are protein-lipid interaction domains of about 100 amino acids in size, forming a β-barrel from two β-sheets and a carboxyl-terminal α-helix [40, 41]. A unique feature of the Tec family tyrosine kinases is the presence of a PH domain. Rlk is an exception as it contains a cysteine palmitoylation motif and no PH domain [42]. The PH domain contains the propensity to bind phospholipids in many forms, including phosphatidylinositol-3,4,5 triphosphate (PIP3), PI(4,5)P2, and PI(4)P, with binding of Itk to PIP3 at highest affinity. Itk binding to the plasma membrane through its PH domain is induced through the activity of PI3K, which phosphorylates PIP2 to PIP3. Addition of PI3K inhibitors prevents Itk from localizing to the plasma membrane and becoming active [43–45]. Therefore, Itk is targeted to the membrane and activated through a PI3K-dependent mechanism. Interestingly, localization to the plasma membrane through the PH domain is not enough for Itk's activation. Pleckstrin homolgy domain deleted Itk replaced with a myristoylation sequence enables Itk to localize to the membrane, but it does not become tyrosine phosphorylated upon TCR stimulation [43]. These data indicate that the PH domain contains some other role vital to Itk's activation other than simple membrane localization. PH domain binding to PIP3 localizes Itk in proximity of its activating kinase, Lck, thereby increasing Itk's kinase activity [43, 44, 46]. Also, this domain is integral for binding to the nuclear membrane when the molecule is shuttled to the nucleus [47]. Another unique feature of the Tec kinases is the presence of a PH domain FYF motif. This motif in Btk is linked to agammaglobulinaemia in humans; however, this motif's function has not yet been addressed in Itk. Although selective PH domain inhibitors of Btk have been reported, such as the quinone epoxide terreic acid [48], no selective PH domain inhibitors of Itk have been reported. Recently, the PH domain of Itk was shown to bind to inositol-1,3,4,5-tetrakisphophate (IP4) in vitro with a functional in vivo consequence [49]. Molecularly, this causes Itk's disruption of its inducible localization to the T cell/APC contact site and for its activation. Cellularly, this creates a phenotype where thymocytes undergoing positive selection become unable to progress beyond the CD4+CD8+ double positive stage of development. This necessitates Itk's involvement in T cell development, which requires an alternative role for Itk's PH domain. These combined data suggest an intriguing role of the PH domain of Itk both in its molecular activation and Itk's effect on cellular function.
3.2. The Tec Homology (TH) Domain (a.a. 115–163)
Itk's TH domain contains a familial Tec motif of a conserved 27 a.a. sequence at the amino-terminus of the domain [50] as well as a proline rich region (PRR) at a.a. 153–163. This domain is integral to the activity of Itk as it is thought that this domain binds to its own SH3 domain in an autoinhibited state [51]. Disruption of this association, either by competitive interaction with another peptide sequence or by mutation, is surmised to relieve this autoinhibition and activate the kinase. However, deletion of only the PRR has an opposite effect, it reduces Itk's basal activity by 50% [52]. Additionally, it has been reported that this domain contains a familial Zn++ binding motif, which is critical for oligomerization of Itk at the cell surface [53]. As of yet, no protein has been shown to functionally bind to the TH domain of Itk. Proteins shown to bind in vitro have later been shown to bind indirectly in vivo, either through another Itk structural domain or through another molecule entirely [54]. Given the paradoxical nature between the structure and the function of this domain, more work should be done to identify what the TH domain is doing in Itk.
3.3. The Src Homology 3 (SH3) Domain (a.a. 174–230)
SH3 domains allow for protein-protein interactions to proline peptide sequences (either (R/K)XXPXXP or PXXPXR motifs, where X is any amino acid) facilitating protein function or cellular localization. Not only is the SH3 domain of Itk involved with the binding of internal and external PRRs, but it is also involved in the negative regulation of its own catalytic activity. For example, elimination of the SH3 domain or mutation of a Tec family conserved tryptophan (W208K) that is required for SH3 binding to PRR causes a spontaneous activation of the enzyme, presumably through the release of autoinhibition [55]. Paradoxically, auto-phosphorylation on tyrosine 180 leads to the activation of Itk [8, 56], so elimination of this domain should theoretically lead to an inactive condition. However, elimination of this domain and mutational disruption most likely leads to a conformational release of autoinhibition, allowing the kinase domain to become exposed and catalytically active. It is also possible that the phosphorylation within this domain prevents the binding of a repressor protein though no such protein has been found. There are a plethora of proteins that bind the SH3 domain of Itk in vitro [57], including WASp, Sam68, Cbl, SLP-76, and Vav1, many of which have not been confirmed in vivo. One exception is the recent report of the in vivo interaction of Itk with SLP-76 [58]. Utilizing a cell-permeable peptide to competitively inhibit the Itk-SH3 interaction with SLP-76 PRR, Itk activity and functional T cell response was affected, indicating an important role of the Itk-SH3 domain interaction with SLP-76. The interaction between Itk's SH3 domain and the nuclear import chaperone karyopherin α (Rag cohort 1α, Rch1α) leads to Itk's transport into the nucleus [47]. This interaction requires Rch1α's proline 242, as a mutant Rch1α (P242A) decreased nuclear localization of Itk and diminished IL-2 production. As described later, this is important as Itk not only serves as a cellular membrane proximal kinase linking early signal transduction events, but it also serves to regulate transcriptional activation of certain proteins in the nucleus.
3.4. The Src Homology 2 (SH2) Domain (a.a. 237–329)
SH2 domains allow for inducible protein-protein interactions with phosphotyrosine-containing peptide sequences [59, 60]. Itk's binding to tyrosine phosphorylated substrates not only provides the mechanism for it to inducibly interact with protein partners, but it has shined light on its own localization and consequent activation. Mutation of the SH2 domain of Itk, either through deletion or point mutation of the tyrosine binding pocket, leads to the inactivation of Itk [55]. Itk has been reported to bind to SLP-76 [54, 61], LAT, [44, 55], and Vav1 [62] through its SH2 domain, although it has not yet been determined to which of these molecules the SH2 domain of Itk has a higher affinity. Itk also binds to PLCγ1 through its SH2 domain, though it is not resolved as to whether it is direct [39], or indirect through SLP-76 [9, 63]. Importantly, it has been shown that Itk is negatively regulated through its SH2 domain by the prolyl-isomerase cyclophilin A (CypA), whose binding to Itk can be competed against by Cyclosporin A leading to Itk's activation [64]. Peptidylprolyl isomerases, such as CypA, convert proline peptide bonds to cause a cis- or trans-conformational switch in the protein with which it interacts. CypA binds to a proline residue in the SH2 domain of Itk (P287), promoting the cis-intramolecular interaction with the SH3 domain. This proline residue switch is unique to Itk, as it is not a shared phenomenon with the other Tec kinases [65]. When CypA is relieved of binding to Itk, an isomeric switch within the SH2 domain causes Itk to take on a transconformation, allowing it to bind to other proteins. These data imply that CypA is a negative regulator of Itk activity. Consistent with this idea, T cells deficient for CypA display an overall pattern of increased signaling in response to TCR stimulation, with an increase of PLCγ1 phosphorylation in particular [66]. Given the inducible relieving of repression and consequent functional binding to many key signaling molecules, the SH2 domain of Itk is of vital importance for its activity.
3.5. The Src Homology 1 (SH1) Domain (a.a. 363–612)
The sting for Itk's kinase activity resides within its SH1 domain. Herein lies its ATP binding pocket, allowing it to enzymatically transfer terminal ATP phosphate to its protein target(s). Also within this domain is tyrosine 511 which needs to become transphosphorylated by Lck for the initial activation of Itk [67]. X-ray crystal structures of the kinase domain of Itk have been resolved both in the non-phosphorylated and in the phosphorylated states [68]. Interestingly, phosphorylation of tyrosine 511 did not induce a conformational change in this domain indicating that although phosphorylation of this residue is required for the activity of this enzyme, it does not do so through a phosphorylation-dependent conformational switch. Rather, phosphorylation of the kinase domain could lead to some secondary function. Further, the isolated kinase domain of Itk is similar to the other Tec kinases in that they are kinetically inactive [69]. This is in stark contrast to any other SH1 containing kinase. This indicates that other domains of Itk help to regulate the kinase. Finally, the known targets of Itk kinase domain transphosphorylation are PLCγ1 tyrosines 775 and 783 [63], T-bet tyrosine 525 [70], Tim-3 tyrosine 265 [71], and TFII-I tyrosine 248 [72].
4. Upstream Regulators of Itk
The activation of Itk is a complex orchestration of events (Figure 2). This kinase is activated through a myriad of surface receptors, including the TCR/CD3 signaling complex, co-receptors, chemokine receptors, and heterotrimeric G-protein-coupled receptors (GPCRs). Prior to the activation of the T cell, Itk resides in the cytoplasm in a closed, autoinhibited state. This inhibitory conformation occurs through the cisbinding of its SH3 domain to the PRR of the TH domain [51]. More recently, however, it was found that the SH2 and SH3 domains of Itk could dimerize with each other in a trans-head-to-tail manner that could preclude or mitigate the cis-binding of a single Itk molecule to itself [73]. Further, it was found that full-length Itk self-associates intermolecularly [74]. This intermolecular clustering negatively regulates Itk and, once disrupted, leads to Itk activity. Continuing to determine the conformational state and activation of full-length Itk is of critical importance, as the information will be utilized for inhibitor development.
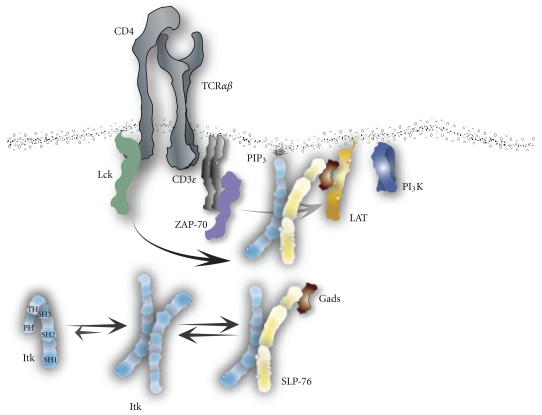
Figure 2.
Mode of Itk activation through the T cell receptor. While residing in the cytoplasm, Itk preferentially takes on an inhibited homodimer conformation and in some instances as an inhibited independent cis-conformation. Upon T cell engagement, Itk is able to bind SLP-76 through its SH3 domain and shuttled to the LAT signalosome. Once there, activated ZAP-70 phosphorylates SLP-76 on tyrosine 145 which Itk then binds to with its SH2 domain to solidify the connection at the signalosome. At which time Itk localizes to the plasma membrane by binding PI3K-generated PIP3 with its PH domain and Itk then becomes tyrosine phosphorylated by Lck on tyrosine 511. This leads to Itk's catalytic activity that first phosphorylates itself on tyrosine 180 culminating in a fully activated enzyme.
Itk associates with SLP-76 while in the cytoplasm, and is shuttled to the membrane-associated LAT signalosome by SLP-76 upon TCR/CD3 stimulation [54]. While at the LAT signalosome, Itk becomes phosphorylated at tyrosine 511 of the SH1 domain by Lck [8, 67]. Transphosphorylation activates Itk kinase activity, whereupon the kinase autophosphorylates itself at tyrosine 180 of the SH3 domain [8]. Cis-phosphorylation fully opens the protein, allowing the PH domain to bind PI3K phosphorylated lipids at the membrane and therefore acts as a fully active kinase within the LAT signalosome. Cells lacking Lck or containing a mutant Itk phenylalanine substitution for tyrosine at the transphosphorylation position 511 leads to a kinetically inactive Itk [67]. Further, mutation of Itk at the auto-phosphorylation site tyrosine 180 to phenylalanine impairs Itk's activity in response to stimulation [8]. This Itk auto-phosphorylated tyrosine lies within the substrate binding sequence of the SH3 domain [51], leading one to speculate that this tyrosine regulatory site is not just important for enzymatic activity, but for protein-protein interactions as well. Similarly, Btk's autophosphorylation site at tyrosine 223 only binds to WASp while in the non-phosphorylated state, but only binds to Syk while in the phosphorylated state [75]. It would be reasonable to speculate that Itk acts in a similar fashion.
4.1. T Cell Receptor Activation
TCR engagement leads to the pivotal activation of two molecules that are integrally important for the activation of Itk, Lck, and PI3K. Activation of Lck through the dephosphorylation of its inhibitory tyrosine by CD45 causes a rapid phosphorylation of receptor components as well as associating molecules such as ZAP-70 and downstream adaptor molecules LAT and SLP-76. Recruitment of Itk to the TCR-nucleated signalosome is mediated through SLP-76, which brings Itk from the cytoplasm to the plasma membrane where Itk PH domain can bind to PI3K phosphorylated phospholipids [58]. Recruitment by SLP-76 to the ZAP-70 primed LAT signalosome leads Itk to be transphosphorylated by Lck. This combination of events leads to the fully activated state of Itk, one that is relieved of autoinhibited restraints, and one that can phosphorylate downstream targets.
4.2. Co-Receptor Activation
Upon CD28 stimulation, Itk binds to CD28 and becomes tyrosine phosphorylated by Lck [76–78]. Itk's interaction with the cytoplasmic polyproline motif of CD28 through its SH3 domain facilitates both the activation of Itk and the tyrosine phosphorylation of CD28 on all four tyrosines found in its cytoplasmic tail [79–81]. This activation of Itk helps to amplify TCR signals, which has a profound effect on Ca2+ mobilization [82]. This association was originally proposed to illuminate Itk as a negative regulator of T cell signaling through CD28, as mice lacking Itk have an increased proliferative response to CD28 stimulation [83]. However, more recent findings indicate that Itk is not essential for CD28 signaling [84]. In this work, investigators used purified CD4+ T cells from Itk−/− mice and noted that these cells already have an activated or memory phenotype that becomes enhanced upon stimulation. It was therefore found that CD28 signaling was normal in Itk−/− cells and Itk does not act as a negative regulator of CD28 stimulation. Similar to the binding of Itk to CD28, Itk has been reported to bind to the PRR on the cytoplasmic portion of CD2, enabling Itk to become activated by Lck upon CD2 cross-linking [85, 86]. Itk's explicit association with these co-receptors inspire further evaluation as it relates to signal transduction pathway cross-talk.
4.3. Chemokine and Hetero-Trimeric G-Protein Coupled Receptors
Both sets of binding G-protein subunits can functionally associate with Itk. The G-protein βγ subunits have been shown to bind to Itk through Itk's PH domain and can promote Itk activity [87]. Chemokines are small proteins that are inducibly secreted by resident cells to promote the recruitment and migration of lymphocytes to an area of infection. Chemokine stimulation linking G-protein coupled receptors to actin polymerization events have shown that Itk can mobilize to the plasma membrane and become tyrosine phosphorylated upon Stromal cell-derived Factor 1-alpha (SDF-1α, also known as CXCL12) chemokine stimulation [88, 89]. Itk's response to SDF-1α is sensitive to both PI3K and pertussis toxin from the bacterium Bordetella pertussis, which inactivates Gα i. Additionally, overexpression of Gα transducin leads to the dominant-negative inhibition of endogenous Gβγ subunits, which affects recruitment of Itk to the plasma membrane in HeLa cells [88]. Since PI3K can be activated through Gβγ, it follows that recruitment of Itk to the plasma membrane upon chemokine stimulation is dependent on a Gβγ-PI3K pathway. This was shown to be true in a recent paper detailing the T cell specific adapter protein (TSAd) promoting the migration of T cells through the interaction of the Gβ subunit, leading to CXCL12-induced Itk phosphorylation, actin polymerization, and cellular migration [90]. The Schwartzberg group also showed that mutant Itk can block the chemokine-induced polarization and activation of the Rho GTPases Cdc42 and Rac [88]. Consistent with the molecular findings, T cells from Itk−/− mice are deficient in their ability to home to the lymph node, and as such, there are reduced numbers of T cells in the lymph nodes in comparison to the numbers of T cells in the spleens of these mice [88]. Further, Itk deficient T cells display an impairment in their ability to migrate to the lungs in response to CXCR4 ligand [89]. This finding has been confirmed in mice treated with a kinase-domain Itk-specific inhibitor, whose T cells were also reduced in ability to home to the lungs [91]. All of the above Itk-activating pathways coordinate to tune the immune response leading to downstream effector function.
5. Downstream Itk Effectors
Intriguingly, Itk is a known activator of many different downstream targets leading not only to the immediate activation of certain signal transduction pathways, but also to transcriptional activation of certain genes corresponding to the development of T cell subsets.
5.1. PLCγ1 and Calcium Mobilization
As mentioned above, Itk is a critical activator of PLCγ1. T cells from Itk−/− mice display diminished tyrosine phosphorylation of PLCγ1 [92] and of tyrosine 783 in particular [63], the critical activating site for this lipase. The consequence of PLCγ1 inactivation is lack of downstream secondary messenger IP3 binding to IP3R preventing the release of intracellular Ca2+ stores [92, 93] and the inhibition of calcium release activated channels (CRAC) at the plasma membrane [93]. CRAC-mediated capacitative influx of Ca2+ from extracellular sources prolongs the signaling necessary for later action, including transcriptional activity. This sustained Ca2+ influx is necessary for long-term signaling in the T cell as well as the formation and the continued presence of the immunological synapse [94]. Accordingly, Itk-deficient T cells are severely diminished in their short-term and long-term Ca2+ flux abilities. The failure of sustained Ca2+flux leads to the downstream inactivation of Erk [92], which manifests itself into a lack of NFAT translocation to the nucleus to initiate its transcriptional activity. Kinase-inactive mutants of Itk cannot phosphorylate PLCγ1 and therefore, downstream activation of Erk and of TCR-induced NFAT reporter activation is compromised [54]. This inactivation is also due to the lack of PLCγ1 generated secondary messenger DAG, a necessary component for the activation of the DAG-binding domain containing protein RasGRP, which is required for Erk pathway activation in T cells [95, 96]. There is some functional redundancy to the Tec kinases in T cells, as PLCγ1 tyrosine phosphorylation, and subsequent Ca2+ flux is only reduced, and not ablated in cells lacking Itk, suggesting a functional role for Rlk and Tec in these processes [92, 93]. T cells from Rlk or Tec knockout mice do not display these phenotypes, as PLCγ1 phosphorylation and Ca2+ flux are intact. However, T cells from double knockouts of Itk and Rlk diminish these two characteristics even further [92]. Further, overexpression of Tec or Rlk in the absence of Itk can functionally rescue the Itk-deficient phenotype in T cells [37].
Itk has been established to regulate Ca2+ flux in T cells through PLCγ1, the lipase that directly leads to the release of Ca2+ from ER stores. However, this does not explain Itk effect on prolonged Ca2+ flux. The recently characterized CRAC channels would further explain the relationship between Itk and sustained Ca2+ signaling. How ER-released Ca2+ stores regulate CRAC channel operation was recently identified to be due to an ER-resident Ca2+ sensor named STIM1 [97]. STIM1 knockdown leads to a complete abrogation of store-operated calcium entry in T cells. Furthermore, upon TCR-induced activation of the T cell, STIM1 colocalizes with a plasma membrane resident CRAC channel named Orai1 at the immunological synapse, which leads to a sustained Ca2+ flux for the T cell [98]. Orai1 is a tetraspanin pore-forming subunit of the CRAC channel, which when absent or mutated also causes an abrogation of sustained Ca2+ flux [99, 100]. This mutation (R91W) in Orai1 manifests in human disease, as one aspect for the development of SCID is due to the attenuation of Ca2+ flux after T cell activation [101]. Given Itk's strong role in sustained Ca2+ flux, it would be of interest to determine whether Itk has an effect on the activity of either STIM1 or Orai1. Regardless of a direct or indirect relationship, it might still provide a more specific method for temporary therapeutic relief of some inflammatory responses, such as graft rejection in transplantation, psoriasis, arthritis, or colitis. Current therapies for these disorders involve the use of cyclosporin A and FK506 to inhibit calcineurin activity leading to reduced Ca2+ flux and reduced T cell activity. Unfortunately, since calcineurin is widely expressed in most cell types, these treatments often lead to unwanted and sometimes severe side effects. If one could directly affect Ca2+ flux in T cells using Itk inhibitors, without affecting other cells, it would be extremely beneficial for the treatment of these disorders.
5.2. Actin Reorganization
The Tec family kinases were initially implicated in the regulation of the actin cytoskeleton through work done in Drosophila. The Tec family kinase Tec29 was found to be required for the growth of ring canals, a necessary bridge between the nurse cells and oocyte during fly embryogenesis [102, 103]. The ring canals in the Tec29 mutant egg chambers failed to form correctly, thereby failing to nourish the maturing oocyte. This data shows an evolutionarily conserved function for Tec kinases in actin regulation.
Within mammalian T cells, it was discovered by two groups that Itk was involved in TCR-induced actin polymerization [104, 105]. These data showed that in T cells lacking Itk, actin directed processes (such as lamellipodial formation and directed actin polymerization) were impaired. The significance of this deficiency is striking, as the defects in actin polymerization and polarization in Itk−/− T cells is complete, whereas the Itk defect in Ca2+ flux is only reduced. Additionally, this defect is most likely entirely due to the absence of Itk, as T cells deficient for both Itk and Rlk are just as defective in actin polymerization as Itk singly deficient T cells, suggesting no functional redundancy [105]. Further, overexpression of Itk leads to an increase in membrane ruffling (lamellipodia) [44]. This regulation of actin is through the interaction of Itk with two molecules directly involved with actin polymerization, Vav1, and WASp [62, 106]. Vav molecules are GTP exchange factors (GEFs) that exchange GDP for GTP to activate members of the Rho GTPase family, particularly that of Cdc42 in T cells. Vav1 deficient T cells not only display a defect in the ability to activate the Rho proteins, but also show very striking actin defects. These cells fail to polymerize actin, show impaired integrin adhesion and clustering, and display broader cellular defects than that of T cells lacking either Itk or WASp [107, 108]. Initially, it was determined that Itk affected Vav1 localization, as T cells deficient in Itk failed to localize Vav1 when engaging antigen-pulsed APCs [105]. This defect had a functional consequence, as activated Cdc42 no longer localized to the contact site as measured with a biosensor. Itk was discovered to constitutively associate with Vav1 [62], an association that was dependent upon Itk's SH2 domain. This interaction confirms a hypothesis that Itk has a kinase-independent function [109], thus broadening the role of this kinase beyond the simplistic idea that a kinase must only act as a kinase, that is, to phosphorylate targets. Notable, however, is the finding that Vav1 still becomes tyrosine phosphorylated in cells lacking Itk (although it has not been determined whether tyrosine 174 phosphorylation is defective, the key activating tyrosine in Vav1 [110]), a finding compounded by the data that Itk activation is also abnormal in cells lacking Vav1 [111]. Lately, Itk−/−Vav1−/− mice were generated and were shown to be strongly reduced in the number of double-positive thymocytes and displayed impaired positive selection [112]. This increased developmental defect shows that Itk and Vav1 share some mechanistic similarities affecting T cell development as well.
The Wiskott-Aldrich Syndrome protein (WASp) is the gene product whose deficiency leads to Wiskott-Aldrich Syndrome, a fulminant state that includes lymphopenia mediated by a defect in actin regulation. WASp is vital in actin regulation as it activates the Arp2/3 complex which helps to nucleate new actin filaments [113–117]. WASp is indirectly dependent upon Vav1 for its activation as it needs to bind to GTP bound Cdc42 to release its autoinhibition and activate the Arp2/3 complex, leading to the nucleation of new actin filaments [24]. Itk has been shown to bind WASp, both in vitro through its SH3 domain [57] and in vivo using full-length molecules [106]; however, the functional outcome of this interaction is muddied because Itk seems to be required for WASp's localization [105], but not for its phosphorylation [118], a requirement necessary for WASp's full activity [119, 120]. Again, this implies a kinase-independent function for Itk's role in actin polymerization.
The intricate dance between Itk, Vav1, and WASp, as it relates to TCR-induced actin polymerization remains an interesting topic, as it seems that Itk affects both Vav1 and WASp in a kinase-independent manner. This does not, however, preclude that Itk might have a kinase-specific role for the continued activation of these molecules. If Vav1 is probed for its phosphorylation on its activating tyrosine 174 [121] and if WASp is probed for its phosphorylation on its activating tyrosine Y291 [119] in the presence or absence of Itk, one will get a better idea of Itk's role in a kinase-dependent or kinase-independent manner. Furthermore, it has been reported that the Tec family kinase Btk not only binds actin directly through its PH domain [122], but it can also tyrosine phosphorylate WASp as well [123, 124]. Also, given that the tyrosine kinase Abl can bind and phosphorylate actin directly [125], and that the tyrosine kinase Fyn can phosphorylate WASp [118], it may be useful to pursue whether Itk can perform any of these duties as well.
The effects of Itk seen on actin polymerization in T cells also dismisses the idea that the cellular defects seen in Itk−/− cells is due merely to the molecular inactivation of PLCγ1. The decrease in actin polymerization can affect signal strength and signal duration, as well as affect integrin-mediated adhesion leading to the known defects in Itk−/− T cells including altered CD4/CD8 ratios, specific decrease in cytokine production, and in the proliferation of these cells. It is likely that the defect in cytoskeletal reorganization contributes to the decreased response of Itk−/− T cells as a result of inefficient immunological synapse formation [126].
5.3. Adaptor Proteins
As mentioned above, Itk associates with a plethora of adaptor molecules, most notably, SLP-76. Itk needs SLP-76 for its phosphorylation and for its localization to the T cell/APC contact site [9, 58, 63]. The interaction between Itk and SLP-76 is pivotal, as it helps to integrate Itk's functional relationship with many molecules, including LAT, PLCγ1, and Vav1. The interaction of Itk with SLP-76 is a complex undertaking, as it is surmised through in vitro studies that the interaction is a cooperative one involving SH3 domain of Itk binding to PRR of SLP-76, and then SH2 domain of Itk binding to phosphorylated tyrosine 145 of SLP-76, once it has been phosphorylated by ZAP-70 [54]. This multivalent binding of Itk to SLP-76 provides the means for Itk to associate with another adaptor molecule necessary for its activation, LAT. TCR-induced SLP-76 signalosome bridging to the LAT signalosome is mediated by the adaptor protein Gads [127]. SH3 domain of Gads binding to a PRR motif within SLP-76 separate from that of Itk indirectly connects SLP-76 to LAT when the latter becomes phosphorylated and the SH2 domain of Gads can bind that site. Although Itk does not bind Gads directly, Itk activation is therefore dependent on Gads ability to unite SLP-76 and LAT. Itk has been reported to bind LAT directly in vitro using GST-fusion proteins of the Itk SH2 domain [128]. This is supported by the evidence that Itk needs to be localized to the LAT signalosome for its activation [55]. Further, the association of Itk with LAT may have functional conformational consequences for Itk's activation [129]. This would imply an additional role for LAT, one that involves more than Itk localization. Therefore, the question remains whether the binding of Itk to SLP-76 or LAT is more important for Itk activation. The answer probably is both. SLP-76 and LAT are integral for the activity of a T cell, so consequently both are necessary for the activation of Itk. Lastly, Itk has also been reported to bind another adaptor molecule, Rlk/Itk binding protein (RIBP) [130, 131], which has been reported to negatively regulate Itk function, but the mechanism of this has yet to be determined.
Recently, Itk's association with SLP-76 in vivo has been addressed through use of an inhibiting cell-penetrating peptide [58]. This paper provided the first demonstration of the functional importance of the Itk-SH3 domain interaction with SLP-76 as it relates to Itk's localization, activation, and ultimate production of TH2 cytokines. This provides a mechanism of stable Itk-SH3 binding to SLP-76-PRR as a prerequisite for the subsequent binding of the Itk-SH2 domain to the phosphorylated tyrosine 145 on SLP-76, ultimately coordinating to localize and properly activate Itk [132].
5.4. Regulation of Cellular Adhesion
In order for T cells to initiate and maintain contact with an APC, functional adhesion through cell surface integrins are necessary. This integrin adhesion requires a functional actin cytoskeleton and also requires signaling through the TCR. Data from Jurkat T cells expressing mutant kinase domain Itk shows that Itk is integral in the activation of β1 integrins through the T cell receptor in an “inside-out” signaling function [133]. Further, blocking TCR-induced T cell activation through inhibition of Itk leads to a block in β1 and β2-integrin adhesion, and it affects the recruitment of LFA-1 to the site of TCR stimulation [133]. This observation was carried into primary mouse T cells by showing that TCR-induced upregulation of β2 integrin adhesion is also severely reduced in cells lacking Itk [134]. Thus, the TCR-induced maintenance of the T cell interaction with APC is very much dependent on Itk's kinase activity. Although the exact inter-molecular mechanism of how this cellular interaction regulated through Itk remains unknown; many Itk-associating signaling molecules involved in actin reorganization are also associated with integrin adhesion. Molecules such as PI3K, Vav1, PKCθ, and ADAP are all connected to integrin signaling, with Itk partnering PI3K, Vav1, and PKCθ. It is currently unknown whether Itk associates with ADAP, although given its extraordinary complicity with integrin signaling and its association with SLP-76, it would be a natural target to explore for Itk's regulation of integrin signaling.
5.5. Transcriptional Regulation
NFATc fails to translocate to the nucleus when Itk−/− T cells become activated [135], as a result of diminished Ca2+ movement in these cells. This is an expected result because NFAT is activated through dephosphorylation by the Ca2+ sensitive phosphatase, calcineurin [136]. NFAT is regulated in a highly dynamic Ca2+ dependent manner because as intracellular Ca2+ levels drop, NFAT quickly becomes phosphorylated and exits from the nucleus. Consequently, for NFAT-dependent transcription to occur, quick pulses of ER-derived Ca2+ are not enough to sustain transcription. Rather, a prolonged surge of Ca2+ from extracellular sources is needed for NFAT to stay active and promote transcription [137]. Since Itk is involved in capacitative Ca2+ flux, Itk is also involved in prolonged NFAT transcription. NFκB appears not to be defective in Itk-deficient cells, which is an interesting result as this transcription factor needs PKC to activate it, which it cannot do without sufficient DAG present [135]. DAG is needed to activate PKC leading to Erk, and ultimately, to NFκB activation. Though not measured directly, DAG activity is diminished as PKCθ-mediated activation of Erk is reduced in Itk−/− cells, and therefore one would expect that NFκB activity would also be compromised. Additionally, the activation of the transcription factor AP-1 is reported to be dependent on Itk activity, as its Erk dependent ability to bind DNA is reduced [138]. Itk is also known to directly phosphorylate the transcription factor T-bet on tyrosine 525 [36, 139], which may cause the most direct means in controlling TH1 development. When T-bet becomes phosphorylated, it proteolytically breaksdown, leaving GATA-3 expressed, promoting a GATA-3-driven TH2 response. As mentioned earlier, Itk can localize to the nucleus upon activation, putatively phosphorylating T-bet at this site. However, the exact cellular location of this phosphorylation event as well as the functional significance of Itk localization to the nucleus is still unknown. The functional consequence of Itk's phosphorylation of T-bet, however, will be explained in the context of T cell differentiation.
6. In Vivo Role of Itk
At first glance, the physiological defects in cells lacking Itk would be considered modest since a deficiency in Itk has only recently been implicated in any known human disease [140]. However, mice lacking Itk display intriguing and beguiling results when it comes to cellular function. When compared to other signaling molecules involved in TCR-induced T cell signal transduction, Itk defects mirror some, though not all, defects seen with nearby molecules. The degree of Itk defects is also variable, placing Itk more as a modulator of T cell signal pathways, rather than a bridge through which all signals must pass. Table 1 summarizes the in vivo effects of Itk knockout mice and compares them to other knockout mice of related molecules (Lck [141], ZAP-70 [142, 143], Rlk [92], Tec [144], Itk/Rlk double knockout [92], LAT [145–147], SLP-76 [148, 149], PLCγ1 [150], WASp [151, 152], PKC-θ [153–157], Vav1 [107, 108, 158–162], ItpkB [163, 164], Gads [165], CypA [66, 166]).
Table 1.
Comparison of phenotypes between TCR-inducible signaling molecules. Table depicting developmental, biochemical, and phenotypic defects observed in mice deficient for the indicated gene in the first column. A “Yes” response denotes confirmation of the defect, while “N.A.” indicates “not applicable” and “N.D.” indicates “not determined”. References for the defects are listed in the last column.
| Developmental defects | Biochemical defects | In vivo phenotype defects | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KO mouse | Stage pre- TCR | Affected | PLCγl phos | Ca++ flux | Erk phos | NFAT activ | IL-2 prod | Proliferation | Actin polym | Adhesion | Activated phenotype | AICD | TH2 response | Pathogen clearance | Eosinophilia | |
| Itk | Mild | DP-SP | Yes | Mild | Yes | Yes | Yes | Mild | Yes | Yes | Yes | Yes | Yes | Yes | Mild | [36, 92, 167, 170, 202, 204, 205] |
| RIk | No | No | No | No | No | No | Mild | No | No | No | N.D. | N.D. | N.D. | N.D. | N.D. | [92] |
| Tec | No | No | N.D. | N.D. | N.D. | N.D. | No | No | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | [144] |
| Itk/Rlk | Yes | DP-SP | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | N.D. | [92] |
| Lck | Yes | DN1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N.D. | N.A. | N.A. | N.A. | N.A. | N.A. | [141] |
| ZAP-70 | Yes | DN3-DN4 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N.D. | N.A. | N.A. | N.A. | N.A. | N.A. | [142, 143] |
| LAT Y136F | Yes | DN3-DN4 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Increase | N.D. | Yes | [145–147] |
| SLP-76 | Yes | DN1 | Yes | Yes | Yes | Yes | N.D. | Yes | Yes | N.D. | N.A. | N.A. | N.A. | N.A. | N.A. | [148, 149] |
| Vav1 | Mild | DP-SP | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N.D. | N.D. | Yes | N.D. | N.D. | [107, 108, 158–162] |
| ItpkB | Yes | DP-SP | Yes | No | Yes | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | [49, 163, 164] |
| Gads | Yes | DN3-DN4 | Yes | Yes | Yes | Yes | Yes | Yes | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | [165] |
| PLCγl | Mild | DP-SP | Yes | Yes | N.D. | Yes | Yes | Yes | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | [150] |
| WASp | Mild | No | N.D. | Mild | No | Yes | Yes | Yes | Yes | No | Yes | N.D. | Yes | N.D. | N.D. | [151, 152] |
| PKCθ | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | N.D. | N.D. | Yes | Yes | Yes | N.D. | [153–157] |
| CypA | No | No | No | No | No | Yes | Yes | Yes | N.D. | N.D. | N.D. | N.D. | Yes | N.D. | N.D. | [66, 166] |
6.1. Development
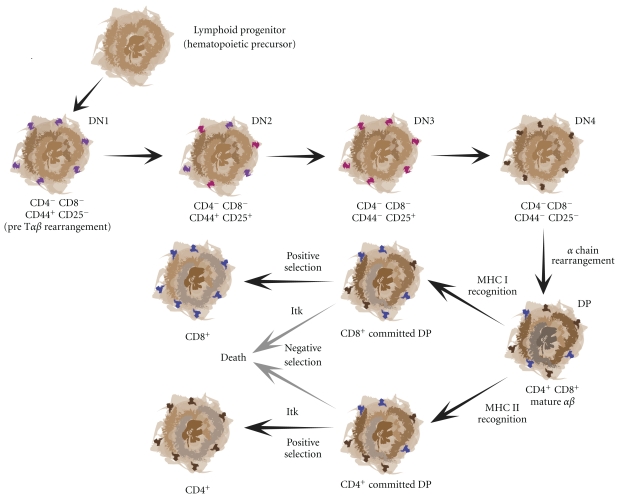
Itk−/− mice display decreased numbers of maturing thymocytes reduced proliferative responses and showed a profound defect in CD4+ T cell development Figure 3 [167]. Itk−/− mice have half the number of CD4+ T cells, which when related to Itk molecular signaling, may be due not only to signal strength, but to signal duration as well [126]. This again signals Itk's status as a molecular rheostat. Proteins upstream and downstream of Itk, Lck, and Erk1/2, respectively, have both been shown to be important in lineage commitment [168]. Therefore, it makes sense that Itk would have defects in this area as well. However, both CD4+ and CD8+ T cells do emanate from the Itk−/− thymus, but at altered ratios due to lower CD4+ numbers and normal CD8+ numbers (though this does not mean that the CD8+ cells are normal, as explained later). Thymic development in both fetal and adult Itk−/− mice is normal when analyzed at CD4−CD8− (double-negative, DN) and CD4+CD8+ (double-positive, DP) stages, although there is evidence that the time of progression from DN to DP stages is longer in Itk−/− thymocytes when compared to wild-type cells [169]. There is a reduction seen in peripheral CD4+ T cells [167], which is due to an impairment in positive selection in class II MHC TCRs in Itk−/− T cells [170]. There is little defect seen in positive selection using the conventional H-Y TCR system [92, 167]. Class I MHC H-Y TCRs in Itk−/− T cells result in decreased efficiency of negative selection [171]. Alternatively, CD8+ T cell peripheral numbers are higher than controls, indicating that negative selection in the absence of Itk has failed. Both positive and negative selection need a functional calcium flux leading to the activation of Erk1/2 for positive selection [172–174] and p38 activation for negative selection [175]. Itk−/− T cells do not possess an optimal calcium flux and fail to activate Erk1/2, but p38 activation is still intact. Furthermore, in the paper by Huang et al., positive selection is affected while negative selection is spared when Itk becomes inactive due to the lack of secondary messenger IP4 [49]. These data indicate that Itk would have a more profound impact on positive selection rather than negative selection. This is confirmed when in the absence of Itk, the deletion of self-reactive thymocytes under strong stimulus conditions is impaired, but not under weak stimulus conditions. Only when Itk-deficient thymocytes continue to progress to a later stage of development do they succumb to negative selection signals [170].
Figure 3.
Thymic T cell development. Cartoon diagram of the development of a T cell from a bone marrow-derived hematopoietic progenitor to that of a mature CD4+ or CD8+ T cell capable of mounting an immune response in the periphery. Itk is necessary for both positive and negative selection, as it is able to modulate the strength of signal emanating from the T cell receptor.
Although the total numbers of CD8+ T cells from Itk-deficient mice appear to be normal, conventional CD8+ T cells are severely diminished. Instead, an innate or memory cellular phenotype exists in these mice causing the CD8+ T cell numbers to appear normal [176, 177]. CD8+ thymocytes from Itk−/− mice have a memory cell surface phenotype (CD44hi, CD62Lhi, CD122hi) and include natural killer cell markers, such as NK1.1. Additionally, Itk−/− CD8+ T cells spontaneously express IFNγex vivo and upregulate antiapoptosis genes such as BCL-XL upon IL-15 stimulation, which is similar to memory cells. Furthermore, independent thymic development of CD8+ T cells in Itk−/− mice is observed regardless of the type of cell presenting MHC class I, whereas conventional CD8+ thymic derived T cells are only selected through interaction with thymic epithelial MHC class I presentation. Finally, Itk−/− CD8+ T cells are dependent on the presence of IL-15 for their survival, again similar to a memory cell phenotype [178]. The work to describe the existence of these innate CD8+ T cells emanating from Itk−/− mice was done in the Schwartzberg lab where they showed these cells arise in Itk−/− fetal thymic organ cultures [176]. Furthermore, a molecular marker for memory CD8+ T cells, eomesodermin, is upregulated in the absence of Itk [177]. This is interesting because eomesodermin is known to upregulate the expression of both the memory cell marker CD122 and the expression of IFNγ [179]. Finally, the rise of these innate-like CD8+ T cells is dependent on the adaptor SAP, like NKT cells [180]. Therefore, Itk-deficient mice provide an interesting insight into the development of CD8+ memory T cells.
6.2. Differentiation
T helper cell differentiation is a necessary and crucial developmental process that selectively hones the T cell response towards certain pathogens (Figure 4). Initially, these differentiated cells were divided into two types, T helper 1 (TH1) and T helper 2 (TH2). This delineation is evolving to include T helper 17 (TH17) and regulatory T cells (TReg) as well.
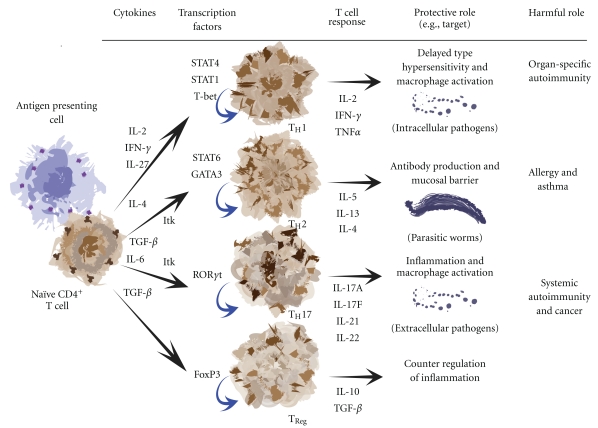
Figure 4.
T cell differentiation and their responses to pathogens. Cartoon diagram displaying the interaction of a naïve CD4+ T cell with a dendritic cell APC leading to differentiation into T helper subsets. The development of these subsets allows for protective immunity against specific pathogens. Itk is needed to promote TH2 and TH17 differentiation.
During TH1 differentiation, IL-12, IL-27, or IFNγ signaling through STAT4 and STAT1 induces the expression of the transcription factor T-bet, which can directly drive TH1 development by increasing IFNγ production and inhibiting IL-4 production. TH2 differentiation, by contrast, is dependent on the IL-4-induced activation of STAT6 leading to the activity of the transcription factor GATA-3, causing an increase in IL-4 production and a decrease in IFNγ production. TH2 differentiation was recently described to occur in two steps when examined in mice expressing an IL-4 dual reporter gene [181]. The first is the TH2 competent stage where cells initially upregulate IL-4 and GATA-3 gene expression. The second is the TH2 effector stage where cells support the production and release of TH2 cytokines necessary for effector function. This two-step TH2 differentiation model was extended by use of an IL-4-GFP reporter system crossed into Itk-deficient mice [182]. In these studies, it was found that in the absence of Itk, TH2 differentiation could proceed normally, but when these differentiated cells needed to produce and secrete TH2 effector cytokines, they could not do so even with repeated stimulation. Additionally, under non-skewing conditions (no exogenous IL-4 added ex vivo to the developing cultures to promote TH2 differentiation) Itk−/− cells display defects in TH2 competent cells, as they preferentially develop into IFNγ producing TH1 cells [36]. This is an interesting result, as IL-2 receptor signaling is necessary for IL-4 transcription [183], and since Itk−/− mice fail to produce substantial amounts of IL-2 upon stimulation, IL-4 production could be impaired due to the lack of autocrine IL-2 signaling necessary to stabilize transcription factor access to the Il4 gene leading to TH2 differentiation [184]. Furthermore, the relative ratios of Itk and Rlk during T helper cell differentiation can also be useful in determining which pathway the cell will take. As mentioned earlier, naïve CD4+ T cells express 3–5 times more Itk than Rlk when measured by qRT-PCR. When the T cell becomes activated through its T cell receptor, Rlk becomes downregulated, leaving 100 times more Itk than Rlk present [36]. While in this activated state exposure to cytokines secreted by resident non-T cell leukocytes provides a skewing environment that drives T helper cell differentiation, which is greatly influenced by the increased ratio of Itk.
TH1 and TH2 effector cells are also characterized by the expression of two transcription factors, T-bet in TH1 cells, GATA-3 in TH2 cells. T-bet is required for the differentiation of naïve T helper cells into TH1 cells and expression of IFNγ [185], while GATA-3 regulates chromatin remodeling at the locus that encodes the cytokines IL-4, IL-5, and IL-13, and consequently promotes TH2 differentiation [186, 187]. Itk plays an active role in the expression of these transcription factors, as it phosphorylates T-bet on tyrosine 525 [36, 70]. Phosphorylation of T-bet leads to its proteolytic breakdown, leaving only GATA-3 to be expressed, and therefore, a TH2 effector cell differentiated phenotype.
An intriguing question asked recently is whether Itk is involved in the regulation of TH17 cellular function. TH17 cells normally mediate the host defensive response to extracellular bacterial infections and are reportedly involved in the pathogenesis of autoimmune diseases through the overexpression of proinflammatory cytokines. The signature cytokines of TH1 and TH2 cells, IFNγ and IL-4 respectively, have both been shown to inhibit TH17 differentiation [188, 189]. Markedly, while IFNγ normally upregulates T-bet, the expression of T-bet is significantly lower in TH17 cells [190, 191]. Further, the promotion of TH17 development leads to the pathogenesis of experimental autoimmune encephalomyelitis [192]. The TH17 signature cytokine IL-17 is overexpressed in the airways of asthmatics where it induces the production of IL-8 chemoattractant needed by neutrophils, which are phagocytic granulocytes involved in eliminating bacterial infections [193]. These findings draw a connection between Itk, TH17 cells, asthma, and autoimmunity through the transcription factor T-bet. Since Itk phosphorylates and affects the downregulation of T-bet, it would be expected that Itk promotes the development of TH17 cells. This is indeed the case as shown recently by Gomez-Rodriguez et al. [194]. It was found in this work that Itk−/− T cells produce less IL-17A than their wild-type counterparts through a reduction in IL17a transcripts, though interestingly enough, IL17f transcript production was intact as was the expression of the transcription factor that regulates TH17 differentiation, RORγt. This is a fascinating result that implies TH17 differentiated T cells can be further subdivided into functional subsets [195]. Lastly, since the presence of Itk helps to promote IL-17A production, the recent finding that IL-17A provides a protective function in a colitis model system suggests that Itk too can be important in the mediation of intestinal inflammation [196].
Another re-emerging set of differentiated CD4+ T cells that have garnered lots of interest lately are regulatory T cells, or TReg cells. TReg cells have a critical function in the regulation of peripheral tolerance. It may be that TReg and TH17 cells are reciprocally regulated through the activation and deactivation of transcription factors, much like inverse the relationship between TH1 and TH2 cells [197]. The main cytokine produced by TReg cells is IL-10. This cytokine inhibits the expression of many proinflammatory cytokines and chemokines, helping to decrease the number of circulating leukocytes from entering the inflamed tissue. Notably, another significant defect in Itk-deficient T cells is the failure to produce IL-10 in response to activation [138]. This was previously interpreted to be part of a diminished TH2 function. However, given what is now known about the TReg signature cytokine being IL-10, it would be interesting to determine the function of these TReg cells in the absence of Itk. Speculatively, Itk−/− T cells could have a diminished TReg response leading to hyperinflammatory conditions.
The regulation of the T lymphocyte cytoskeleton by Itk is through an intricate dance between two additional cytoskeletal molecules, Vav1 and WASp. Although Itk may not be integral in the phosphorylation of WASp [118], it is intimately involved in locating WASp to the immunological synapse thereby affecting WASp activity [105]. Recently, it has been discovered that WASp is important in the development and function of TReg cells, in both mice and in humans [198–201]. These studies confirm that WASp-deficiency curbed the differentiation of these cells, as well as abrogated their suppressive cellular activity, such as suppressing the production of TGFβ and IL-10. Given the relationship between Itk adaptor function and the localization of WASp necessary for its activity, a similar interaction in TReg may indicate that Itk may very well be involved in the management of TReg activity, thus affecting autoimmune disorders.
6.3. Cytokine Production
IL-2 production in T cells lacking Itk is decreased in response to stimulation through the T cell receptor, which affects these cells ability to proliferate [92, 167, 202]. Exogenous IL-2 enables these cells to overcome this proliferative defect, a response that originally led to the name of this kinase [27, 92].
Activation of naïve CD4+ lymphocytes causes their differentiation into either TH1 or TH2 effector cells, which produce distinct sets of cytokines. TH1 cells preferentially produce IFNγ, TNFα, and IL-2, while TH2 cells primarily produce IL-4, IL-5, and IL-13. The balance between these two sets of CD4+ effector cells allows for the tuning of the immune response to the state of infection. Conversely, when this balance is upset, self-reactive disease states can ensue. An excess of TH1 cells can lead to autoimmune states, while an excess of TH2 cells can lead to hypersensitivity. Therefore, T helper cell differentiation and cytokine expression regulation is of paramount importance for an adequate immune response. Itk−/− mice display a peculiar cytokine production profile, as the CD4+ T cells emanating from these mice produce relatively normal levels of TH1 cytokines (e.g., TNFα and IFNγ), while TH2 cytokine expression (IL-4, IL-5, and IL-13) is diminished [135, 203]. IL-4 expression can be rescued by the retroviral expression of Itk in Itk-deficient cells, indicating that Itk is the sole reason for the defect in these cells and not something due to the development of these cells in the absence of Itk [135]. Since Itk−/− cells have an impaired NFAT transcriptional activity, it is therefore expected that IL-4 and IL-13 cytokine production would be hindered as both of those cytokines are dependent upon NFAT activity [204].
6.4. Pathogen Clearance
During the course of a viral infection CD8+ T cells differentiate into activated killer cytotoxic T lymphocytes (CTL) whose main functions are the lysis of infected cells and the secretion of antiviral cytokines, such as IFNγ and TNFα. CD8+ T cell function in cells lacking Itk has yielded perplexing results, as Itk−/− mice were initially thought to have only a mild defect in their response to viral infection [205]. When infected with either lymphocytic choriomeningitis virus (LCMV), vaccinia virus (VV), or vesicular stomatitis virus (VSV), CTL responses were reduced two to six-fold. However, the kinetics of viral clearance was the same in Itk−/− mice as in wild-type mice. Further, in the case of anti-VSV-antibodies, the serum titers of these antibodies were normal in Itk−/− mice when compared to wild-type mice when each had been infected with VSV. A very interesting recent finding is the determination that Itk can affect the replication of human immunodeficiency virus (HIV). Through use of Itk-specific siRNA or by using an Itk chemical inhibitor, production of HIV protein p24, actin-dependent HIV viral entry, and HIV transcription were all reduced [206]. Although this research does not imply Itk in the direct clearance of HIV virally infected cells by CTLs, it does show that by inhibiting Itk activity, one can ameliorate a viral infection.
T helper cell differentiation is crucial for the clearance of pathogens. TH1 cells are important in promoting responses to clear intracellular pathogens, whereas TH2 cells support productive humoral immune responses against extracellular pathogens. Itk−/− mice display an increased susceptibility to parasitic infection, as displayed in their inability to clear the intracellular pathogen Toxoplasma gondii (T. gondii) [92], a parasite that normally induces a protective TH1 response in wild-type mice. This is odd because T. gondii requires a fast and robust IFNγ response by the host to prevent lethality, which Itk−/− mice produce. This may be due to the Itk−/− mouse strain used for these studies since there are differences in TH1 and TH2 differentiation among mouse strains [207, 208]. During a Leishmania major (L. major) infection, wild-type C57Bl/6 mice are able to produce TH1 cytokines resulting in the clearance of the parasite, whereas wild-type BALB/c mice primarily produce TH2 cytokines, which causes them to succumb to the infection. During an infection that requires a TH1 response by the host to clear the infection, Itk−/− mice on C57Bl/6 background elicit a protective TH1 response [135]. However, BALB/c mice deficient for Itk are unable to normally produce a TH1 response, and could not produce a TH2 response due to the absence of Itk, resulting in the inability of the mice to clear the infection. The discrepancy between these results may be because T. gondii infection requires a rapid TH1 response, while L. major can be cleared by a slow, less robust response. Therefore, the kinetics of infection and response may dictate the lethality of the pathogenic infection.
In an attempt to address this issue, the Fowell group backcrossed the Itk−/− mice on IL-4-GFP reporter mouse background [209] to examine an IL-4 producing TH2 response during L. major infection. IL-4 producing CD4+ T cells were present at the site of infection, indicating that differentiation and migration to site of infection were not a problem. However, the amount of IL-4 produced, particularly upon restimulation, was insufficient in the Itk−/− mice when compared to the wild-type mice [182]. This elegantly determines that Itk is not necessarily required for differentiation, but is required in the production of this cytokine.
Meanwhile, TH2 effector function for protective immunity of extracellular pathogens is more clear. Infection with Nippostrongylus brasilinensis (N. brasilinensis) causes a protective TH2 response in BALB/c mice, while Itk−/− BALB/c mice were unable to clear the nematode due to a suboptimal TH2 response [135]. Similarly, Itk−/− mice on a C57Bl/6 strain background were unable to mount a protective TH2 response to the helminth Schistosoma mansoni (S. mansoni) due to a severe reduction in TH2 cytokine production of IL-4 and IL-5 [138].
6.5. Allergy Induced Bronchial Asthma
The connection between Itk and allergic responses was first made in a clinical study on patients with atopic dermatitis, a disease state characterized by an itchy rash and inflammation caused by an excess of TH2 cell response [210]. There is an increased incidence of Itk expression in these patients correlating to the severity of the disease state. This corresponds well with elevated Itk expression levels in mouse TH2 cells [36]. Furthermore, the linkage between atopy and Itk can be traced genetically to the Itk genomic locus as there are single nucleotide polymorphisms at this locus in atopic patients [211]. TH2 cellular responses are involved in the pathology of allergic asthma, as the number of TH2 cells recruited to the lungs (an immune privileged site) is increased, as well as the expression of TH2 cytokines and the consequent movement of responding cells to the lungs resulting in increased inflammation and mucus production [212, 213]. Itk's first direct molecular and cellular correlation to asthma was made by Mueller and August where they induced airway hyperresponsiveness in mice and found that Itk−/− mice displayed a reduced number of T cells and eosinophils infiltrating the lungs when compared to similarly treated wild-type mice [203]. This finding included a reduction in lung inflammation, diminished mucus production, and reduction in the TH2 cytokine expression of IL-5 and IL-13. This was later extended by Ferrara et al. in the finding that Itk−/− mice display a diminished tracheal response to allergen challenge using the same airway hyperresponsiveness model system [214]. Mast cells degranulation was also found to be impaired in Itk−/− mice in the same model system [215]. On a related issue, mice lacking the transcription factor T-bet, which Itk normally phosphorylates to promote T-bet's degradation, spontaneously develop airway hyperresponsiveness and display enhanced inflammation and an increase in secreted TH2 cytokines [216]. To support this idea, the August lab recently published work that the Itk kinase domain activity is necessary for the development of allergic asthma [217]. Interestingly, T cells from these Itk kinase domain lacking mice displayed a profound defect in cytokine mediated T cell migration. Given these results, competitive inhibitors that selectively target Itk would be of beneficial therapeutic value in the treatment of asthma. In fact, one study has shown that by inhibiting Itk kinase activity, lung inflammation in mice can be ameliorated [91].
6.6. Apoptosis
Activation induced cell death (AICD) is the process by which cells are instructed to commit suicide, or apoptose, in response to an extracellular signal. This process is genetically linked as most cells contain signaling pathways always on the ready for the signal to apoptose. Functionally, this process is crucial in the life of a lymphocyte, as stimulation-induced clonal expansion leads to an enormous number of cells generated to combat an infection, the clearance of the invaders leads to many cells that are no longer needed. Apoptosis serves as a means to eliminate these extra cells, which (if no more pathogen is present) can possibly be destructive. During instructive thymic development, T lymphocytes that give too robust a signal to self-antigen during positive selection are signaled to apoptose in order to limit self-reactive T cells from entering the periphery. The apoptotic elimination of clonally expanded T cells after an infection is also necessary to prevent autoimmunity, as an abundance of no longer needed cells could cause destruction of tissue with similar epitopes [218, 219]. Itk is keenly involved in the apoptosis of T cells. Itk−/− T cells have a decreased susceptibility to CD3-induced apoptosis [92]. Thymocyte deletion, particularly that of double-positive T cells, is also defective in cells lacking Itk [171]. Furthermore, Itk is involved with the expression of Fas-ligand as well as the interaction between Fas-receptor and its downstream cell death induction molecules [202]. Itk, however, has not been shown to be involved in anti-Fas engagement leading to apoptosis. Whether Itk influences the apoptosis of the large population of CD8+ memory-like T cells remains to be seen.
6.7. In Vivo Comparison to Other Signaling Molecules
Itk-deficient cells display many commonalities with cells deficient in other signaling molecules within the TCR-induced signalosome (Table 1). LAT−/− mice do not develop mature T cells, although transgenic point mutant Y136F mice (the LAT tyrosine motif that PLCγ1 binds to [220]) have a similar phenotype to that of Itk−/− mice [145, 146]. These mice have impaired Ca2+ mobilization, TH2 cytokine production, and increased amounts of IgE in the serum. JNK−/− mice are also incapable of clearing a L. major infection, indicating that Itk could also act through the JNK pathway. These JNK-deficient mice have a reduced IL-4 yield, yet are able to produce IFNγ when stimulated in vitro under nonskewing conditions much like that of Itk−/− mice. Mice deficient in cyclophilin A (CypA) spontaneously develop allergic disease marked by increases in IgE antibody titer in serum. These mice also have an increased infiltration of mast cells and eosinophils into the lungs [66]. TH2 cells emanating from these mice are hypersensitive to TCR stimulation, and although Itk activity has not been directly assessed in these cells, given CypA repressive function on Itk, it would be assumed that Itk could be hyperactive in these cells. Vav1 and PKCθ deficient T cells have a decrease in IL-2 production, and consequently, IL-4 production as well [154, 155, 161, 162, 221]. Downstream, these cells are defective in Ca2+ mobilization leading to a defect in NFAT transcriptional activation, these cells are similar to Itk−/− cells as they are defective in AP-1 transcription factor activation, and these cells are defective in NFκB activity. Additionally, mice defective for the expression of NFAT1 have a decreased TH2 cytokine expression profile [204]. All of these molecules provide evidence linking the involvement of Itk in their cellular function.
6.8. Itk Function in Unconventional and Non-T Cells
Although much of the research on Itk has been produced in conventional αβ T cells, Itk is expressed in natural killer (NK) cells, natural killer T (NKT) cells, and mast cells. natural killer cells are lymphocytes that can eliminate virally infected cells or transformed cells, independent of an antigen stimulus as in CTLs. The NK cellular response in Itk−/− or in Itk−/−Rlk−/− double knockout cells was first reported to be normal, as interpreted from the robust response to LCMV infection in these mice [35, 205]. However, recently it was determined that Itk both positively regulates NK cell cytotoxicity in response to FcR stimulation and negatively regulates NK cell cytotoxicity upon NKG2D receptor activation [222]. This indicates a more complex role for Itk in NK function, one that deserves further exploration. Mast cells are tissue-resident granulocytes that are the effector cells in immediate hypersensitivity reactions such as allergic rhinitis. The role of Itk in mast cells is blurred by the presence of Btk, where Btk seems to be the dominant Tec kinase in these cells. Itk-deficient mast cells elicit little difficulty in producing a cellular response, while Btk-deficient mast cells have much difficulty doing the same in most experimental cases of hypersensitivity [223]. However, in the specific context of an acute allergic response in the lung, Mast cell degranulation in Itk−/− mice was significantly reduced, irrespective of late phase TH2-dependent inflammatory defects [215]. This is due to saturating levels of IgE occupancy of FcεRI in these mice, which may interfere with the degranulation response of newly secreted IgE [224]. NKT cells are a rare heterogenous subset of T cells that are important for the initiation and regulation of an immune response through the ability to immediately secrete large quantities of cytokines, particularly IFNγ and IL-4. Itk has recently been shown to be important for the development and the homeostasis of NKT cells [225, 226]. Interestingly, not only are NKT cell numbers reduced, but the continued homeostasis of these cells is dependent on Itk as NKT cells are diminished even further in Itk−/− mice as they age when compared to strain-matched control mice. Itk is involved in the production of both IFNγ and IL-4 in NKT cells [227], as Itk-deficient NKT cells fail to produce either cytokine thus making the cells functionally defective. This is an interesting finding because this is different from the TH2-skewed phenotype seen in T cells from Itk-deficient mice where only IL-4 production is affected. Lately it was found that although Itk is necessary for the development of αβ T cells, it was not dispensible for γδ T cells [228]. Itk may act as a negative regulator for these cells, as their numbers were increased with respect to wild-type mice, particularly within the γδ NKT subset. These cells are activated and provide T cell help as seen by elevated levels of IgE [228, 229].
7. Conclusions
Itk can be an ideal therapeutic target for the regulation of TH2 cell-mediated diseases. Since Itk is the Tec kinase primarily expressed in TH2 cells, the selective inhibition of its activity could affect TH2 cell function, including cytokine expression, without affecting immune responses against other infections, such as viral or TH1 cell-mediated pathogens. As such, Itk is a TCR proximal rheostat serving as a critical molecule that affects signaling and development in subtle ways, helping to evaluate the strength of the TCR signal in order to produce a T cell response that is appropriate for the presented situation.
Acknowledgments
This work was supported by Grants AI073636 and AI082321 to C. D. Tsoukas from the National Institutes of Health. J. A. Grasis was supported by a fellowship from the ARCS Foundation Inc. The authors wish to thank GloriaMay Machado for help with the artistry of the figures.
References
- 1.Huang Y, Wange RL. T cell receptor signaling: beyond complex complexes. The Journal of Biological Chemistry. 2004;279(28):28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- 2.Williams BL, Irvin BJ, Sutor SL, et al. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-γ1 and Ras activation. EMBO Journal. 1999;18(7):1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelosi M, Di Bartolo V, Mounier V, et al. Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. The Journal of Biological Chemistry. 1999;274(20):14229–14237. doi: 10.1074/jbc.274.20.14229. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92(1):83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 5.Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Science's STKE. 2000;2000(63, article re1) doi: 10.1126/stke.2000.63.re1. [DOI] [PubMed] [Google Scholar]
- 6.Ishiai M, Kurosaki M, Inabe K, Chan AC, Sugamura K, Kurosaki T. Involvement of LAT, Gads, and Grb2 in compartmentation of SLP-76 to the plasma membrane. Journal of Experimental Medicine. 2000;192(6):847–856. doi: 10.1084/jem.192.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nature Reviews Immunology. 2006;6(1):67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox HM, Berg LJ. Itk phosphorylation sites are required for functional activity in primary T cells. The Journal of Biological Chemistry. 2003;278(39):37112–37121. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 9.Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-γ1. The Journal of Biological Chemistry. 2007;282(5):2937–2946. doi: 10.1074/jbc.M606697200. [DOI] [PubMed] [Google Scholar]
- 10.Serrano CJ, Graham L, DeBell K, et al. A new tyrosine phosphorylation site in PLCγ1: the role of tyrosine 775 in immune receptor signaling. Journal of Immunology. 2005;174(10):6233–6237. doi: 10.4049/jimmunol.174.10.6233. [DOI] [PubMed] [Google Scholar]
- 11.Poulin B, Sekiya F, Rhee SG. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-γ1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4276–4281. doi: 10.1073/pnas.0409590102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annual Review of Immunology. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 13.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annual Review of Immunology. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 14.Altman A, Villalba M. Protein kinase C-θ (PKCθ): it’s all about location, location, location. Immunological Reviews. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 15.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Reviews Immunology. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 16.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Molecular and Cellular Biology. 2005;25(11):4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macián F, López-Rodríguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20(19):2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths EK, Penninger JM. ADAP-ting TCR signaling to integrins. Science's STKE. 2002;2002(127):p. RE3. doi: 10.1126/stke.2002.127.re3. [DOI] [PubMed] [Google Scholar]
- 19.Peterson EJ, Woods ML, Dmowski SA, et al. Coupling of the TCR to integrin activation by SLAP-130/Fyb. Science. 2001;293(5538):2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends in Cell Biology. 2008;18(10):486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annual Review of Immunology. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Fan J, Woodley DT. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001;20(44):6403–6417. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- 23.Zeng R, Cannon JL, Abraham RT, et al. SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site. Journal of Immunology. 2003;171(3):1360–1368. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 24.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404(6774):151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 25.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nature Reviews Immunology. 2005;5(4):284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 26.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annual Review of Immunology. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 27.Siliciano JD, Morrow TA, Desiderio SV. itk, A T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(23):11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heyeck SD, Berg LJ. Developmental regulation of a murine T-cell-specific tyrosine kinase gene, Tsk. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):669–673. doi: 10.1073/pnas.90.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada N, Kawakami Y, Kimura H, et al. Structure and expression of novel protein tyrosine kinases, Emb and Emt, in hematopoietic cells. Biochemical and Biophysical Research Communications. 1993;192(1):231–240. doi: 10.1006/bbrc.1993.1404. [DOI] [PubMed] [Google Scholar]
- 30.Gibson S, Leung B, Squire JA, et al. Identification, cloning, and characterization of a novel human T-cell- specific tyrosine kinase located at the hematopoietin complex on chromosome 5q. Blood. 1993;82(5):1561–1572. [PubMed] [Google Scholar]
- 31.Ohta Y, Haire RN, Amemiya CT, et al. Human Txk: genomic organization, structure and contiguous physical linkage with the Tec gene. Oncogene. 1996;12(4):937–942. [PubMed] [Google Scholar]
- 32.Tamagnone L, Lahtinen I, Mustonen T, et al. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9(12):3683–3688. [PubMed] [Google Scholar]
- 33.Smith CIE, Islam TC, Mattsson PT, Mohamed AJ, Nore BF, Vihinen M. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. BioEssays. 2001;23(5):436–446. doi: 10.1002/bies.1062. [DOI] [PubMed] [Google Scholar]
- 34.Mano H. The Tec family protein-tyrosine kinases: a subset of kinases for a subset of signalings. International Journal of Hematology. 1999;69(1):6–12. [PubMed] [Google Scholar]
- 35.Lucas JA, Miller AT, Atherly LO, Berg LJ. The role of Tec family kinases in T cell development and function. Immunological Reviews. 2003;191:119–138. doi: 10.1034/j.1600-065x.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 36.Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004;21(1):67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Molecular and Cellular Biology. 2004;24(6):2455–2466. doi: 10.1128/MCB.24.6.2455-2466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang WC, Collette Y, Nunès JA, Olive D. Tec kinases: a family with multiple roles in immunity. Immunity. 2000;12(4):373–382. doi: 10.1016/s1074-7613(00)80189-2. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Villar JJ, Kanner SB. Regulated association between the tyrosine kinase Emt/Itk/Tsk and phospholipase-Cγ1 in human T lymphocytes. Journal of Immunology. 1999;163(12):6435–6441. [PubMed] [Google Scholar]
- 40.Rebecchi MJ, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Annual Review of Biophysics and Biomolecular Structure. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- 41.Hyvönen M, Saraste M. Stucture of the PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia. EMBO Journal. 1997;16(12):3396–3404. doi: 10.1093/emboj/16.12.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debnath J, Chamorro M, Czar MJ, et al. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Molecular and Cellular Biology. 1999;19(2):1498–1507. doi: 10.1128/mcb.19.2.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ching KA, Kawakami Y, Kawakami T, Tsoukas CD. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. Journal of Immunology. 1999;163(11):6006–6013. [PubMed] [Google Scholar]
- 44.Shan X, Czar MJ, Bunnell SC, et al. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Molecular and Cellular Biology. 2000;20(18):6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.August A, Sadra A, Dupont BO, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the pleckstrin homology domain of inducible T cell kinase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(21):11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. The Journal of Biological Chemistry. 2001;276(19):16201–16206. doi: 10.1074/jbc.M100873200. [DOI] [PubMed] [Google Scholar]
- 47.Perez-Villar JJ, O’Day K, Hewgill DH, Nadler SG, Kanner SB. Nuclear localization of the tyrosine kinase ltk and interaction of its SH3 domain with karyopherin α (Rch1α) International Immunology. 2001;13(10):1265–1274. doi: 10.1093/intimm/13.10.1265. [DOI] [PubMed] [Google Scholar]
- 48.Kawakami Y, Hartman SE, Kinoshita E, et al. Terreic acid, a quinone epoxide inhibitor of Bruton’s tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2227–2232. doi: 10.1073/pnas.96.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang YH, Grasis JA, Miller AT, et al. Positive regulation of Itk PH domain function by soluble IP. Science. 2007;316(5826):886–889. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 50.Vihinen M, Nilsson L, Smith CI. Tec homology (TH) adjacent to the PH domain. FEBS Letters. 1994;350:263–265. doi: 10.1016/0014-5793(94)00783-7. [DOI] [PubMed] [Google Scholar]
- 51.Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. Regulatory intramolecular association in a tyrosine kinase of the tec family. Nature. 1997;385(6611):93–97. doi: 10.1038/385093a0. [DOI] [PubMed] [Google Scholar]
- 52.Hao S, August A. The proline rich region of the Tec homology domain of ITK regulates its activity. FEBS Letters. 2002;525(1–3):53–58. doi: 10.1016/s0014-5793(02)03066-1. [DOI] [PubMed] [Google Scholar]
- 53.Qi Q, August A. The Tec family kinase itk exists as a folded monomer in vivo. The Journal of Biological Chemistry. 2009;284(43):29882–29892. doi: 10.1074/jbc.M109.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor- initiated signaling cascade. The Journal of Biological Chemistry. 2000;275(3):2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 55.Ching KA, Grasis JA, Tailor P, Kawakami Y, Kawakami T, Tsoukas CD. TCR/CD3-induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. Journal of Immunology. 2000;165(1):256–262. doi: 10.4049/jimmunol.165.1.256. [DOI] [PubMed] [Google Scholar]
- 56.Nore BF, Mattsson PT, Antonsson P, et al. Identification of phosphorylation sites within the SH3 domains of Tec family tyrosine kinases. Biochimica et Biophysica Acta. 2003;1645(2):123–132. doi: 10.1016/s1570-9639(02)00524-1. [DOI] [PubMed] [Google Scholar]
- 57.Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ. Identification of Itk/Tsk Src homology 3 domain ligands. The Journal of Biological Chemistry. 1996;271(41):25646–25656. doi: 10.1074/jbc.271.41.25646. [DOI] [PubMed] [Google Scholar]
- 58.Grasis JA, Guimond DM, Cam NR, et al. In vivo significance of ITK-SLP-76 interaction in cytokine production. Molecular and Cellular Biology. 2010;30(14):3596–3609. doi: 10.1128/MCB.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends in Cell Biology. 2001;11(12):504–511. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 60.Pawson T. Dynamic control of signaling by modular adaptor proteins. Current Opinion in Cell Biology. 2007;19(2):112–116. doi: 10.1016/j.ceb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Su YW, Zhang Y, Schwelkert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. European Journal of Immunology. 1999;29(11):3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 62.Dombroski D, Houghtling RA, Labno CM, et al. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. Journal of Immunology. 2005;174(3):1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 63.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase ltk by the peptidyl-prolyl isomerase cyclophilin A. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):1899–1904. doi: 10.1073/pnas.042529199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mallis RJ, Brazin KN, Fulton DB, Andreotti AH. Structural characterization of a proline-driven conformational switch within the Itk SH2 domain. Nature Structural Biology. 2002;9(12):900–905. doi: 10.1038/nsb864. [DOI] [PubMed] [Google Scholar]
- 66.Colgan J, Asmal M, Neagu M, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21(2):189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. The Journal of Biological Chemistry. 1997;272(40):25401–25408. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 68.Brown K, Long JM, Vial SCM, et al. Crystal structures of interleukin-2 tyrosine kinase and their implications for the design of selective inhibitors. The Journal of Biological Chemistry. 2004;279(18):18727–18732. doi: 10.1074/jbc.M400031200. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins J, Marcy A. Characterization of Itk tyrosine kinase: contribution of noncatalytic domains to enzymatic activity. Protein Expression and Purification. 2001;22(2):211–219. doi: 10.1006/prep.2001.1447. [DOI] [PubMed] [Google Scholar]
- 70.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307(5708):430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 71.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochemical and Biophysical Research Communications. 2006;351(2):571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 72.Sacristán C, Schattgen SA, Berg LJ, Bunnell SC, Roy AL, Rosenstein Y. Characterization of a novel interaction between transcription factor TFII-I and the inducible tyrosine kinase in T cells. European Journal of Immunology. 2009;39(9):2584–2595. doi: 10.1002/eji.200839031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brazin KN, Fulton DB, Andreotti AH. A specific intermolecular association between the regulatory domains of a Tec family kinase. Journal of Molecular Biology. 2000;302(3):607–623. doi: 10.1006/jmbi.2000.4091. [DOI] [PubMed] [Google Scholar]
- 74.Min L, Wu W, Joseph RE, Fulton DB, Berg L, Andreotti AH. Disrupting the intermolecular self-association of Itk enhances T cell signaling. Journal of Immunology. 2010;184(8):4228–4235. doi: 10.4049/jimmunol.0901908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morrogh LM, Hinshelwood S, Costello P, Cory GOC, Kinnon C. The SH3 domain of Bruton’s tyrosine kinase displays altered ligand binding properties when auto-phosphorylated in vitro. European Journal of Immunology. 1999;29(7):2269–2279. doi: 10.1002/(SICI)1521-4141(199907)29:07<2269::AID-IMMU2269>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 76.Gibson S, August A, Branch D, Dupont B, Mills GB. Functional LCK is required for optimal CD28-mediated activation of the TEC family tyrosine kinase EMT/ITK. The Journal of Biological Chemistry. 1996;271(12):7079–7083. doi: 10.1074/jbc.271.12.7079. [DOI] [PubMed] [Google Scholar]
- 77.August A, Gibson S, Kawakami Y, Kawakami T, Mills GB, Dupont B. CD28 is associated with and induces the immediate tyrosine phosphorylation and activation of the Tec family kinase ITK/EMT in the human Jurkat leukemic T-cell line. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(20):9347–9351. doi: 10.1073/pnas.91.20.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raab M, Cai YC, Bunnell SC, Heyeck SD, Berg LJ, Rudd CE. p56(Lck) and p59(Fyn) regulate CD28 binding to phosphatidylinositol 3- kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(19):8891–8895. doi: 10.1073/pnas.92.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marengère LEM, Okkenhaug K, Clavreul A, et al. The SH3 domain of Itk/Emt binds to proline-rich sequences in the cytoplasmic domain of the T cell costimulatory receptor CD28. Journal of Immunology. 1997;159(7):3220–3229. [PubMed] [Google Scholar]
- 80.Gibson S, Truitt K, Lu Y, et al. Efficient CD28 signalling leads to increases in the kinase activities of the TEC family tyrosine kinase EMT/ITK/TSK and the SRC family tyrosine kinase LCK. Biochemical Journal. 1998;330(3):1123–1128. doi: 10.1042/bj3301123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.King PD, Sadra A, Teng JMC, et al. Analysis of CD28 cytoplasmic tail tyrosine residues as regulators and substrates for the protein tyrosine kinases, EMT and LCK. Journal of Immunology. 1997;158(2):580–590. [PubMed] [Google Scholar]
- 82.Michel F, Attal-Bonnefoy G, Mangino G, Mise-Omata S, Acuto O. CD28 as a molecular amplifier extending TCR ligation and signaling capabilities. Immunity. 2001;15(6):935–945. doi: 10.1016/s1074-7613(01)00244-8. [DOI] [PubMed] [Google Scholar]
- 83.Liao XC, Fournier S, Killeen N, Weiss A, Allison JP, Littman DR. Itk negatively regulates induction of T cell proliferation by CD28 costimulation. Journal of Experimental Medicine. 1997;186(2):221–228. doi: 10.1084/jem.186.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li CR, Berg LJ. Cutting edge: Itk is not essential for CD28 signaling in naive T cells. Journal of Immunology. 2005;174(8):4475–4479. doi: 10.4049/jimmunol.174.8.4475. [DOI] [PubMed] [Google Scholar]
- 85.King PD, Sadra A, Han A, et al. CD2 signaling in T cells involves tyrosine phosphorylation and activation of the Tec family kinase, EMT/ITK/TSK. International Immunology. 1996;8(11):1707–1714. doi: 10.1093/intimm/8.11.1707. [DOI] [PubMed] [Google Scholar]
- 86.King PD, Sadra A, Teng JMC, Bell GM, Dupont B. CD2-mediated activation of the Tec-family tyrosine kinase ITK is controlled by proline-rich stretch-4 of the CD2 cytoplasmic tail. International Immunology. 1998;10(7):1009–1016. doi: 10.1093/intimm/10.7.1009. [DOI] [PubMed] [Google Scholar]
- 87.Langhans-Rajasekaran SA, Wan Y, Huang XY. Activation of Tsk and Btk tyrosine kinases by G protein βγ subunits. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(19):8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takesono A, Horai R, Mandai M, Dombroski D, Schwartzberg PL. Requirement for Tec kinases in chemokine-induced migration and activation of Cdc42 and Rac. Current Biology. 2004;14(10):917–922. doi: 10.1016/j.cub.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Fischer AM, Mercer JC, Iyer A, Ragin MJ, August A. Regulation of CXC chemokine receptor 4-mediated migration by the Tec family tyrosine kinase ITK. The Journal of Biological Chemistry. 2004;279(28):29816–29820. doi: 10.1074/jbc.M312848200. [DOI] [PubMed] [Google Scholar]
- 90.Berge T, Sundvold-Gjerstad V, Granum S, et al. T cell specific adapter protein (TSAd) interacts with Tec kinase ITK to promote CXCL12 induced migration of human and murine T cells. PLoS One. 2010;5(3, article e9761) doi: 10.1371/journal.pone.0009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin TA, McIntyre KW, Das J, et al. Selective Itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry. 2004;43(34):11056–11062. doi: 10.1021/bi049428r. [DOI] [PubMed] [Google Scholar]
- 92.Schaeffer EM, Debnath J, Yap G, et al. Requirement for Tec kinases Rlk and ltk in T cell receptor signaling and immunity. Science. 1999;284(5414):638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 93.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. Journal of Experimental Medicine. 1998;187(10):1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295(5559):1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 95.Dower NA, Stang SL, Bottorff DA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature Immunology. 2000;1(4):317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 96.Ebinu JO, Stang SL, Teixeira C, et al. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95(10):3199–3203. [PubMed] [Google Scholar]
- 97.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca channel function. Journal of Cell Biology. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lioudyno MI, Kozak JA, Penna A, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 100.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 101.Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca channel activation and altered K channel gating in T cells from immunodeficient patients. Journal of Experimental Medicine. 2005;202(5):651–662. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guarnieri DJ, Dodson GS, Simon MA. SRC64 regulates the localization of a Tec-family kinase required for Drosophila ring canal growth. Molecular Cell. 1998;1(6):831–840. doi: 10.1016/s1097-2765(00)80082-9. [DOI] [PubMed] [Google Scholar]
- 103.Roulier EM, Panzer S, Beckendorf SK. The Tec29 tyrosine kinase is required during Drosophila embryogenesis and interacts with Src64 in ring canal development. Molecular Cell. 1998;1(6):819–829. doi: 10.1016/s1097-2765(00)80081-7. [DOI] [PubMed] [Google Scholar]
- 104.Grasis JA, Browne CD, Tsoukas CD. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. Journal of Immunology. 2003;170(8):3971–3976. doi: 10.4049/jimmunol.170.8.3971. [DOI] [PubMed] [Google Scholar]
- 105.Labno CM, Lewis CM, You D, et al. Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP. Current Biology. 2003;13(18):1619–1624. doi: 10.1016/j.cub.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsoukas CD, Grasis JA, Browne CD, Ching KA. Inducible T cell tyrosine kinase (ITK): structural requirements and actin polymerization. Advances in Experimental Medicine and Biology. 2006;584:29–41. doi: 10.1007/0-387-34132-3_3. [DOI] [PubMed] [Google Scholar]
- 107.Fischer KD, Kong YY, Nishina H, et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Current Biology. 1998;8(10):554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 108.Holsinger LJ, Graef IA, Swat W, et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Current Biology. 1998;8(10):563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 109.Tsoukas CD, Grasis JA, Ching KA, Kawakami Y, Kawakami T. Itk/Emt: a link between T cell antigen receptor-mediated Ca events and cytoskeletal reorganization. Trends in Immunology. 2001;22(1):17–20. doi: 10.1016/s1471-4906(00)01795-6. [DOI] [PubMed] [Google Scholar]
- 110.Miletic AV, Sakata-Sogawa K, Hiroshima M, et al. Vav1 acidic region tyrosine 174 is required for the formation of T cell receptor-induced microclusters and is essential in T cell development and activation. The Journal of Biological Chemistry. 2006;281(50):38257–38265. doi: 10.1074/jbc.M608913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reynolds LF, Smyth LA, Norton T, et al. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. Journal of Experimental Medicine. 2002;195(9):1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raberger J, Boucheron N, Sakaguchi S, Penninger JM, Ellmeier W. Impaired T-cell development in the absence of Vav1 and Itk. European Journal of Immunology. 2008;38(12):3530–3542. doi: 10.1002/eji.200838388. [DOI] [PubMed] [Google Scholar]
- 113.Ochs HD, Notarangelo LD. Structure and function of the Wiskott-Aldrich syndrome protein. Current Opinion in Hematology. 2005;12(4):284–291. doi: 10.1097/01.moh.0000168520.98990.19. [DOI] [PubMed] [Google Scholar]
- 114.Thrasher AJ. WASp in immune-system organization and function. Nature Reviews Immunology. 2002;2(9):635–646. doi: 10.1038/nri884. [DOI] [PubMed] [Google Scholar]
- 115.Snapper SB, Rosen FS. The Wiskott-Aldrich Syndrome Protein (WASP): roles in signaling and cytoskeletal organization. Annual Review of Immunology. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- 116.Marchand JB, Kaiser DA, Pollard TD, Higgs HN. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nature Cell Biology. 2001;3(1):76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- 117.Higgs HN, Pollard TD. Regulation of actin filament network formation through Arp2/3 complex: activation by a diverse array of proteins. Annual Review of Biochemistry. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 118.Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich Syndrome Protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. Journal of Experimental Medicine. 2004;199(1):99–111. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cory GOC, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. The Journal of Biological Chemistry. 2002;277(47):45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 120.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Molecular Cell. 2003;11(5):1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 121.López-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR. Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Molecular and Cellular Biology. 2000;20(5):1678–1691. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yao L, Janmey P, Frigeri LG, et al. Pleckstrin homology domains interact with filamentous actin. The Journal of Biological Chemistry. 1999;274(28):19752–19761. doi: 10.1074/jbc.274.28.19752. [DOI] [PubMed] [Google Scholar]
- 123.Guinamard R, Aspenström P, Fougereau M, Chavrier P, Guillemot JC. Tyrosine phosphorylation of the Wiskott-Aldrich Syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Letters. 1998;434(3):431–436. doi: 10.1016/s0014-5793(98)01016-3. [DOI] [PubMed] [Google Scholar]
- 124.Baba Y, Nonoyama S, Matsushita M, et al. Involvement of Wiskott-Aldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood. 1999;93(6):2003–2012. [PubMed] [Google Scholar]
- 125.Woodring PJ, Litwack ED, O’Leary DDM, Lucero GR, Wang JYJ, Hunter T. Modulation of the F-actin cytoskeleton by c-Abl tyrosine kinase in cell spreading and neurite extension. Journal of Cell Biology. 2002;156(5):879–892. doi: 10.1083/jcb.200110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Singer A. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Current Opinion in Immunology. 2002;14(2):207–215. doi: 10.1016/s0952-7915(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 127.Liu SKW, Berry DM, McGlade CJ. The role of Gads in hematopoietic cell signalling. Oncogene. 2001;20(44):6284–6290. doi: 10.1038/sj.onc.1204771. [DOI] [PubMed] [Google Scholar]
- 128.Shan X, Wange RL. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. The Journal of Biological Chemistry. 1999;274(41):29323–29330. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 129.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochemical Journal. 2001;356(2):461–471. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajagopal K, Sommers CL, Decker DC, et al. RIBP, a novel Rlk/Txk- and Itk-binding adaptor protein that regulates T cell activation. Journal of Experimental Medicine. 1999;190(11):1657–1668. doi: 10.1084/jem.190.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perchonock CE, Pajerowski AG, Nguyen C, Shapiro MJ, Shapiro VS. The related adaptors, adaptor in lymphocytes of unknown function X and Rlk/Itk-binding protein, have nonredundant functions in lymphocytes. Journal of Immunology. 2007;179(3):1768–1775. doi: 10.4049/jimmunol.179.3.1768. [DOI] [PubMed] [Google Scholar]
- 132.Jordan MS, Smith JE, Burns JC, et al. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28(3):359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of β1 integrins by the T-cell receptor. EMBO Journal. 2001;20(6):1232–1244. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Finkelstein LD, Shimizu Y, Schwartzberg PL. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. Journal of Immunology. 2005;175(9):5923–5930. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- 135.Fowell DJ, Shinkai K, Liao XC, et al. Impaired NFATc translocation and failure of Th2 development in Itk- deficient CD4+ T cells. Immunity. 1999;11(4):399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 136.Feske S. Calcium signalling in lymphocyte activation and disease. Nature Reviews Immunology. 2007;7(9):690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 137.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nature Immunology. 2001;2(4):316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 138.Schaeffer EM, Yap GS, Lewis CM, et al. Mutation of Tec family kinases alters T helper cell differentiation. Nature Immunology. 2001;2(12):1183–1188. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 139.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 140.Huck K, Feyen O, Niehues T, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. Journal of Clinical Investigation. 2009;119(5):1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Molina TJ, Kishihara K, Siderovski DP, et al. Profound block in thymocyte development in mice lacking p56(lck) Nature. 1992;357(6374):161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 142.Negishi I, Motoyama N, Nakayama KI, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376(6539):435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 143.Wiest DL, Ashe JM, Howcroft TK, et al. A spontaneously arising mutation in the DLAARN motif of murine ZAP-70 abrogates kinase activity and arrests thymocyte development. Immunity. 1997;6(6):663–671. doi: 10.1016/s1074-7613(00)80442-2. [DOI] [PubMed] [Google Scholar]
- 144.Ellmeier W, Jung S, Sunshine MJ, et al. Severe B cell deficiency in mice lacking the Tec kinase family members Tec and Btk. Journal of Experimental Medicine. 2000;192(11):1611–1623. doi: 10.1084/jem.192.11.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sommers CL, Park CS, Lee J, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296(5575):2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 146.Aguado E, Richelme S, Nuñez-Cruz S, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296(5575):2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 147.Zhang W, Sommers CL, Burshtyn DN, et al. Essential role of LAT in T cell development. Immunity. 1999;10(3):323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 148.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, Geha RS. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94(2):229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 149.Clements JL, Yang B, Ross-Barta SE, et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281(5375):416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 150.Aifantis I, Gounari F, Scorrano L, Borowski C, Von Boehmer H. Constitutive pre-TCR signaling promotes differentiation through Ca mobilization and activation of NF-κB and NFAT. Nature Immunology. 2001;2(5):403–409. doi: 10.1038/87704. [DOI] [PubMed] [Google Scholar]
- 151.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskoff-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9(1):81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 152.Cotta-de-Almeida V, Westerberg L, Maillard MH, et al. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(39):15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun Z, Arendt CW, Ellmeier W, et al. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature. 2000;404(6776):402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 154.Pfeifhofer C, Kofler K, Gruber T, et al. Protein kinase Cθ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. Journal of Experimental Medicine. 2003;197(11):1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase Cθ . Journal of Immunology. 2004;173(10):6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 156.Marsland BJ, Soos TJ, Späth G, Littman DR, Kopf M. Protein kinase C θ is critical for the development of in vivo T helper (Th)2 cell but not Th-1 cell responses. Journal of Experimental Medicine. 2004;200(2):181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sakowicz-Burkiewicz M, Nishanth G, Helmuth U, et al. Protein kinase C-θ critically regulates the proliferation and survival of pathogen-specific T cells in murine listeriosis. Journal of Immunology. 2008;180(8):5601–5612. doi: 10.4049/jimmunol.180.8.5601. [DOI] [PubMed] [Google Scholar]
- 158.Krawczyk C, Oliveira-dos-Santos A, Sasaki T, et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16(3):331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- 159.Wülfing C, Bauch A, Crabtree GR, Davis MM. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(18):10150–10155. doi: 10.1073/pnas.97.18.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Costello PS, Walters AE, Mee PJ, et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang R, Alt FW, Davidson L, Orkin SH, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374(6521):470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 162.Tarakhovsky A, Turner M, Schaal S, et al. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374(6521):467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 163.Wen BG, Pletcher MT, Warashina M, et al. Inositol (1,4,5) trisphosphate 3 kinase B controls positive selection of T cells and modulates Erk activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5604–5609. doi: 10.1073/pnas.0306907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pouillon V, Hascakova-Bartova R, Pajak B, et al. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nature Immunology. 2003;4(11):1136–1143. doi: 10.1038/ni980. [DOI] [PubMed] [Google Scholar]
- 165.Yoder J, Pham C, Iizuka YM, et al. Requirement for the SLP-76 adaptor GADS in T cell development. Science. 2001;291(5510):1987–1991. doi: 10.1126/science.1057176. [DOI] [PubMed] [Google Scholar]
- 166.Colgan J, Asmal M, Yu B, Luban J. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. Journal of Immunology. 2005;174(10):6030–6038. doi: 10.4049/jimmunol.174.10.6030. [DOI] [PubMed] [Google Scholar]
- 167.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3(6):757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 168.Germain RN. T-cell development and the CD4—CD8 lineage decision. Nature Reviews Immunology. 2002;2(5):309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 169.Lucas JA, Felices M, Evans JW, Berg LJ. Subtle defects in pre-TCR signaling in the absence of the Tec kinase Itk. Journal of Immunology. 2007;179(11):7561–7567. doi: 10.4049/jimmunol.179.11.7561. [DOI] [PubMed] [Google Scholar]
- 170.Lucas JA, Atherly LO, Berg LJ. The absence of Itk inhibits positive selection without changing lineage commitment. Journal of Immunology. 2002;168(12):6142–6151. doi: 10.4049/jimmunol.168.12.6142. [DOI] [PubMed] [Google Scholar]
- 171.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. Journal of Experimental Medicine. 2000;192(7):987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annual Review of Immunology. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 173.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7(5):609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 174.Fischer AM, Katayama CD, Pagès G, Pouysségur J, Hedrick SM. The role of Erk1 and Erk2 in multiple stages of T cell development. Immunity. 2005;23(4):431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 175.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9(4):565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 176.Broussard C, Fleischacker C, Horai R, et al. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25(5):93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 177.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25(1):79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 178.Dubois S, Waldmann TA, Müller JR. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(32):12075–12080. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature Immunology. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 180.Horai R, Mueller KL, Handon RA, et al. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27(5):775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23(4):419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Au-Yeung BB, Katzman SD, Fowell DJ. Cutting edge: Itk-dependent signals required for CD4+ T cells to exert, but not gain, Th2 effector function. Journal of Immunology. 2006;176(7):3895–3899. doi: 10.4049/jimmunol.176.7.3895. [DOI] [PubMed] [Google Scholar]
- 183.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. Journal of Experimental Medicine. 2005;202(6):793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cote-Sierra J, Foucras G, Guo L, et al. Interleukin 2 plays a central role in Th2 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(11):3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Szabo SJ, Sullivan BM, Sternmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in Th1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science. 2002;295(5553):338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 186.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T1-T2 responses. Nature Immunology. 2004;5(11):1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 187.Lee HJ, Takemoto N, Kurata H, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. Journal of Experimental Medicine. 2000;192(1):105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 189.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mathur AN, Chang HC, Zisoulis DG, et al. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108(5):1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gocke AR, Cravens PD, Ben LH, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. Journal of Immunology. 2007;178(3):1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 193.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1β-mediated CXCL-8 release from human airway smooth muscle cells. American Journal of Physiology. 2007;292(4):L1023–L1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 194.Gomez-Rodriguez J, Sahu N, Handon R, et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31(4):587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ishigame H, Kakuta S, Nagai T, et al. Differential Roles of Interleukin-17A and -17F in Host Defense against Mucoepithelial Bacterial Infection and Allergic Responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 196.O’Connor W, Jr., Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature Immunology. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector T17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 198.Adriani M, Aoki J, Horai R, et al. Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clinical Immunology. 2007;124(1):41–48. doi: 10.1016/j.clim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maillard MH, Cotta-de-Almeida V, Takeshima F, et al. The Wiskott-Aldrich syndrome protein is required for the function of CD4+CD25+Foxp3+ regulatory T cells. Journal of Experimental Medicine. 2007;204(2):381–391. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marangoni F, Trifari S, Scaramuzza S, et al. WASP regulates suppressor activity of human and murine CD4+CD25+FOXP3+ natural regulatory T cells. Journal of Experimental Medicine. 2007;204(2):369–380. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Humblet-Baron S, Sather B, Anover S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. Journal of Clinical Investigation. 2007;117(2):407–418. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Miller AT, Berg LJ. Defective Fas ligand expression and activation-induced cell death in the absence of IL-2-inducible T cell kinase. Journal of Immunology. 2002;168(5):2163–2172. doi: 10.4049/jimmunol.168.5.2163. [DOI] [PubMed] [Google Scholar]
- 203.Mueller C, August A. Attenuation of immunological symptoms of allergic asthma in mice lacking the tyrosine kinase ITK. Journal of Immunology. 2003;170(10):5056–5063. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 204.Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9(5):627–635. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- 205.Bachmann MF, Littman DR, Charlene Liao X. Antiviral immune responses in Itk-deficient mice. Journal of Virology. 1997;71(10):7253–7257. doi: 10.1128/jvi.71.10.7253-7257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Readinger JA, Schiralli GM, Jiang JK, et al. Selective targeting of ITK blocks multiple steps of HIV replication. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(18):6684–6689. doi: 10.1073/pnas.0709659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annual Review of Immunology. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 208.Morokata T, Ishikawa J, Ida K, Yamada T. C57BL/6 mice are more susceptible to antigen-induced pulmonary eosinophilia than BALB/c mice, irrespective of systemic T helper 1/T helper 2 responses. Immunology. 1999;98(3):345–351. doi: 10.1046/j.1365-2567.1999.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15(2):303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 210.Matsumoto Y, Oshida T, Obayashi I, et al. Identification of highly expressed genes in peripheral blood T cells from patients with atopic dermatitis. International archives of allergy and immunology. 2002;129(4):327–340. doi: 10.1159/000067589. [DOI] [PubMed] [Google Scholar]
- 211.Graves PE, Siroux V, Guerra S, Klimecki WT, Martinez FD. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain - IL-2-inducible T-cell kinase gene cluster in chromosome 5q33. Journal of Allergy and Clinical Immunology. 2005;116(3):650–656. doi: 10.1016/j.jaci.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 212.Wong WSF, Leong KP. Tyrosine kinase inhibitors: a new approach for asthma. Biochimica et Biophysica Acta. 2004;1697(1-2):53–69. doi: 10.1016/j.bbapap.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 213.Barnes PJ. Cytokine modulators as novel therapies for asthma. Annual Review of Pharmacology and Toxicology. 2002;42:81–98. doi: 10.1146/annurev.pharmtox.42.111901.111143. [DOI] [PubMed] [Google Scholar]
- 214.Ferrara TJ, Mueller C, Sahu N, Ben-Jebria A, August A. Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase. Journal of Allergy and Clinical Immunology. 2006;117(4):780–786. doi: 10.1016/j.jaci.2005.12.1330. [DOI] [PubMed] [Google Scholar]
- 215.Forssell J, Sideras P, Eriksson C, et al. Interleukin-2-inducible T cell kinase regulates mast cell degranulation and acute allergic responses. American Journal of Respiratory Cell and Molecular Biology. 2005;32(6):511–520. doi: 10.1165/rcmb.2004-0348OC. [DOI] [PubMed] [Google Scholar]
- 216.Finotto S, Neurath MF, Glickman JN, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295(5553):336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 217.Sahu N, Mueller C, Fischer A, August A. Differential sensitivity to Itk kinase signals for T helper 2 cytokine production and chemokine-mediated migration. Journal of Immunology. 2008;180(6):3833–3838. doi: 10.4049/jimmunol.180.6.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28(2):197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28(2):218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 220.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. The Journal of Biological Chemistry. 2001;276(31):29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 221.Fischer KD, Zmuidzinas A, Gardner S, Barbacid M, Bernstein A, Guidos C. Defective T-cell receptor signalling and positive selection of Vav-deficient CD4+ CD8+ thymocytes. Nature. 1995;374(6521):474–477. doi: 10.1038/374474a0. [DOI] [PubMed] [Google Scholar]
- 222.Khurana D, Arneson LN, Schoon RA, Dick CJ, Leibson PJ. Differential regulation of human NK cell-mediated cytotoxicity by the tyrosine kinase Itk. Journal of Immunology. 2007;178(6):3575–3582. doi: 10.4049/jimmunol.178.6.3575. [DOI] [PubMed] [Google Scholar]
- 223.Felices M, Falk M, Kosaka Y, Berg LJ. Tec kinases in T cell and mast cell signaling. Advances in Immunology. 2007;93:145–184. doi: 10.1016/S0065-2776(06)93004-1. [DOI] [PubMed] [Google Scholar]
- 224.Iyer AS, August A. The tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses. Journal of Immunology. 2008;180(12):7869–7877. doi: 10.4049/jimmunol.180.12.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. Journal of Immunology. 2002;169(5):2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 226.Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. Journal of Immunology. 2008;180(5):3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 227.Au-Yeung BB, Fowell DJ. A key role for Itk in both IFN gamma and IL-4 production by NKT cells. Journal of Immunology. 2007;179(1):111–119. doi: 10.4049/jimmunol.179.1.111. [DOI] [PubMed] [Google Scholar]
- 228.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(20):8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Qi A, Xia M, Hu J, et al. Enhanced development of CD4+ γδ T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114(3):564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]