Abstract
The Rab11 Family Interacting Proteins (Rab11-FIPs) are hypothesized to regulate sequential steps in the apical recycling and transcytotic pathways of polarized epithelial cells. Previous studies have suggested that Rab11-FIP proteins assemble into multi-protein complexes regulating plasma membrane recycling. Rab11-FIP2 interacts with both myosin Vb and Rab11. Recent investigations have noted that that Rab11-FIP2 mutants [Rab11-FIP2(129–512), also designated Rab11-FIP2(ΔC2) and Rab11-FIP2(S229A, R413G), also designated Rab11-FIP2(SARG)], are potent inhibitors of transcytosis in polarized MDCK cells. Interestingly, Rab11-FIP2(ΔC2), but not Rab11-FIP2(SARG), also altered the morphology of the EEA-1 positive early endosomal compartment. These findings suggested that Rab11-FIP2 mutants could differentiate different points along the recycling pathway. We therefore sought to investigate whether Rab11-FIP2 is a general regulator of the early endosomal system. Both Rab11-FIP2 mutants altered the localization and co-localized with dynein heavy chain. In contrast, both clathrin heavy chain and AP-1 accumulated with membranes containing Rab11-FIP2(SARG), but not with Rab11-FIP2(ΔC2). Expression of Rab11-FIP2(ΔC2), but not Rab11-FIP2(SARG), caused clustering of early endosomal markers Rab5b, Epsin 4 and IQGAP1, around a collapsed Rab11-FIP2 containing membranous cisternum. Interestingly, neither Rab11-FIP2 mutant had any effect on the distribution of Rab5a, a classical early endosome marker. The results support the view that Rab11-FIP2 may influence microtubule-dependent centripetal movement of subsets of early endosomes as well as processing through the common and recycling endosomal systems.
Key words: Rab11-FIP2, Rab11, trafficking, apical recycling, endosome, MDCK cells, clathrin, dynein, Rab5, epsin
Introduction
The internalization of proteins from the plasma membranes of polarized cells and their recycling to appropriate cell surfaces represents a dynamic process requiring complex coordination of both sorting and trafficking decisions. A preponderance of recent data suggests that these complex sorting decisions are made within a dynamic system of tubulovesicular endosomal membranes that span a spectrum of discernible compartments from early endosomes to apical recycling system membranes. Over the past ten years, a number of studies have led to the recognition that Rab11 family members regulate the plasma membrane recycling system. Rab11a containing vesicles are concentrated in the apical regions of epithelial cells.1 The polarized cell utilizes an elaborate endosomal structure involving progression through an early sorting endosome, recycling endosome and apical recycling endosome (ARE).2 Rab11a containing tubular membranes localize to the ARE near the centrosome beneath the apical membrane. The integrity and function of the ARE is dependent upon intact microtubules; and destabilization of the microtubule cytoskeleton causes Rab11 positive vesicles to disperse, while stabilization causes aggregation of vesicles near tight junctions.3
Rab11 family members interact with a group of interacting proteins (Rab11-FIPs): Rab11-FIP1, Rab11-FIP2, Rab11-FIP3,4 Rab11-FIP4,5 Rab11-FIP5/pp75/Rip11,6 and Rab11-FIP1C/RCP.7 They each interact with Rab11 at their carboxyl-termini through predicted coiled-coil regions containing an amphipathic a helical Rab11 Binding Domain (RBD).4,8 Rab11a also interacts with the tail region of myosin Vb, which is essential for exit from the plasma membrane apical recycling system.9 The members of the Myosin V Family are unconventional myosins, which function in subcellular localization of organelles and intracellular transport.10 Thus, myosin Vb plays an essential role in Rab11a vesicle trafficking. Rab11-FIP2 also interacts with myosin Vb,11 suggesting that the formation of a ternary complex between Rab11a, Rab11-FIP2 and myosin Vb is a critical regulatory complex in plasma membrane recycling.
While an association of Rab11-FIP2 with recycling system membranes is well-established, other studies indicate that Rab11-FIP2 also serves functions outside the recycling membranes. Thus, Rab11-FIP2 suppresses EGFR uptake and binds α-adaptin, which associates with clathrin coated pits.12 Rab11-FIP2 also contains three NPF motifs that can act as binding domains for Eps15 and EHD proteins.13 These findings suggest that Rab11-FIP2 plays a critical role in early as well as recycling endosome processing. We have recently reported the characterization of two dominant negative Rab11-FIP2 mutants, Rab11-FIP2(129–512), designated Rab11-FIP2(ΔC2) and Rab11-FIP2(S229A, R413G), designated Rab11-FIP2(SARG).14 These mutants could still interact with Rab11 family members (Rab11a, Rab11b and Rab25), but they did not demonstrate direct association with other endosomal Rab proteins, including Rab4, Rab5, Rab7 or Rab8a. The two mutants allowed us to identify separable aspects of the roles of Rab11-FIP2 in polarized MDCK cells. Both mutants caused a collapse of Rab11a-containing membranes into tubular cisternae and were potent inhibitors of basolateral to apical transcytosis. However, Rab11-FIP2(ΔC2), which lacks its amino terminal C2 domain, also caused an aggregation of the EEA1 positive early endosomal system, while Rab11-FIP2(SARG) expression did not alter the distribution of early endosomes. These findings suggested that Rab11-FIP2 mutants were acting at different levels within the plasma membrane recycling system. Thus, Rab11-FIP2 may be a multifunctional regulator of trafficking along the entire endosomal system.
We have now examined the influence of Rab11-FIP2 on a range of putative regulators of the early and recycling endosomal system. This comparison has revealed that Rab11-FIP2(ΔC2) can alter most early endosome components with the exception of Rab5a. In addition, we found that several panendosome proteins, including clathrin heavy chain and dynein heavy chain accumulate with both overexpressed Rab11-FIP2 mutants. The results support the concept that Rab11-FIP2 has multiple regulatory roles throughout the endosomal trafficking pathways.
Results
Effects of Rab11-FIP2 mutants on the microtubule motor, dynein.
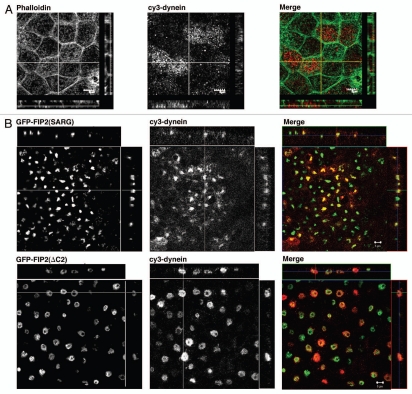
Movement along microtubules is essential for the function of the endocytic pathway (reviewed in ref. 15) and early endosomes move towards the centrosome after formation. Previous work has shown that Rab11-FIP5 associates with kinesin16 and recent work has shown that kinesin and dynein often work in concert.17 McCaffrey and colleagues have also recently reported that Rab11-FIP3 interacts with dynein intermediate chains.18,19 The location of Rab11-FIP2 mutants within the collapsed recycling cisternae near the centrosomes suggested that centripetal dynein motor activity might be concentrating components in a perinuclear membrane nexus. We therefore sought to evaluate whether the Rab11-FIP2 mutants could also alter dynein distribution. In non-transfected cells, dynein heavy chain staining was distributed throughout the cytosol (Fig. 1A). As previously described, EGFP-FIP2(ΔC2) localized to a doughnut shaped membrane cisternae, while EGP-FIP2(SARG) localized to a more collapsed membranous cisternum (Fig. 1B). Interestingly, Figure 1 demonstrates that both Rab11-FIP2 mutants caused prominent accumulation of dynein in the Rab11-FIP2-containing collapsed tubular cisternae. These results indicate that dynein heavy chain is associated with movement of vesicles into the Rab11-FIP2-containing recycling system.
Figure 1.
Rab11-FIP2 mutants alter the localization of the microtubule motor protein, dynein heavy chain. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). (A) Parental T23 MDCK cells were stained with Alexa-488-phalloidin (pseudo-colored green) and antibodies against dynein (pseudo-colored red) and imaged by confocal microscopy. Dynein was observed in association in vesicles at the periphery of the cells, especially at the lateral borders. (B) T23 MDCK cells stably expressing either EGFP-Rab11-FIP2(SARG) or EGFP-Rab11-FIP2(ΔC2) were stained with antibodies against dynein and imaged by confocal microscopy. Expression of both Rab11-FIP2 mutants caused accumulation of dynein within the collapsed membrane cisternae. Images were taken with a 100x lens. Scale bars represent 5 µm.
Effects of Rab11-FIP2 mutants on coat proteins.
The endosomal system is classically associated with the coating and uncoating of vesicles during the dynamic steps in endocytic process. Thus, we have examined two of these coat proteins, clathrin heavy chain and adaptor-related protein complex 1 (AP-1), for association with Rab11-FIP2-containing membranes. Clathrin is associated with membrane trafficking steps throughout the cell. In non-transfected T23 MDCK cells, clathrin was distributed throughout the cell in small punctate vesicles (Fig. 2A). Rab11-FIP2(ΔC2) expression elicited the accumulation of clathrin heavy chain containing vesicles around EGFP-containing collapsed membranous structures, and some limited co-localization was observed directly with the Rab11-FIP2-containing membranes (Fig. 2B). More prominent co-localization, however, was observed with Rab11-FIP2(SARG)-containing membranes. These findings are consistent with our previous findings where coated tubular extensions were observed by electron microscopy in the collapsed cisternae especially in MDCK cells expressing Rab11-FIP2(SARG).14
Figure 2.
Rab11-FIP2 mutants alter the distribution of clathrin heavy chain. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). (A) Parental T23 MDCK cells were stained with Alexa-488-phalloidin (pseudo-colored green) to show the cell boundaries and clathrin heavy chain (pseudo-colored red) and imaged by confocal microscopy. Punctate clathrin staining was observed on vesicles throughout the cytoplasm. (B) T23 MDCK cells stably expressing EGFP-Rab11-FIP2(SARG) and EGFP-Rab11-FIP2(ΔC2) were stained for endogenous clathrin heavy chain (pseudo-colored red) and imaged by confocal microscopy. Clathrin heavy chain staining was accumulated with the EGFP-Rab11-FIP2(SARG) mutant in its collapsed cisternum. In EGFP-FIP2(ΔC2)-expressing cells, clathrin staining was observed predominantly as vesicles surrounding the Rab11-FIP2 containing membranes. Images were taken with a 100x lens with a 3x zoom. Scale bars represent 5 µm.
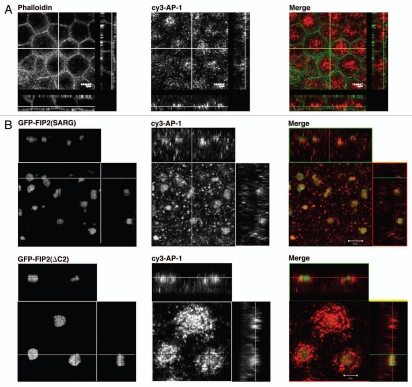
AP-1 is also associated with multiple trafficking pathways within cells in association with clathrin and we have previously reported the presence of AP-1 on recycling tubulovesicles of gastric parietal cells.20,21 In non-transfected MDCK cells, AP-1 was distributed in punctate structures in the subapical region of the cells (Fig. 3). In cells expressing Rab11-FIP2(ΔC2), we observed a further accumulation of AP-1-labeled vesicle elements in this region clustered around the EGFP-Rab11-FIP2(ΔC2)-containing collapsed cisternum. In contrast, we found that AP-1 accumulated prominently with Rab11-FIP2(SARG). Taken together, these results indicate that clathrin and AP-1 are more strongly associated with recycling system membranes in the later stages of trafficking.
Figure 3.
Rab11-FIP2 mutants alter AP-1 distribution. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). (A) Parental T23 MDCK cells were stained with Alexa-488-phalloidin (pseudo-colored green) to show the cell boundaries and antibodies against AP-1 (pseudo-colored red) and imaged by confocal microscopy. AP-1 staining vesicles were observed diffusely within the subapical cytoplasm. (B) T23 MDCK cells stably expressing EGFP-Rab11-FIP2(SARG) or EGFP-Rab11-FIP2(ΔC2) were stained for endogenous AP-1 (pseudo-colored red) and imaged by confocal microscopy. AP-1 showed a prominent accumulation with EGFP-Rab11-FIP2(SARG) containing cisternae. In Rab11-FIP2(ΔC2)-expressing cells, AP-1-containing vesicles were clustered around the Rab11-FIP2-containing cisternum. Images were taken with a 100x lens with a 3x zoom. Scale bars represent 5 µm.
Rab11-FIP2 mutant effects on Rab5 distribution.
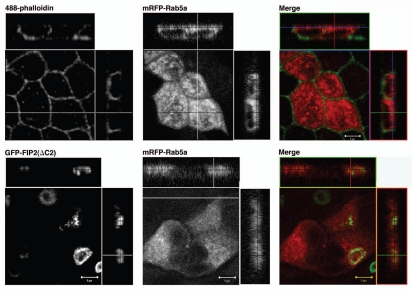
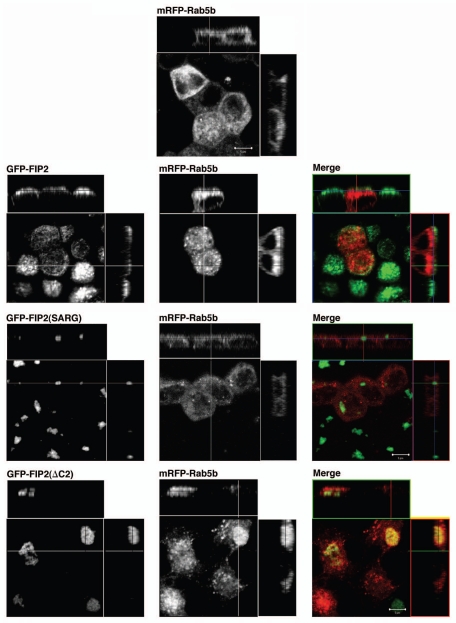
Our previous studies had shown that Rab11-FIP2(ΔC2) altered the distribution of EEA1 positive endosomes, but Rab11-FIP2(SARG) had no effect on EEA1 distribution.14 We therefore sought to examine whether Rab11-FIP2(ΔC2) had a general effect on all early endosomes. We first examined the localization of a more traditional early endosome protein, Rab5a, by transient expression of mRFP-Rab5a in parental T23 MDCK cells and the Rab11-FIP2(ΔC2) cell line. As expected, in parental MDCK cells, mRFP-Rab5a was distributed in small vesicles throughout the cytoplasm (Fig. 4). However, surprisingly, in marked contrast to our previous observations with EEA1,14 Rab5a distribution was not altered by Rab11-FIP2(ΔC2) expression (Fig. 4). Expression of Rab11-FIP2(SARG) also had no effect of the distribution of mRFP-Rab5a (data not shown). Rab5 has three isoforms, Rab5a, Rab5b and Rab5c, coded for by three separate genes.22 Thus, we also examined the effects of Rab11-FIP2 overexpression on Rab5b (Fig. 5). Co-transfection of mRFP-Rab5b into either parental T23 MDCK cells or wild-type Rab11-FIP2-overexpressing cells revealed a diffuse punctate distribution of Rab5b consistent with localization on early endosomal structures. However, in cells expressing Rab11-FIP2(ΔC2), Rab5b containing vesicles were more concentrated towards the membranous cisternae, but did not directly colocalize with Rab11-FIP2(ΔC2). Rab11-FIP2(SARG) expression did not alter the distribution of Rab5b-containing membranes (Fig. 5). In our previous investigations, we observed that the Rab11-FIP2(ΔC2) and Rab11-FIP2(SARG) defined spatially distinct compartments in electron micrographs.14 Interestingly, Z-axis views in Figure 5 demonstrate that, while mRFP-Rab5b-containing membranes were located on the more basal sides of the wild-type Rab11-FIP2 and Rab11-FIP2(SARG)-containing compartments, mRFP-Rab5b vesicles clustered around the apical aspects of the Rab11-FIP2(ΔC2)-containing compartment. These data suggested that a sub-population of early endosomes was affected by Rab11-FIP2.
Figure 4.
Rab11-FIP2 does not alter the localization of Rab5a. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). Parent T23 MDCK cells and T23 MDCK cells stably expressing EGFP-Rab11-FIP2(ΔC2) were transiently transfected with mRFP-Rab5a and imaged by confocal microscopy. Upper parts: Parent T23 MDCK cells were co-stained with Alexa-488-phalloidin (pseudo-colored green) and showed normal diffuse localization of mRFP-Rab5a. Lower parts: The mRFP-Rab5a did not show a relocalization towards the Rab11-FIP2 containing cisternae in cells overexpressing Rab11-FIP2(ΔC2). Images were taken with a 100x lens with a 3x zoom. Scale bars represent 5 µm.
Figure 5.
Differentiable influences Rab11-FIP2 mutants on mRFP-Rab5b. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). Parental MDCK-T23 cells and cells overexpressing EGFP-Rab11-FIP2 constructs were transiently transfected with mRFP-Rab5b. Top part: In parental T23 cells, mRFP-Rab5b was distributed in fine punctate structures throughout the cells. Lower parts: T23 MDCK cells stably expressing wild-type EGFP-FIP2 and each of the mutants (EGFP-FIP2(SARG) and EGFP-FIP2(ΔC2)) were transiently transfected with mRFP-Rab5b and imaged by confocal microscopy. Overexpression of either wild-type Rab11-FIP2 or Rab11-FIP2(SARG) had no effect on the distribution of Rab5b. However, overexpression of EGFP-FIP2(ΔC2) caused a redistribution of mRFP-Rab5b-containing vesicles towards the Rab11-FIP2 containing membrane cisternae. Nevertheless, little direct co-localization was observed. Images were taken with a 100x lens with a 3x zoom. Scale bars represent 5 µm.
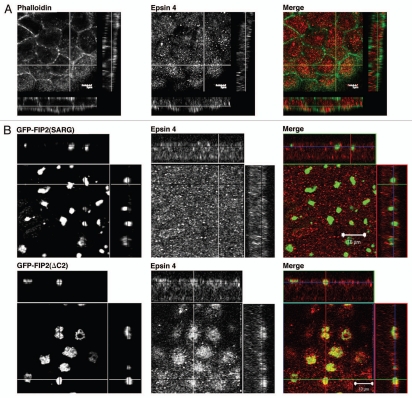
Rab11-FIP2(ΔC2) alters Epsin 4 distribution.
Given the results with Rab5 isoforms, we sought to evaluate the effects of Rab11-FIP2(ΔC2) expression on other markers of early endosomes. Epsin 4 is an adaptor protein associated with early endosomes used in retrograde trafficking.23 In parental T23 MDCK cells, Epsin 4 was distributed diffusely throughout the cytoplasm (Fig. 6A). However, expression of Rab11-FIP2(ΔC2) caused accumulation of Epsin 4 in vesicles surrounding the collapsed GFP-labeled membranous cisternum. Overexpression of Rab11-FIP2(SARG) had no effect on the localization of Epsin 4 (Fig. 6B).
Figure 6.
Differentiable influences of Rab11-FIP2 mutants on Epsin 4 localization. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). (A) Parental T23 MDCK cells were stained with Alexa-488-phalloidin (pseudo-colored green) and Epsin 4 (pseudo-colored red) antibodies and imaged by confocal microscopy to show endogenous ubiquitous localization. Epsin 4 was diffusely distributed throughout the cytoplasm. (B) Similarly, in T23 cells stably expressing EGFP-FIP2(SARG), Epsin 4 was distributed throughout the cytoplasm. However, overexpression of Rab11-FIP2(ΔC2) caused accumulation of Epsin 4-containing vesicles around the collapsed Rab11-FIP2-containing cisternae. All images are derived from 100x images. Scale bars are 10 µm.
As noted previous, we originally observed that expression of the Rab11-FIP2(ΔC2) mutant in polarized MDCK cells caused accumulation of EEA-1 staining vesicles around the collapsed EGFP-labeled cisternum.14 We therefore sought to compare the re-distribution of epsin 4 and EEA-1 containing vesicles (Fig. 7). As above, expression of Rab11-FIP2(SARG) had no significant effect on either epsin 4 or EEA-1 containing membranes. Both epsin 4 and EEA-1 staining membranes were distributed throughout the cells, although epsin 4 membranes were sometimes more clustered in the subapical region. In contrast, expression of Rab11-FIP2(ΔC2) caused a marked concentration of both epsin 4 and EEA-1 staining membranes in apposition with the EGFP-labeling collapsed cisternum (Fig. 7). These results suggest that Rab11-FIP2(ΔC2) can influence the distribution of multiple endosome pathways.
Figure 7.
Rab11-FIP2(ΔC2) alters the distribution of both Epsin 4 and EEA-1. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). T23 cells stably expressing either (upper parts) EGFP-FIP2(SARG) or (lower parts) EGFP-FIP2(ΔC2) were stained with antibodies against Epsin 4 (Cy3 secondary, pseudo-colored red) and EEA-1 (Cy5 secondary, pseudo-colored blue) antibodies and imaged by confocal microscopy. EGFP-FIP2(ΔC2) expression caused accumulation of both EEA-1 and Epsin 4 around the collapsed membrane cisternum. Images were taken with a 100x lens with a 3x zoom. Scale bars represent 5 µm.
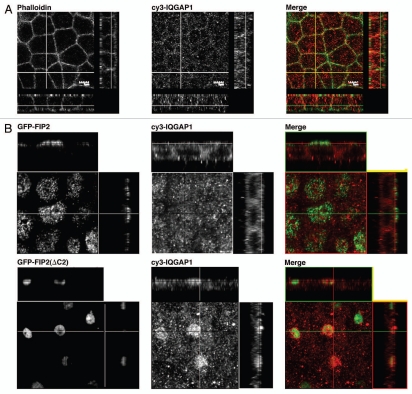
Rab11-FIP2(ΔC2) alters IQGAP1 distribution.
We also evaluated the association of Ras GTPase-activating-like protein IQGAP1 with Rab11-FIP2. IQGAP1 has been implicated in a number of membrane trafficking pathways including endocytosis.24 In non-transfected MDCK cells or MDCK cells expressing EGFP-Rab11-FIP2 wild-type, IQGAP1 staining was present diffusely throughout the cells (Fig. 8). However, in MDCK cells expressing the Rab11-FIP2(ΔC2) mutant, IQGAP1 containing membrane vesicles accumulated in the perinuclear region (Fig. 8). IQGAP1 localization was altered in the Rab11-FIP2(ΔC2) cells in a similar manner to other early endosome-associated proteins. All of these studies indicated that Rab11-FIP2 could affect the morphology of a subpopulation of early endosomal membranes.
Figure 8.
Rab11-FIP2(ΔC2) alters the distribution of IQGAP1. Confocal fluorescence microscopic images of polarized MDCK cells. X-Y plane images are shown flanked by X-Z projections (horizontal) and Y-Z projections (vertical). (A) Parental T23 MDCK cells were stained with Alexa-488-phalloidin (pseudo-colored green) and antibodies against IQGAP1 (pseudo-colored red) and imaged by confocal microscopy. IQGAP was distributed throughout the cytosol with some concentration near lateral membranes. (B) In T23 cells stably expressing wild-type EGFP-FIP2, IQGAP1 was distributed diffusely within the cytoplasm. However, overexpression of Rab11-FIP2(ΔC2) caused the accumulation of vesicles containing IQGAP1 around the collapsed membrane cisternae containing EGFP-Rab11-FIP2. Images were taken with a 100x lens with a 3x zoom. Scale bars represent 5 µm.
Discussion
Rab11-FIP2 is a multifunctional regulator of multiple trafficking pathways. Recent investigations have implicated Rab11-FIP2 in the regulation of early endocytosis, plasma membrane recycling and the establishment of polarity in epithelial cells.12,14,25 This range of functions suggests that Rab11-FIP2 participates in multiple steps along the endocytotic and recycling pathways. In this study, we have sought to identify the range of endocytic regulators that can be influenced by Rab11-FIP2. We have previously characterized two dominant negative mutants of Rab11-FIP2, Rab11-FIP2(ΔC2) and Rab11-FIP2(SARG), which both inhibit transcytotic trafficking and elicit tubulation and collapse of the Rab11a-containing recycling system.14 Nevertheless, differences in membrane morphology and the position of inhibited membrane cisternae led to the suggestion that the two mutants block trafficking through the recycling system at differentiable levels. Consistent with this hypothesis, only Rab11-FIP2(ΔC2) also caused an aggregation of EEA-1-containing early endosome vesicles in proximity to the collapsed recycling system tubular cisternae.14 In the present investigation, these two patterns of recycling system inhibition allowed functional dissection of the possible roles of putative Rab11-FIP2 interacting proteins along particular aspects of the endocytic recycling pathway. Three patterns have emerged from our analysis. The first pattern included direct accumulation of proteins with both Rab11-FIP2 mutants, as exemplified by dynein and clathrin. This pattern is most consistent with that seen for Rab11a and cargoes within the slow plasma membrane recycling system, such as polymeric IgA receptor.26 In a second pattern, we observed little direct colocalization of early endosomal proteins within membranes containing either Rab11-FIP2 mutant, but did find an accumulation of vesicles containing these early endosomal markers clustering around the collapsed Rab11-FIP2(ΔC2)-containing cisternae. These results suggest Rab11-FIP2(ΔC2) can affect the transition of early endosomal elements into later trafficking steps, either to late endosomes or recycling endosomes. Finally, in a third pattern, we observed significant accumulation of both clathrin and AP-1 with Rab11-FIP2(SARG), but less with Rab11-FIP2(ΔC2). In the case of Rab11-FIP2(ΔC2) expression, we also observed some accumulation of vesicles around the collapsed membranes that was morphologically similar to the elements observed with early endosomal proteins. All of these investigations indicate that Rab11-FIP2 has broad influences on the entire endosomal pathway.
The strong association of dynein heavy chain with both of the dominant negative Rab11-FIP2 mutants implicates dynein in the trafficking of endosomes through centripetal movement along microtubules. We have previously shown that Rab11-FIP2 and Rab11a interact with the actin motor protein myosin Vb.9 Overexpression of the tail region of myosin Vb lacking the motor domain blocks Rab11 mediated trafficking events. However, we have noted that treatment of MDCK cells with the microtubule-stabilizing drug taxol caused relocation of Rab11a-containing recycling vesicles to the apical corners of polarized cells.3 Polarized MDCK cells treated with the microtubule depolymerizing drug nocodazole showed dispersal of the apical recycling endosome as marked by Rab11a.3 These two pieces of data strongly implicate the importance of the microtubule cytoskeleton and microtubule motors in addition to the actin motor myosin Vb in regulation of endocytosis and plasma membrane recycling. Recent investigations have shown that Rab11-FIP5 associates directly with kinesin II and regulates plasma membrane recycling, presumably the outgoing centrifugal movement back to the plasma membrane.16 McCaffrey and colleagues have recently noted an association of Rab11-FIP3 with dynein intermediate chain.18,19 The results here therefore suggest that the Rab11-FIP2 mutants cause retention of dynein on the inhibited recycling system elements perhaps through the retention of Rab11-FIP3.
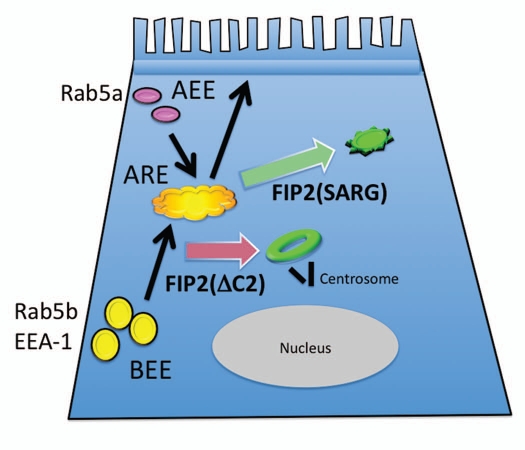
The results here showed that Rab11-FIP2(ΔC2), but not Rab11-FIP2(SARG), can alter the distribution of a number of early endosomal proteins. While Rab11-FIP2(ΔC2) expression affected most of the early endosome proteins we have studied including Rab5b, it had no influence on the distribution of Rab5a. Rab5b is a Rab5 family member that interacts with EEA1 and localizes presumably to early endosome, but has a different GTPase activating protein than Rab5a.27 Each of the Rab5 family members are phosphorylated by different kinases suggesting that they may be regulated differentially and thus regulate different aspects of early endosome-mediated trafficking.28 To our knowledge, this is the first study to find a functional difference between Rab5b and Rab5a. These studies indicate that separate populations of recycling vesicles exist in cells (Fig. 9). Our previous investigations have demonstrated that both of the Rab11-FIP2 mutants used in these studies prominently inhibited basolateral to apical transcytosis.14 However, neither of these mutants altered apical recycling of polymeric IgA receptor. It is tempting to suggest that in polarized cells, different populations of early endosomes may define separate trafficking pathways, perhaps leading either to slow versus fast recycling or to late endosomes and degradation. Alternatively, since Rab11-FIP2(ΔC2) does not affect apical recycling, it is possible that Rab5a regulates endocytosis at the apical pole, while Rab5b regulates endocytosis at the basolateral membrane (Fig. 9). Overall, these investigations indicate that the endosomal systems in polarized epithelial cells likely contain discrete domains that provide for specializations within the dynamic recycling pathways. Further investigations will be required to determine more precisely how specific Rab5 isoforms may define specializations within the early endosomal pathways and how Rab11-FIP proteins may regulate particular aspects of membrane recycling.
Figure 9.
Scheme for the influence of Rab11-FIP2 mutants on trafficking in polarized MDCK cells. While both Rab11-FIP2(ΔC2) and Rab11-FIP2(SARG) cause collapse of the apical recycling endosome (ARE) in a compressed membranous nexus, the morphologies are dissimilar. Rab11-FIP2(ΔC2) causes the formation of a doughnut shaped tubular cisternum that is located next to the centrosome (black bars depict the centrioles) (red arrow). In contrast, Rab11-FIP2(SARG) causes the formation of a tighter and more peripheral membrane nexus (green arrow). Additionally, it appears that the influence of Rab11-FIP2(ΔC2) is felt on earlier steps and also impacts on a range of early endosomal trafficking pathways. In contrast, Rab11-FIP2(SARG) seems to divert trafficking out of the ARE at a later stage and does not perturb early endosomal systems. Apical early endosomal (AEE) trafficking leading to apical recycling appears to avoid the influence of Rab11-FIP2 mutants, while the pathway initiated by basolateral early endosomes (BEE) for basolateral to apical transcytosis is strongly inhibited by both Rab11-FIP2 mutants. These findings support a hypothesis that Rab5a may regulate AEE function and apical recycling, while Rab5b and EEA-1 mediate BEE function and transcytosis.
In summary, we have determined that Rab11-FIP2 can influence a spectrum of regulators of endosomal trafficking. These results in polarized MDCK cells suggest that Rab11-FIP2 has roles in the modulation of trafficking not only within recycling endosome systems, but also in elements of early endosomal and pre-recycling trafficking. The findings demonstrate that Rab11-FIP2 is likely involved in multiple aspects of microtubule-dependent trafficking along the entire endosomal trafficking axis. Thus, discrete steps within the recycling system may be regulated by interaction of Rab11-FIP proteins with distinct sets of regulators that will define promotion of cargoes into and out of the membrane recycling pathways.
Materials and Methods
Materials.
Rabbit anti-Rab11a (VU57) antibodies were developed against the amino terminus of human Rab11a and were specific for Rab11a versus Rab11b and Rab25.29 The commercial antibodies used were goat anti-dynein (Santa Cruz, sc-7527-R), anti-clathrin heavy chain (BD Transduction Labs, 610500) and anti-AP-1 (Sigma, A4200). In addition, we received anti-Epsin 4 from Dr. Margaret Robinson (MRC Laboratory of Molecular Biology) and anti-IQGAP1 from Dr. David Sacks (Harvard University).
Vectors.
Human Rab5a and Rab5b sequences were amplified from full length human ESTs with Pfu Ultra Polymerase (Agilent, 600380-51) using sense primers with in-frame EcoRI sites and anti-sense primers with SalI sites. To construct the mRFP-C2 vector plasmid, the coding region of mRFP was inserted in pEGFP-C2 (Clontech 6083-1) backbone between the Nhe I and Bgl I restriction sites. The fidelity of cloned sequences was confirmed by DNA sequencing (Vanderbilt Molecular Biology Core Facility).
Cell culture.
Doxycycline-inhibitable expression vectors and stable inducible cell lines were generated as previously described in reference 25. Parental T23 MDCK cells30 as well as the stably transfected cell lines described previously in reference 14, were grown in D-MEM (Cellgro, 15-017-CV) supplemented with 10% characterized fetal bovine serum (Hyclone, SH30396.03), penicillin-streptomycin (Cellgro, 30-002-CI), 2 mM L-glutamine (Cellgro, 25-005-CI) and 0.1 mM MEM non-essential amino acids (Cellgro, 25-025-CI). Media for cell lines also contained 0.5 mg/ml G418 sulfate (Cellgro, 30-234-CR) and 0.25 ng/ml hygromycin (Cellgro, 30-240-CR). In the stable cell lines, expression of the EGFP chimeras was inhibited with doxycycline (20 ng/ml) (Calbiochem, 324385). To examine EGFP protein expression, cells were grown on 0.4 µm Transwell filters (Costar, 3470) without doxycycline in tetracycline-screened fetal bovine serum (HyClone) media for 2–4 d. Transient transfection of mRFP Rab5a and Rab5b was performed using Effectene (Qiagen, 301425) following the manufacturer's protocol.
Immunofluorescence.
Staining for endogenous protein localization was performed as previously described for Rab11a.25 Cells were stained for dynein, IQGAP1, AP-1 and clathrin heavy chain as previously described in reference 31. Briefly, cells were fixed in 70% ethanol for 20 min, permeabilized with 0.25% Triton X-100 for 10 min and blocked in 2% BSA in PBS for at least one hour. Primary antibody was added in 1% BSA in a humid chamber overnight. Cells were washed with PBS and then incubated with secondary antibody (Jackson ImmunoResearch: Cy3 donkey anti-rabbit, 711-166-152; Cy3 donkey anti-goat, 705-166-147; Cy3 donkey anti-mouse 715-166-151; Cy5 donkey anti-mouse 715-175-151) in 1% BSA for 1 h. For visualization of F-actin, Alexa-488-phalloidin (Invitrogen, A-12379) was added (1:100) with the secondary antibodies. Cells were washed and then stained with 4,6-diamidino-2-phenylindole (DAPI) in sodium phosphate. Filters were cut out of the transwells and mounted with Prolong Gold + DAPI solution (Invitrogen, P36931). Cells were imaged on a Zeiss LSM510 confocal microscope or on an Olympus FV1000 confocal microscope using a 100x lens.
Acknowledgments
We thank Dr. M. Robinson for the gift of antibody reagent against Epsin 4 and Dr. David Sacks for the gift of antibody reagent against IQGAP1. Confocal images were generated through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126 and the Digestive Diseases Research Center, P30 DK058404). This work was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK070856 and DK48370 (to J.R.G.).
References
- 1.Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol Gastrointest Liver Physiol. 1996;270:515–525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- 2.Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized madin-darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, et al. Rab11a and Rab25 association with the apical recycling system of polarized MDCK cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, et al. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DM, Lindsay AJ, Hendrick AG, McCaffrey MW. The novel Rab11-FIP/Rip/RCP family of proteins displays extensive homo- and hetero-interacting abilities. Biochem Biophys Res Commun. 2002;292:909–915. doi: 10.1006/bbrc.2002.6736. [DOI] [PubMed] [Google Scholar]
- 6.Prekeris R, Klumperman J, Scheller RH. A Rabll/Rip11 complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay AJ, Hendrick AG, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, et al. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem. 2002;277:12190–12199. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- 8.Prekeris R, Davies JM, Scheller RH. Identificaition of a novel Rab11/25 binding domain in eferin and Rip proteins. J Biol Chem. 2001;276:38966–38970. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- 9.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, et al. Myosin Vb is associated with and regulates plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsakraklides V, Krogh K, Wang L, Bizario JC, Larson RE, Espreafico EM, et al. Subcellular localization of GFP-myosin-V in live mouse melanocytes. J Cell Sci. 1999;112:2853–2865. doi: 10.1242/jcs.112.17.2853. [DOI] [PubMed] [Google Scholar]
- 11.Hales CM, Vaerman JP, Goldenring JR. Rab11-Family interacting protein 2 (Rab11-FIP2) assocaites with myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 12.Cullis DN, Philip B, Baleja JD, Feig LA. Rab11-FIP2, an adaptor protein connecting cellular components involved in internalization and recycling of epidermal growth factor receptors. J Biol Chem. 2002;277:49158–49166. doi: 10.1074/jbc.M206316200. [DOI] [PubMed] [Google Scholar]
- 13.Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducharme NA, Williams JA, Oztan A, Apodaca G, Lapierre LA, Goldenring JR. Rab11-FIP2 regulates differentiable steps in transcytosis. Am J Physiol Cell Physiol. 2007;293:1059–1072. doi: 10.1152/ajpcell.00078.2007. [DOI] [PubMed] [Google Scholar]
- 15.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 16.Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, et al. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loubery S, Wilhelm C, Hurbain I, Neveu S, Louvard D, Coudrier E. Different microtubule motors move early and late endocytic compartments. Traffic. 2008;9:492–509. doi: 10.1111/j.1600-0854.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 18.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 binds dynein light intermediate chain 2 and its overexpression fragments the Golgi complex. Biochem Biophys Res Commun. 2010;394:387–392. doi: 10.1016/j.bbrc.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 20.Liu K, Surendhran K, Nothwehr SF, Graham TR. P4-ATPase requirement for AP-1/clathrin function in protein transport from the trans-Golgi network and early endosomes. Mol Biol Cell. 2008;19:3526–3535. doi: 10.1091/mbc.E08-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto CT, Karam SM, Jeng YY, Forte JG, Goldenring JR. Identification of clathrin and clathrin adaptors on tubulovesicles of gastric acid secretory (oxyntic) cells. Am J Physiol Cell Physiol. 1998;274:1017–1029. doi: 10.1152/ajpcell.1998.274.4.C1017. [DOI] [PubMed] [Google Scholar]
- 22.Bucci C, Lutcke A, Steele-Mortimer O, Olkonnen VM, Dupree P, Chiarello M, et al. Co-operative regulation of endocytosis by three Rab5 isoforms. FEBS Lett. 1995;366:65–71. doi: 10.1016/0014-5793(95)00477-q. [DOI] [PubMed] [Google Scholar]
- 23.Saint-Pol A, Yelamos B, Amessou M, Mills IG, Dugast M, Tenza D, et al. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev Cell. 2004;6:525–538. doi: 10.1016/s1534-5807(04)00100-5. [DOI] [PubMed] [Google Scholar]
- 24.Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166:237–248. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducharme NA, Hales CM, Lapierre LA, Ham AJ, Oztan A, Apodaca G, et al. MARK2/EMK1/Par-1Balpha phosphorylation of Rab11-family interacting protein 2 is necessary for the timely establishment of polarity in Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3625–3637. doi: 10.1091/mbc.E05-08-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducharme NA, Jin M, Lapierre LA, Goldenring JR. Assessment of Rab11-FIP2 interacting proteins in vitro. Methods Enzymol. 2005;403:706–715. doi: 10.1016/S0076-6879(05)03061-2. [DOI] [PubMed] [Google Scholar]
- 27.Callaghan J, Nixon S, Bucci C, Toh BH, Stenmark H. Direct interaction of EEA1 with Rab5b. Eur J Biochem. 1999;265:361–366. doi: 10.1046/j.1432-1327.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 28.Chiariello M, Bruni CB, Bucci C. The small GTPases Rab5a, Rab5b and Rab5c are differentially phosphorylated in vitro. FEBS Lett. 1999;453:20–24. doi: 10.1016/s0014-5793(99)00686-9. [DOI] [PubMed] [Google Scholar]
- 29.Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, et al. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol. 2007;292:1249–1262. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]
- 30.Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocaization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soukoulis V, Reddy S, Pooley RD, Feng Y, Walsh CA, Bader DM. Cytoplasmic LEK1 is a regulator of microtubule function through its interaction with the LIS1 pathway. Proc Natl Acad Sci USA. 2005;102:8549–8554. doi: 10.1073/pnas.0502303102. [DOI] [PMC free article] [PubMed] [Google Scholar]