Abstract
Directional migration is a critical component of cell motility is observed in many diverse processes including embryogenesis, immune surveillance and wound repair. A central aspect of directional migration is cellular polarity, which is established through several signaling pathways that converge on the small GTPases. These factors orchestrate precise spatial and temporal organization of the actin cytoskeleton at the leading edge of the cell, and induce polarized capture and stabilization of microtubules and their associated microtubule organizing center (MTOC). Studies of the regulation of the GTPases have predominantly focused on post-translational mechanisms involving guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). In this commentary, we examine the transcriptional regulation of these factors, focusing on the recently described regulation of RhoGEF19, an activator of RhoA, by the epidermal-specific transcription factor GRHL3, and the importance of this regulatory mechanism in wound repair. Our findings establish novel links between epidermal cell migration in wound healing and the planar cell polarity (PCP) signaling pathway, and establish a paradigm for tissue-specific regulation of Rho GTPase activity.
Key words: Grhl3, RhoGEF19, transcription, epidermis, RhoA, PCP, cell migration, wound healing
Small Rho GTPases in Cell Migration and Wound Healing
Rho proteins are small GTP-binding proteins of the Ras superfamily. The three major members of this subfamily, Rho, Rac and Cdc42, control many cellular functions including adhesion, polarity and cell cycle progression, and thus play pivotal roles in both health and disease.1–3 Another process regulated by the small GTPases is directional cell migration, which has been widely studied in the setting of epithelial wound repair. Two distinct mechanisms underpin the coordinated movement of epithelial sheets during healing. In the first, which is typical for adult skin wounds, closure occurs by protrusion of filopodia and lamellipodia at the wound edge, with cells crawling toward the opposing wound margin. In the second, characterised by embryonic epidermal wound repair, actomyosin cables are organized into a purse string at the wound margin that ultimately pulls the edges together. Both processes require activation of signaling pathways assembled in a coordinating cascade. In response to specific stimuli, the principal effectors, including the small GTPases and different polarity protein complexes engage in cooperative crosstalk to rearrange the cytoskeleton and to drive the physical force required for cell migration.4 Active Rho GTPases regulate establishment of cell polarity by contributing to a gradient of signaling molecules that rearrange the actin cytoskeleton and induce directional migration during wound healing.5 The polarized morphology of migrating cells also involves the reorganization of the microtubular network and the alignment of the Golgi apparatus towards the migrating direction.6 Rho has been implicated in the formation of adherens junctions and localizes at active actomyosin-enriched sites to mediate actomyosin contraction through phosphorylation of myosin light chain (MLC).7 Rac activates actin polymerization during lamellipodia formation, and dominant-negative Rac1 inhibits migration in multiple cell types.8 Cdc42 is found in its active GTP-bound state at the leading edge of migrating cells, and inactivation of Cdc42 inhibits formation of filopodia, cell polarization and directional cell movement.9
Tissue-Specific and Temporal Regulation of Small GTPases
An abundant literature details the post-translational regulation of Rho GTPases by guanine nucleotide exchange factors (GEFs) that catalyze exchange of GDP for GTP, GTPase activating proteins (GAPs) that stimulate the intrinsic GTPase activity to inactivate Rho, and guanine nucleotide dissociation inhibitors (GDIs), whose role appears to be most often as negative-regulators by blocking GDP-dissociation and hence nucleotide exchange of GTPases.10 However, little data exists as to the mechanisms regulating the precise spatio-temporal control of activation of the Rho GTPases that would be required for epithelial repair and other tissue-specific functions. Although several Rho GTPases display tissue-restricted expression patterns,11 and some of these are transcriptionally regulated,12–14 Rho, Rac and Cdc42 are ubiquitously expressed. The promoters of these genes display all the hallmarks of house-keeping genes, suggesting that binding of tissue-restricted transcription factors is not a key feature of their regulation.
We have recently identified a novel mechanism by which precise spatio-temporal regulation of the RhoA GTPase is achieved in the context of epidermal wound repair. Central to this mechanism is the direct transcriptional regulation of the RhoA activator RhoGEF19 by the epidermal-specific factor Grainyhead-like 3 (Grhl3).15 Grhl3 is a member of a highly conserved family of transcription factors critical for development and homeostasis of the surface ectoderm across a wide range of species.16 The antecedent member of the family, Drosophila grainy head (grh) plays central roles in cuticle formation and repair,17,18 and is also involved in the regulation of wing hair and ommatidia orientation as a component of the PCP signaling pathway.19 In mammals, Grhl3 is essential for the formation of the barrier function of the integument, and also in a range of epidermal morphogenetic events including eyelid fusion, neural tube closure and wound repair.20–22 In these latter processes, characterized by the need for orchestrated directional cell movement, Grhl3 functions cooperatively with components of the PCP pathway, emphasizing the functional evolutionary links between the mammalian and Drosophila members of the family.15
In the context of epidermal wound healing, expression of Grhl3 is markedly upregulated in cells at the wound margins. We have shown that this induces the expression of RhoGEF19, a homologue of a RhoA activator involved in PCP signaling in Xenopus. Phylogenetic analysis, ChIP and gene expression in Grhl3-/- mice demonstrate that RhoGEF19 is a direct transcriptional target of GRHL3, providing a mechanism by which activation of RhoA can be achieved in a precise spatio-temporal fashion. Consistent with this, knockdown (-kd) of Grhl3 or RhoGEF19 in keratinocytes induces defects in actin polymerisation, cellular polarity (with loss of organization of Golgi apparatus in the direction of cell movement) and wound healing. Re-expression of RhoGEF19 in Grhl3-kd cells rescues these defects, indicating that RhoGEF19 is an essential GRHL3 target gene in directional cell migration in wound repair.15 These results are noteworthy as they: establish Grhl3 as a novel component of the mammalian PCP pathway; demonstrate for the first time that PCP signaling is critical for epidermal wound repair; establish a novel mechanism by which the precise spatio-temporal expression of small GTPases required for cell migration and polarity in epidermal morphogenetic events can be achieved. On this basis, it is likely that transcriptional regulation of RhoGEF19 will also be important for neural tube closure and eyelid fusion.
Recent studies suggest that transcriptional regulation of the regulators of the small GTPases by tissue-restricted transcription factors could be an emerging paradigm for spatiotemporal-specific remodeling of the actin cytoskeleton, polarity and selective cell migration in diverse tissues. In neural cells, the basic helix-loop-helix transcription factor, oligodendrocyte lineage transcription factor 2 (OLIG2), represses the expression of RhoGAP8 resulting in increased activity of RhoA and enhanced formation of stress fibers and focal adhesions. OLIG2 also alters the expression of three other genes in the Rho signaling pathway (p115RhoGEF, LARG and p190RhoGAP) to regulate cell migration and invasiveness.23 Tsapara et al. have shown the RhoA activator GEF-H1/Lfc is a direct target gene and effector of the TGFbeta signaling pathway. GEF-H1 induction is Smad4-dependent and leads to RhoA activation in different cell lines including the primary RPE cells isolated from porcine eyes and the Madin-Darby canine kidney (MDCK) line.24 In a separate study, Shen et al. found that mRNA and protein levels of Net1, a specific GEF for RhoA, increased upon treatment of the human keratinocytes HaCaT with TGFbeta. The induction of Net1 in Smad3 dominant-negative cells was dramatically decreased in comparison to that of normal cells, suggesting that Smads are also involved in the TGFbeta induction of Net1 to regulate stress fiber formation. Interestingly, the authors reported that Net1 expression was not induced in primary mouse fibroblasts, even though TGFbeta has an effect on the migration of those cells. They suggest that in different cell types different GEFs, GAPs and GDIs serve as targets that can be regulated by the TGFbeta signaling pathway to activate RhoA.25 The RhoGAP member, p73, is a vascular cell-specific GTPase-activating protein and an important modulator of angiogenesis. p73RhoGAP displays GTPase activity to Rho but not to Rac or Cdc42 and its mRNA is upregulated in an angiogenic milieu with little or no regulation seen under non-angiogenic conditions.26 RhoGAP12, is one member of a subfamily that includes, RhoGAP9 and 15, and displays GTPase activity towards Rac1. RhoGAP12 was identified as a specific transcriptional target of HGF, and its mRNA and protein were markedly repressed in response to HGF treatment in invasive cell growth.27 Various GEFs, GAPs and GDIs have been shown to be regulated at the transcription level in Interleukin-2 (IL-2)-stimulated lymphocytes. Although RhoA does not undergo any change in expression, mRNA and protein of ARHGEF3/XPLN and Net1 appear to be induced by IL-2 and those of TRIO to be reduced, leading to RhoA regulation in a cell cycle-dependent manner.28 Analysis of promoters of GEFs, GAPs and GDIs reveal significant complexity with a range of predicted transcription factor binding sites. Further studies to unravel the importance of tissue-specific regulators in different contexts are required.
Upstream Signaling to Grhl3 in Wound Healing?
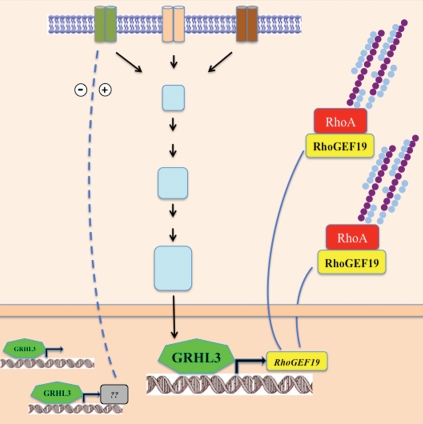
A key question emerging from our studies centers on the nature of the upstream signals at the wound margin that activate the Grhl3/RhoGEF19/RhoA axis that induces directional cell migration. In Drosophila, FGF receptors have emerged as important mediators of cell migration, in which the Rho family of small GTPases provide the link between membrane receptor signaling and the regulation of actin cytoskeleton.29 Grh has been shown to directly stimulate the transcription of an epidermal target gene Stit (FGFR1-like) which encodes a Ret-family receptor tyrosine kinase required for efficient epidermal wound healing.30 The Branchless/FGF signaling was also shown to induce grh expression, as was described for the control of apical membrane growth and tube elongation.31 In mammals, FGF signaling has also been characterized as a positive regulator and signal transducer in wound repair.32 Our model (Fig. 1), suggests that activation of FGF signaling in response to wounding could induce GRHL3 expression, with resultant upregulation of RhoGEF19 and RhoA activiation culminating in coordinated cellular polarity and directional cell migration.
Figure 1.
Schematic representation of receptors signaling to the cytoskeleton. Specific stimuli induce the transcriptional regulation of RhoGEF19 by GRHL3 to control RhoA GTPase and polarity in wound healing.
Acknowledgements
S.M.J. is a Principal Research Fellow of the Australian National Health and Medical Research Council (NHMRC). The work was supported by Project Grants from the NHMRC, The March of Dimes Foundation (S.M.J.).
Extra View to: Caddy J, Wilanowski T, Darido C, Dworkin S, Ting SB, Zhao Q, et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell. 2010;19:138–147. doi: 10.1016/j.devcel.2010.06.008.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/13620
References
- 1.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 2.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 4.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 5.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 6.Nabi IR. The polarization of the motile cell. J Cell Sci. 1999;112:1803–1811. doi: 10.1242/jcs.112.12.1803. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 8.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 9.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 10.Sorokina EM, Chernoff J. Rho-GTPases: new members, new pathways. J Cell Biochem. 2005;94:225–231. doi: 10.1002/jcb.20327. [DOI] [PubMed] [Google Scholar]
- 11.Schiller MR. Coupling receptor tyrosine kinases to Rho GTPases—GEFs what's the link. Cell Signal. 2006;18:1834–1843. doi: 10.1016/j.cellsig.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 13.Vasilaki E, Papadimitriou E, Tajadura V, Ridley AJ, Stournaras C, Kardassis D. Transcriptional regulation of the small GTPase RhoB gene by TGF{beta}-induced signaling pathways. FASEB J. 2010;24:891–905. doi: 10.1096/fj.09-134742. [DOI] [PubMed] [Google Scholar]
- 14.Schiavone D, Dewilde S, Vallania F, Turkson J, Di Cunto F, Poli V. The RhoU/Wrch1 Rho GTPase gene is a common transcriptional target of both the gp130/STAT3 and Wnt-1 pathways. Biochem J. 2009;421:283–292. doi: 10.1042/BJ20090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caddy J, Wilanowski T, Darido C, Dworkin S, Ting SB, Zhao Q, et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell. 2010;19:138–147. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilanowski T, Tuckfield A, Cerruti L, O'Connell S, Saint R, Parekh V, et al. A highly conserved novel family of mammalian developmental transcription factors related to Drosophila grainyhead. Mech Dev. 2002;114:37–50. doi: 10.1016/s0925-4773(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski S, Dierick HA, Bejsovec A. Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics. 2002;161:171–182. doi: 10.1093/genetics/161.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Adler PN. The grainy head transcription factor is essential for the function of the frizzled pathway in the Drosophila wing. Mech Dev. 2004;121:37–49. doi: 10.1016/j.mod.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Hislop NR, Caddy J, Ting SB, Auden A, Vasudevan S, King SL, et al. Grhl3 and Lmo4 play coordinate roles in epidermal migration. Dev Biol. 2008;321:263–272. doi: 10.1016/j.ydbio.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Ting SB, Wilanowski T, Auden A, Hall M, Voss AK, Thomas T, et al. Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nat Med. 2003;9:1513–1519. doi: 10.1038/nm961. [DOI] [PubMed] [Google Scholar]
- 22.Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, Zhao LL, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- 23.Tabu K, Ohba Y, Suzuki T, Makino Y, Kimura T, Ohnishi A, et al. Oligodendrocyte lineage transcription factor 2 inhibits the motility of a human glial tumor cell line by activating RhoA. Mol Cancer Res. 2007;5:1099–1109. doi: 10.1158/1541-7786.MCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 24.Tsapara A, Luthert P, Greenwood J, Hill CS, Matter K, Balda MS. The RhoA activator GEF-H1/Lfc is a transforming growth factor-beta target gene and effector that regulates alpha-smooth muscle actin expression and cell migration. Mol Biol Cell. 2010;21:860–870. doi: 10.1091/mbc.E09-07-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen X, Li J, Hu PP, Waddell D, Zhang J, Wang XF. The activity of guanine exchange factor NET1 is essential for transforming growth factor-beta-mediated stress fiber formation. J Biol Chem. 2001;276:15362–15368. doi: 10.1074/jbc.M009534200. [DOI] [PubMed] [Google Scholar]
- 26.Su ZJ, Hahn CN, Goodall GJ, Reck NM, Leske AF, Davy A, et al. A vascular cell-restricted RhoGAP, p73RhoGAP, is a key regulator of angiogenesis. Proc Natl Acad Sci USA. 2004;101:12212–12217. doi: 10.1073/pnas.0404631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentile A, D'Alessandro L, Lazzari L, Martinoglio B, Bertotti A, Mira A, et al. Met-driven invasive growth involves transcriptional regulation of Arhgap12. Oncogene. 2008;27:5590–5598. doi: 10.1038/onc.2008.173. [DOI] [PubMed] [Google Scholar]
- 28.Mzali R, Seguin L, Liot C, Auger A, Pacaud P, Loirand G, et al. Regulation of Rho signaling pathways in interleukin-2-stimulated human T-lymphocytes. FASEB J. 2005;19:1911–1913. doi: 10.1096/fj.05-4030fje. [DOI] [PubMed] [Google Scholar]
- 29.Montell DJ. Developmental regulation of cell migration. Insight from a genetic approach in Drosophila. Cell Biochem Biophys. 1999;31:219–229. doi: 10.1007/BF02738240. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Tsarouhas V, Xylourgidis N, Sabri N, Tiklova K, Nautiyal N, et al. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol. 2009;11:890–895. doi: 10.1038/ncb1898. [DOI] [PubMed] [Google Scholar]
- 31.Hemphala J, Uv A, Cantera R, Bray S, Samakovlis C. Grainy head controls apical membrane growth and tube elongation in response to Branchless/FGF signalling. Development. 2003;130:249–258. doi: 10.1242/dev.00218. [DOI] [PubMed] [Google Scholar]
- 32.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]