Abstract
The activation of receptor tyrosine kinases, particularly ErbB2, has been linked to the genesis and progression of breast cancer. Two of the central signaling pathways activated by ErbB2 are the Ras/Raf-1/Mek/Erk pathway, which plays an important role in tumor cell growth and migration, and the PI3K/Akt pathway, which plays an important role in cell survival. Recently, we and others have shown that signaling through the Ras-Erk pathway can be influenced by p21-activated kinase 1 (Pak1), an effector of the Rho family GTP ases Rac and Cdc42. Expression of activated forms of Rac promotes activation of Erk through mechanisms involving Pak1 phosphorylation of Raf-1 and Mek1. In addition, Pak1 has also been implicated in the activation of Akt. However, our understanding regarding the degree to which Rho GTPases, and their effectors such as Pak1, contribute to ErbB2-mediated signaling is very limited.
Recent results from our laboratory indicate that ErbB2 expression correlates with Pak activation in estrogen receptor negative human breast tumor samples. Using a three-dimensional (3D) culture of human MCF-10A mammary epithelial cells, we found that activation of Rac-Pak pathway by ErbB2 induces growth factor independent proliferation and promotes disruption of acini-like structures through the activation of the Erk and Akt pathways. We also observed that blocking Pak1 activity by small molecule inhibitors impeded the ability of activated ErbB2 to transform these cells and to activate its associated downstream signaling targets. In addition, we found that suppressing Pak activity in ErbB2-amplified breast cancer cells delayed tumor formation and downregulated Erk and Akt signaling in vivo. These results support a model in which Pak, by activating Erk and Akt, cooperates with ErbB2 in transforming mammary epithelial cells.
Key words: ErbB2, breast cancer, inhibitor, small GTPase, protein kinase, signal transduction, oncogene
Introduction
The ErbB family of receptor tyrosine kinases (RTKs), including EGFR (ErbB1/HER1), ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4), couples binding of extracellular growth factor ligands to intracellular signaling pathways regulating diverse biologic responses, including proliferation, differentiation, cell motility and survival. ErbB RTKs are widely expressed in epithelial, mesenchymal and neuronal cells and signaling through these receptors plays a critical role in determining cell fate in many organ systems, as exemplified by the perinatal (ErbB1) or early embryonic lethality (ErbB2, ErbB3 and ErbB4) of knockout mice because of severe defects in a broad range of organs, including skin, lung, the gastrointestinal tract, brain, heart and peripheral nervous system.1,2 Furthermore, ErbB RTKs are involved in mammary gland development during puberty and pregnancy, as well as in the maintenance of tissue homeostasis. Conversely, dysregulation of the ErbB signaling network is implicated in cancer initiation, tumor growth/progression, metastasis and poor patient outcome. Since they have important functions in regulating tumor proliferation, survival and metastasis, the ErbB family has provided an attractive therapeutic target in breast cancer.3–5 Of the four ErbB proteins, ErbB2 has been of special interest, as it is overexpressied in approximately 25–30% of primary breast cancers and is a significant prognostic factor in terms of nodal status, tumor grade, overall survival and probability of relapse in breast cancer patients.6
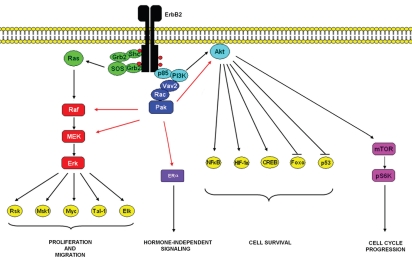
Although ErbB2 activation and constitutive signaling is achieved through overexpression and homodimerization, it functions as the preferred heterodimeric partner of the other three ErbB members.7 ErbB2 binds to a larger subset of phosphotyrosine-binding proteins than the other ligand-binding receptors of the family.8 Furthermore, ErbB2-containing heterodimers are characterized by a higher affinity and broader specificity for various ligands than the other heterodimeric receptor complexes, owing to slow rates of growth-factor dissociation. Also, ErbB2-containing heterodimers undergo slow rates of endocytosis and more frequently recycle back to the cell surface.9,10 These features translate to potent mitogenic signals11 owing to the simultaneous and prolonged recruitment of multiple signaling pathways including the PI3K/Akt and Ras/Raf-1/Mek/Erk pathways (Fig. 1).
Figure 1.
Dimerization of ErbB2 receptors leads to phosphorylation and activation of several intracellular catalytic substrates, including the Ras/Raf/MEK/Erk, PI3K/Akt and other important signaling pathways that regulate apoptosis, protein synthesis and cellular proliferation. Our experimental results summarized in this Extra View, demonstrate that ErbB2 signaling activates a Rac-Pak signaling pathway that contributes to ErbB2 mediated transformation through the Erk and Akt pathways.
While ErbB signaling is quite complex, the Ras/Raf-1/Mek/Erk pathway is one the best characterized downstream signaling routes of these RTKs.12 In addition, blockade of Erk activity by pharmacological inhibitors against MEK13 or by expression of a kinase dead form of Erk14 has been shown to suppress proliferation and tumorigenecity of many human breast cancer cell lines in vitro and in vivo. Since we and others had established that Paks are required for activation of Erk downstream of several RPTKs,15–18 in the scientific paper highlighted here,19 we studied the role of Pak signaling in ErbB2 mediated transformation and its effect in the activation of the Erk pathway as well as on Akt signaling.
Pak and Breast Cancer
Previous reports have shown that Pak family members are overexpressed and/or hyperactivated in various human cancers (reviewed in ref. 20 and 21), including brain, liver, kidney, bladder, pancreas, colon, ovarian and breast tumors among others.22–31 The role of Pak1 in tumor pathogenesis has been characterized in greatest detail in breast cancer. In mammary tumors, deregulation of Pak1 is well documented and correlates with increased invasiveness and cell survival. More than 50% of human breast cancers display overexpression and/or hyperactivation of Pak1, usually in association with amplification of 11q13.5-q14.28,30 Recent reports have shown that expression of a constitutively active (CA) mutant of Pak1 increases cell motility, anchorage-independent growth and invasiveness in MCF-7 breast cancer cells and that transgenic expression of this allele in mouse mammary glands leads to development of mammary tumors and other types of breast lesions.32,33 In addition, Pak1 has been shown to protect mammary epithelial cells from anoikis by inhibiting PARP cleavage,34 as well as acting downstream of α6β4 integrin to confer resistance to apoptosis in 3D cultures of MCF10A cells by activating NFκB.35 In accord with these effects, overexpression of wild type Pak1 correlates with inhibition of hollow lumen formation and distorts normal acinar morphology in 3D conditions.36 Conversely, expression of dominant-negative (DN) Pak1 suppresses motility and invasiveness in different breast cancer cell lines and partially restores normal acinar morphology in the in vitro 3D culture mentioned above.33,36–38
This accumulating evidence indicates that Pak contributes to the transformation of breast epithelial cells. However, the relationship of Pak to ErbB2 signaling is unclear, and since Pak signaling is critical for activation of the Ras/Raf-1/Mek/Erk pathway, we examined the potential role of this kinase in ErbB2 mediated transformation of human breast epithelial cells.
The data published in the article object of this Extra View showed that there is a strong correlation between ErbB2 overexpression and Pak activity in ER-negative human breast cancer specimens, suggesting that ErbB2 may modulate Pak signaling in ER-negative mammary tumors. To clarify the molecular mechanisms by which Pak contributes to ErbB2 signaling in breast carcinogenesis, we grew MCF-10A cells (ER-negative) that stably express an AP1510-activatable, chimeric form of ErbB2 in 3D conditions.39 Using this system, we first demonstrated that activation of ErbB2 promotes a robust activation of endogenous Rac and Pak1. These observations are consistent with the documented activation of these signaling proteins by other RTKs.15,40,41
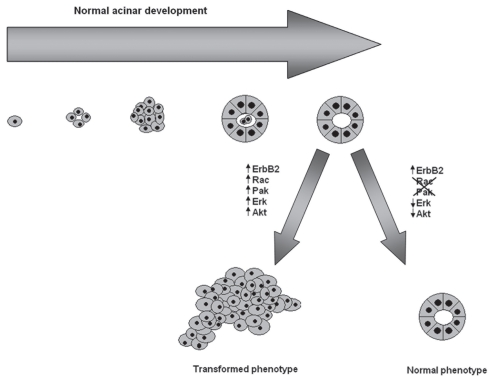
Normally, MCF-10A cells form organized acini that recapitulate the architectural elements of breast acinar development when grown in a 3D setting. As had been reported previously by the Muthuswamy lab, we found that activation of ErbB2 signaling with AP1510 caused characteristic changes in acinar morphogenesis, luminal apoptosis and proliferation, resembling those observed in human breast ductal carcinoma in situ.39 We found that overexpression of dominant negative (DN)-Rac or DN-Pak impeded the ability of ErbB2 to disrupt the architecture of the 3D acinar-like stuctures and blocked the activation of PI3K/Akt and Ras/Raf-1/Mek/Erk signaling. Moreover, we confirmed that these morphological and signaling effects were actually due to the loss of Pak activity by using specific small molecule inhibitors of Rac xml:lang="42 and Pak.43 Conversely, cells expressing constitutive active forms of Rac or Pak showed aberrant morphology, high rates of cell proliferation and suppression of apoptosis and constitutive activation of Akt and Erk signaling even in the absence of activated ErbB2, suggesting that, in this system, Rac-Pak signaling is necessary and sufficient for ErbB2 to induce cell survival, proliferation and a multiacinar phenotype (Fig. 2). We obtained similar results in a more realistic model of ErbB2 function in which transformation of MCF10A cells is driven by ErbB1/ErbB2 heterodimers.
Figure 2.
Schematic representation of normal acinar morphology and the effect of ErbB2 signaling on the acinar architecture. Single mammary ephitelial cells seeded on a basement membrane gel recapitulate numerous features of breast epithelium in vivo, including the formation of acinus-like spheroids with a hollow lumen, apicobasal polarization of cells making up these acini and the basal deposition of basement membrane components. Activation of ErbB2 signaling, promotes the activation of several signaling pathways, including the Ras/Raf-1/MEK/Erk and PI3K/Akt pathways disrupting the normal architecture of the acini. Blockade of Rac and Pak activity by using dominant negative mutants as well as small molecule inhibitors restores the normal acinar morphology.
Our results in the 3D breast epithelial cell culture system showed that Pak plays a positive role in ErbB2 signaling. Using this model, we were able to dissect the signaling events that underlie these important effects, but how does Pak contribute to oncogenesis in mammary cells in vivo? To define the role of Pak in the proliferation of ErbB2-expressing human breast cancer cells we used a xenograft model. ErbB2-positive MDA-MB-631/DYT2 breast cancer cells expressing a Pak-inhibitory domain (PID) were injected into the flanks of SCID mice and we monitored the development of tumors over the course of several weeks. Control cells expressing either GFP or an inactive PID quickly developed into large tumors 3 weeks post-injection. In contrast, PID-expressing cells resulted in much smaller tumors at a considerably delayed rate. Consistent with the results obtained in our 3D cell culture system, loss of Pak activity downregulated Akt and Erk activities in vivo, indicating that suppression of Pak can at least partially block the ability of ErbB2 to induce tumor formation. These results show that Rac-Pak signaling is a key element in ErbB2 function in breast epithelial cells, and that inhibiting Pak activity impedes breast tumor growth in animals.
Next Steps
While a role for Pak1 in ErbB2-mediated transformation in mammary epithelial cells is, we feel, now well-established, the mechanism by which Pak1 contributes to this process, and whether it extends to more realistic breast cancer models, is not. That is, although we found a strong correlation between blocking Pak activity, blocking Erk and Akt activity and blocking transformation by ErbB2, we do not know if these molecular links are germane to the restoration of normal morphology in the MCF-10A system, or to the reduced tumor volume in xenografts. It is entirely possible that other Pak targets are as important or more important to these phenomena. Among the leading candidates are the NFκB and ER signaling pathways (Fig. 1). Pak is known to activate NFκB, thought the precise molecular links have not been established.44 Certainly, Pak-mediated activation of this pathway might be important to its cellular effects. Also, phosphorylation of BAD by Pak1 could contribute to the pro-survival effects of this kinase. Finally, it should be noted that nuclear localization of Pak1 plays a critical role in zebrafish development,45 and that, given the functional similarities of fish and mammalian Pak1, we ought therefore pay special attention to potential nuclear substrates of Pak1 in mammalian cells. The estrogen-receptor (ER) might represent one such nuclear substrate. Recently, Tharakan et al. showed that Pak1 can phosphorylate ER-α at serine residue 305 promoting its activation in a ligand-independent manner, linking this event to tamoxifen resistance in ER positive breast tumors insensitive to hormone based therapies.46
In addition to determining the relevant pathways affected by Pak, we need to establish if loss of Pak affects tumorigenesis in a genetically engineered model of breast cancer. For ErbB2, several such mouse models exist, such as the MMTV-Neu mouse. Breeding these mice with Pak-deficient mice could provide compelling in vivo data regarding the role of Pak1 in ErbB2-driven breast cancer.
A final point: given the high frequency of 11q13 amplification in human breast cancer, it is reasonable to ask if cells from such tumors, irrespective of ErbB2 or ER status, are “addicted” to Pak1. In this case, cell lines established from such tumors (e.g., BT-474 or UACC 893,47) might undergo growth arrest and/or apoptosis in the absence of high-level Pak1 expression or in the presence of a Pak1 inhibitor. If so, this might point to particular tumors amenable to Pak-inhibitor based therapies.
Extra View to: Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, Chernoff J. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29:5839–5849. doi: 10.1038/onc.2010.318.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/14109
References
- 1.Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 3.Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro FDSS. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6:243–257. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- 4.Troyer KL, Lee DC. Regulation of mouse mammary gland development and tumorigenesis by the ERBB signaling network. J Mammary Gland Biol Neoplasia. 2001;6:7–21. doi: 10.1023/a:1009560330359. [DOI] [PubMed] [Google Scholar]
- 5.Normanno N, Bianco C, De Luca A, Maiello MR, Salomon DS. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr Relat Cancer. 2003;10:1–21. doi: 10.1677/erc.0.0100001. [DOI] [PubMed] [Google Scholar]
- 6.Shalaby MR, Shepard HM, Presta L, Rodrigues ML, Beverley PC, Feldmann M, Carter P. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J Exp Med. 1992;175:217–225. doi: 10.1084/jem.175.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RB, Gordus A, Krall JA, MacBeath G. A quantitative protein interaction network for the ErbB receptors using protein microarrays. Nature. 2006;439:168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- 9.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 10.Lenferink AE, Pinkas-Kramarski R, van de Poll ML, van Vugt MJ, Klapper LN, Tzahar E, et al. Differential endocytic routing of homo- and heterodimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 12.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 13.Hoeflich KP, O'Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basallike breast cancer models. Clin Cancer Res. 2009;15:4649–4664. doi: 10.1158/1078-0432.CCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 14.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem. 2005;280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 16.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEKI-ERK complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao K, Kobayashi S, Jaffer ZM, Huang Y, Volden P, Chernoff J, Liang Q. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J Mol Cell Cardiol. 2008;44:429–434. doi: 10.1016/j.yjmcc.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 19.Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, Chernoff J. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogen. 2010 doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28:2545–2555. doi: 10.1038/onc.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 22.O'Sullivan GC, Tangney M, Casey G, Ambrose M, Houston A, Barry OP. Modulation of p21-activated kinase 1 alters the behavior of renal cell carcinoma. Int J Cancer. 2007;121:1930–1940. doi: 10.1002/ijc.22893. [DOI] [PubMed] [Google Scholar]
- 23.Aoki H, Yokoyama T, Fujiwara K, Tari AM, Sawaya R, Suki D, et al. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res. 2007;13:6603–6609. doi: 10.1158/1078-0432.CCR-07-0145. [DOI] [PubMed] [Google Scholar]
- 24.Ching YP, Leong VY, Lee MF, Xu HT, Jin DY, Ng IO. p21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res. 2007;67:3601–3608. doi: 10.1158/0008-5472.CAN-06-3994. [DOI] [PubMed] [Google Scholar]
- 25.Mahlamaki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432–439. doi: 10.1593/neo.04130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, et al. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Nishiyama H, Kawanishi H, Matsui S, Guilford P, Reeve A, Ogawa O. p21-activated kinase 1: a new molecular marker for intravesical recurrence after transurethral resection of bladder cancer. J Urol. 2007;178:1073–1079. doi: 10.1016/j.juro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, et al. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003;163:985–992. doi: 10.1016/S0002-9440(10)63458-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson B, Shih Ie M, Wang TL. Different clinical roles for p21-activated kinase-1 in primary and recurrent ovarian carcinoma. Hum Pathol. 2008;39:1630–1636. doi: 10.1016/j.humpath.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279:1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- 31.Bostner J, Ahnstrom Waltersson M, Fornander T, Skoog L, Nordenskjold B, Stal O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene. 2007;26:6997–7005. doi: 10.1038/sj.onc.1210506. [DOI] [PubMed] [Google Scholar]
- 32.Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene. 2006;25:2931–2936. doi: 10.1038/sj.onc.1209309. [DOI] [PubMed] [Google Scholar]
- 33.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, et al. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 34.Menard RE, Jovanovski AP, Mattingly RR. Active p21-activated kinase 1 rescues MCF10A breast epithelial cells from undergoing anoikis. Neoplasia. 2005;7:638–645. doi: 10.1593/neo.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NFkappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–3712. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 36.Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia. 2008;10:314–329. doi: 10.1593/neo.07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000;275:12041–12050. doi: 10.1074/jbc.275.16.12041. [DOI] [PubMed] [Google Scholar]
- 38.Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 39.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menard RE, Mattingly RR. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cellular Signalling. 2003;15:1099–1109. doi: 10.1016/s0898-6568(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidantdependent NFkappaB activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lightcap CM, Kari G, Arias-Romero LE, Chernoff J, Rodeck U, Williams JC. Interaction with LC8 is required for Pak1 nuclear import and is indispensable for zebrafish development. PLoS One. 2009;4:6025. doi: 10.1371/journal.pone.0006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tharakan R, Lepont P, Singleton D, Kumar R, Khan S. Phosphorylation of estrogen receptor alpha, serine residue 305 enhances activity. Mol Cell Endocrinol. 2008;295:70–78. doi: 10.1016/j.mce.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Shadeo A, Lam WL. Comprehensive copy number profiles of breast cancer cell model genomes. Breast Cancer Res. 2006;8:9. doi: 10.1186/bcr1370. [DOI] [PMC free article] [PubMed] [Google Scholar]