Abstract
Antigens of pathogenic microbes that mimic autoantigens are thought to be responsible for the activation of autoreactive T cells. Viral infections have been associated with the development of the neuroendocrine autoimmune diseases type 1 diabetes and stiff-man syndrome, but the mechanism is unknown. These diseases share glutamic acid decarboxylase (GAD65) as a major autoantigen. We screened synthetic peptide libraries dedicated to bind to HLA-DR3, which predisposes to both diseases, using clonal CD4+ T cells reactive to GAD65 isolated from a prediabetic stiff-man syndrome patient. Here we show that these GAD65-specific T cells crossreact with a peptide of the human cytomegalovirus (hCMV) major DNA-binding protein. This peptide was identified after database searching with a recognition pattern that had been deduced from the library studies. Furthermore, we showed that hCMV-derived epitope can be naturally processed by dendritic cells and recognized by GAD65 reactive T cells. Thus, hCMV may be involved in the loss of T cell tolerance to autoantigen GAD65 by a mechanism of molecular mimicry leading to autoimmunity.
Glutamic acid decarboxylase (GAD65) is shared as a major autoantigen by the neuroendocrine autoimmune diseases type 1 (insulin-dependent) diabetes and stiff-man syndrome (SMS) (1). This neuroenzyme is expressed in neurons and β cells. Although type 1 diabetes is believed to result from a T cell-mediated autoimmune destruction of the pancreatic β cells (2, 3), SMS is thought to result from impairment of γ-aminobutyric acid-ergic inhibition of α-motor neurons, presumably involving autoantibodies to GAD65 (4). Type 1 diabetes is developed by 35% of SMS patients with GAD65 autoantibodies (5). Therefore a pathogenic association between these autoimmune diseases is conceivable. Molecular mimicry has been postulated to represent the environmental cause of autoimmune diseases (6, 7). Both type 1 diabetes and SMS have been associated with microbial infections (8, 9).
To identify a viral or bacterial antigen that mimics autoantigen GAD65 we determined the recognition pattern of a GAD65-specific, HLA-DR3-restricted T cell clone by using synthetic peptide libraries that are dedicated to bind to HLA-DR3 (10, 11), which predisposes to type 1 diabetes and SMS (12). This clone (PM1#11) was isolated from a SMS patient with high levels of type 1 diabetes-associated antibodies against GAD65 and islet cells (13), who subsequently developed type 1 diabetes. The epitope recognized was mapped to GAD65 amino acids 339–352 (Table 1). To identify viral or bacterial antigens that matched the recognition pattern, the pattern was used for database searching. Hits matching the pattern were tested for proliferation induction of clone PM1#11.
Table 1.
Omission mixture analysis of synthetic mimicry epitope
| PM1#11 stimulating peptide | Sequence | Omitted in X | 1 μM, cpm |
|---|---|---|---|
| Human GAD65 339–352 | TVYGAFDPLLAVAD | ||
| Library 1 epitope | SIAMAFDPOIPMAA | ||
| Library 2 epitope | TDSLAFEPKVPRRQ | ||
| Omission mixture | TDSXAFEPKVPRRQ | L, I, M, V, A, G, Y, F, C | 8,395 ± 1,536 |
| TDSLXFEPKVPRRQ | A, C | 2,885 ± 1,227 | |
| TDSLAXEPKVPRRQ | F, C | 28 ± 12 | |
| TDSLAFXPZVPRRQ | D, N, E, Q, S, T, A, V, G, C | 166,815 ± 13,517 | |
| TDSLAFEXKVPRRQ | P, C | 40 ± 12 | |
| TDSLAFBPXVPRRQ | K, R, H, P, C | 144,517 ± 7,736 | |
| TDSLAFEPKXPRRQ | L, I, M, V, A, G, C | 151 ± 97 | |
| TDSLAFEPKVXRRQ | P, A, C | 1,223 ± 632 |
The sequences of GAD65 339–352, the library 1 epitope, and the library 2 epitope are aligned. Relative positions 1–8 of the three peptides are highly similar (underlined). An omission mixture analysis of relative positions 1–8 of the library 2 epitope is described. Peptide mixtures based on the library 2 epitope were tested for stimulation of PM1#11 in a T cell proliferation assay at total concentrations of 1 μM (mean of triplicate, ± SD). In each mixture all natural l-amino acids were present at position X (underlined), except the amino acids omitted. At position Z (underlined) all natural l-amino acids, except C and D were present. Background counts were 127 ± 40 cpm (mean ± SD of triplicate). The positive control response against GAD65 339–352 was 166,587 ± 10,336 (mean ± SD of triplicate).
Materials and Methods
Synthetic Peptides.
Peptides were synthesized by solid-phase strategies on an automated multiple peptide synthesizer (SyroII, MultiSynTech, Bochum, Germany) as described (14). The purity of the peptides was determined by analytical reversed-phase HPLC and proved to be at least 80%. The integrity of the peptides was determined by matrix-assisted laser desorption ionization time-of-flight MS on a Lasermat mass spectrometer (Finnigan-MAT, Hemel Hempstead, U.K.).
Proliferation Assay.
CD4+ T cell proliferation assays for testing synthetic peptides were performed, using 1 × 104 T cells and 5×104 irradiated HLA DR3-matched peripheral blood mononuclear cells (PBMCs) in flat-bottomed 96-well plates in 150 μl complete Iscove's modified Dulbecco's medium (GIBCO), containing 10% pooled human serum. For testing protein preparations, 1 × 104 HLA DR3-matched monocyte-derived dendritic cells (purity > 90%) (15) per well were used in U-bottomed 96-well plates instead of PBMCs in flat-bottomed 96-well plates. Phytohemagglutinin (10 μg/ml) and IL-2 (T cell growth factor, 10% Lymphocult, Biotest Diagnostics, Danville, NJ) were used as positive controls for T cell proliferation. 3H-labeled thymidine (0.5 μCi in 50 μl RPMI) was added after 72 h, cells were harvested (Micro Cell Harvester, Skatron, Sterling, VA), and activity of the T cell DNA was counted after another 18 h (LKB 1205 Betaplate, Liquid Scintillation Counter).
ELISPOT Analysis.
A total of 20,000 T cells of clone PM1#11 and 50,000 irradiated HLA-matched antigen-presenting cells were preincubated with medium alone, PMA/ionomycine, GAD65 autoantigen, or cytomegalovirus (CMV) protein for 48 h. Then, nonadherent cells were washed and 3,500 cells were transferred to wells of ELISA plates precoated with high-affinity mAbs against IL-10, IL-13, or IFN-γ, to which the cytokine produced during incubation will bind. After incubation for 4 h (IL-10, IL-13, and IFN-γ), cells were lysed and debris was washed away. Areas to which the cytokine was bound were detected with a combination of biotinylated anticytokine antibody and gold-labeled antibiotin antibody. Finally, a silver enhancement reagent was added yielding black zones (spots), which reveal the sites of cytokine secretion. Numbers of spots and surface area were calculated automatically by using Olympus microimager software (means of triplicates ± SD).
Database Searching.
The nonredundant protein database (March 1998) was retrieved from the European Molecular Biology Laboratory by ftp (http://mac-mann6.embl-heidelberg.de/massspec/software.html) (Heidelberg, Germany). The database consisted of 301,822 protein entries. Pattern searches were performed in the database by using peptidesearch (16).
Cloning, Expression, and Purification of Protein.
Human fibroblasts infected with human CMV (hCMV) strain AD169 were kindly provided by M. Harmsen (University Hospital, Groningen, The Netherlands). Primers for a PCR on part of the hCMV major DNA-binding protein gene (coding for amino acids 601–738, including epitope amino acids 674–687, excluding the DNA-binding domain and large hydrophobic regions) were designed to generate a PCR product containing two BamHI restriction sites. The PCR product was ligated in the pGEM-T Easy vector (Promega) and transformed to Escherichia coli XL1-blue. The obtained construct was digested with BamHI and ligated into the pET-19b vector (Qiagen, Chatsworth, CA) downstream of the His-tag coding part. The integrity of the construct was confirmed by DNA sequencing. Plasmid DNA containing the proper fragment in the desired orientation was transformed to E. coli BL21-DE. Clones were grown in LB medium to an OD of 0.6 at 600 nm, induced with 1 mM isopropyl β-d-thiogalactoside for 18 h at 25°C and analyzed for recombinant His-tagged fusion protein production by SDS/PAGE. Inclusion bodies containing the His-tagged fusion protein were isolated as described (17) and washed with 0.5% Trition X-100 in water. Inclusion bodies were dissolved in 8 M ureum and loaded on a mono-S cation exchange-column. A 30-min gradient was applied, using 5% acetic acid in water and 0→2 M NaCl. The fraction containing the fusion protein was further purified by reversed-phase chromatography using a C4 protein column (Vydac) (0.1% trifluoroacetic acid in water, 5→95% acetonitrile, 30 min), lyophilized, and dissolved in 0.005% trifluoroacetic acid in water to a final concentration of 2.0 mg/ml. The purity and integrity of the fusion protein (Mr = 18.8 kDa) were confirmed by SDS/PAGE and protein sequencing by Edman degradation of amino acids 1–50 (amino acids 1–28 are His-tag and linker, amino acids 29–50 are amino acids 601–622 of the hCMV major DNA-binding protein) (G1005A, Hewlett–Packard). The integrity was further confirmed by MS after trypsin digestion (Q-tof, Micromass, Manchester, U.K.).
Results
Determination of PM1#11 Recognition Pattern.
Two dedicated synthetic peptide libraries (complexity 4 × 106 peptides per library) containing different HLA DR3 binding motifs (10, 11) were synthesized and screened with PM1#11 as described (10, 14). From each library a synthetic mimicry epitope was identified (Table 1). Similarity between GAD65 339–352 and the two library epitopes was observed for relative positions 1–8. This similarity included identical amino acids at relative positions 2, 3, and 5 (A, F, and P, respectively). To determine the ligand restrictions of PM1#11, an omission mixture analysis (10) was performed for relative positions 1–8 of the library 2 peptide (Table 1). This analysis was based on the predictability of T cell ligands by analysis of individual amino acid positions (18) and the interdependence of relative positions 4 and 6 for peptide binding to HLA-DR3 (12). The analysis led to the definition of a database search pattern, reflecting the recognition pattern of PM1#11 (Table 2).
Table 2.
Alignment of recognition pattern with hCMV 674–687 related sequences
| T cell recognition pattern | |
|---|---|
| XXX LAFXPXLA XXX | |
| I IP | |
| M M | |
| V V | |
| A A | |
| G G | |
| G | |
| Y | |
| F | |
| Major DNA binding protein | hCMV 674–687 related sequences |
| hCMV | 674-PYA VAFQPLLA YAY-687 |
| Herpes simplex virus type 1 | 660-PYA CGPCPLLQ LLG-673 |
| Herpes simplex virus type 2 | 660-PYT CGPCPLLQ LLA-673 |
| Herpes simplex virus type 6 | 619-PYA TAFSPFLT FSY-632 |
| Herpes simplex virus type 7 | 619-PLS LAFSPFFV FTY-632 |
| Varicella-zoster virus | 659-PYS GAFCPITN FLV-672 |
The T cell recognition pattern determined (Table 1) was used for searching a nonredundant protein database. The stimulatory peptide derived from this search, hCMV major DNA-binding protein was compared to other herpes virus major DNA-binding proteins. The epitope region of hCMV major DNA-binding protein was aligned with the equivalent regions of the other herpes viruses.
Peptide-Crossreactivity of PM1#11.
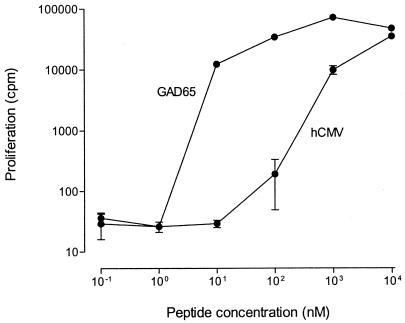
A nonredundant protein database containing 301,822 proteins was searched with the PM1#11 recognition pattern using the computer program peptide search (kindly provided by M. S. Wilm, European Molecular Biology Laboratory, Heidelberg, Germany) (16). Of the 259 hits obtained including GAD65 of four different species, viral and bacterial hits were selected. Peptides (14-mers) of these sequences were synthesized and tested for stimulation of PM1#11 in a T cell proliferation assay. A peptide derived from hCMV major DNA-binding protein UL57 (19) (amino acids 674–687; Swiss Prot entry P17147) stimulated the GAD65-specific T cell clone PM1#11 (Fig. 1), whereas all other peptides were completely unable to induce a proliferative response. Because the hCMV major DNA-binding protein of 134 kDa that is required for DNA replication is homologous to other herpes virus DNA-binding proteins, we subsequently aligned these six proteins for comparison with the epitope region of the hCMV major DNA-binding protein (Table 2). The peptides derived from all other herpes viruses were substantially different from the hCMV major DNA-binding protein epitope (amino acids 674–687) and did not match the PM1#11 recognition pattern (Table 2). As expected, these herpes virus-derived peptides did not stimulate clone PM1#11 in a T cell proliferation assay (data not shown). This finding implies that the T cell-stimulating hCMV epitope is not conserved in other herpes viruses despite homology at the protein level. Thus, crossreactivity of the GAD65 reactive T cell clone is specific for hCMV.
Figure 1.
Recognition of hCMV 674–687 and GAD65 339–352. HCMV 674–687 = PYAVAFQPLLAYAY and GAD65 339–352 = TVYGAFDPLLAVAD. Proliferative responses of clone PM1#11 to a range of peptide concentrations are indicated (means ± SD of triplicate tests).
To ensure that the PM1#11 crossreacting response was indeed clonal, PM1#11 was anergized by preincubation of the T cells with either GAD65 peptide 339–352 or hCMV major DNA-binding protein peptide 674–687, in the absence of antigen-presenting cells. Presentation of the peptide epitope by HLA-DR3 expressed on the activated T cell clone without appropriate costimulation leads to induction of anergy of T cells bearing T cell receptors with this epitope specificity. Indeed, clone PM1#11 lost its ability to respond to either peptide after prepulsing with each of the peptides separately (Fig. 2), proving further evidence of crossreactivity of PM1#11 at the single cell level.
Figure 2.
Clonality of PM1#11 crossreacting response to GAD65 and hCMV. T cells were prepulsed overnight with either GAD65 339–352 or hCMV major DNA-binding protein 674–687 (10 μM). After washing the T cells, cells were stimulated with DR3-matched irradiated peripheral blood mononuclear cells and either GAD65 339–352 or hCMV major DNA-binding protein 675–687 (1 μM) and cultured as described. As a control for the ability to proliferate, prepulsed cells were stimulated with IL-2. In a negative control experiment (data not shown), PM1#11 was mixed with a coxsackie B4 virus-specific T cell clone and prepulsed with either the coxsackie B4 virus-derived epitope or GAD65 339–352. The response of the T cell mixture, after washing, to GAD65 339–352 was completely abrogated after prepulsing with GAD65 339–352, but not after prepulsing with coxsackie B4A virus-derived epitope. In all experiments, responses are the means ± SD of triplicate tests. Mean background of triplicates after prepulsing never exceeded 500 cpm.
Protein-Crossreactivity of PM1#11.
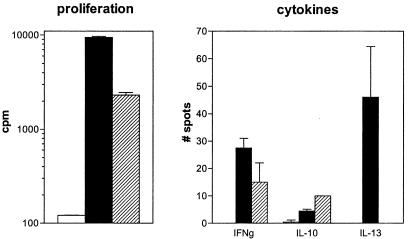
To investigate whether the hCMV major DNA-binding protein-derived peptide 674–687 can be naturally processed we cloned a fragment of the protein (amino acids 601–738) and tested a fusion protein containing the fragment for activation of clone PM1#11. Because it is believed that potentially autoreactive T cells are primed by environmental antigens via dendritic cells (20), we used these cells for protein processing and presentation. Proliferation and production of cytokines could be demonstrated against both GAD65 and the viral protein fragment (50 μg/ml), although the proliferative response to the viral protein was lower and in the absence of IL-13 production (Fig. 3). Monoclonal antibodies directed against HLA-DR completely blocked the response against antigen.
Figure 3.
Recognition of hCMV and GAD65 protein. Crossreactivity of T cell clone PM1#11 with recombinant protein of GAD65 (filled bars) and hCMV (hatched bars) compared with medium alone (i.e., T cells and dendritic cells without antigen; open bars) was tested by proliferation and cytokine production (ELISPOT analysis). IL-4 production was undetectable (data not shown).
Discussion
We conclude that clonal T cells recognizing GAD65 339–352 crossreact with hCMV major DNA-binding protein pUL57 peptide 674–687 and that this peptide is efficiently processed for presentation by HLA-DR3. pUL57 had previously been defined as major target antigen for the IgM antibody response, illustrating its immunogenic nature (21). The synthetic peptide library approach used for the definition of natural T cell antigens is based on functional and structural similarity rather than sequence homology between library-derived mimicry epitopes and database-extracted epitopes (22). The proliferative response of PM1#11 to the GAD65 peptide was achieved at lower peptide concentration compared with the hCMV peptide (Fig. 1). In vivo, the expression of viral antigens might well be one or two log-scales higher than the low expression levels described for GAD65 in pancreatic islet β cells and neuronal tissues (1). In addition, it is conceivable that a crossreactive response is polyclonal, suggesting that T cells recognizing hCMV peptide more efficiently than GAD65 peptide also may exist.
A common association of type 1 diabetes and SMS with hCMV infection received little attention. In view of our findings, a role of hCMV in the pathogenesis of these diseases should be considered. Clinical onset of type 1 diabetes and SMS has been reported to be accompanied by acute hCMV infection (8, 9). Interestingly, the SMS patient from which T cell clone PM1#11 was isolated was diagnosed with an acute hCMV infection at clinical onset of the disease. Recurrent insulitis and autoimmune β cell destruction in pancreatic allografts presented with a predominant fraction of infiltrating T cells reactive to hCMV (23). Also, hCMV infection of mice resulted in the generation of autoantibodies directed to the islets of Langerhans (24).
The mechanism by which hCMV infection contributes to neuroendocrine autoimmunity is unknown. hCMV has been shown to infect β cells (25) and neuronal tissue (26) as well as peripheral blood mononuclear cells (27). This could be either directly cytopathic or lead to induction of virus-specific cytotoxic T cells that kill virus-infected target tissue (28, 29). In each case autoimmunity to GAD65 could be the consequence of spread of the immune response after target cell lysis rather than cause of the immunopathology. Alternatively, systemic hCMV infection could lead to the activation of CD4+ T cells by presentation of hCMV peptide in the context of HLA class II (6, 30). Via molecular mimicry these T cells then could crossreact with GAD65 of neural cells, leading to autoimmune disease.
It has previously been suggested that sequence homology between GAD65 and coxsackie B4 virus may lead to T cell crossreactivity (31) but this has not been supported by functional evidence at clonal level thus far (32). Hence, an association of coxsackie infection with development of type 1 diabetes could result from direct lytic activity to β cells rather than molecular mimicry. It also has been suggested that coxsackie virus-induced type 1 diabetes is initiated by bystander damage by autoreactive T cells after virus infection (33).
In another study, it was shown that a human herpesvirus 6 (HHV-6)-derived peptide was recognized by GAD65 reactive T cells (34). However, an epidemiological association between type 1 diabetes and HHV-6 infection has not been described so far. In addition, in these studies it could not be proven that the HHV-6-derived peptide could be naturally processed.
Our data support a role for hCMV in the pathogenesis of neuroendocrine autoimmune diseases, through T cell crossreactivity with GAD65. Primary prevention of these autoimmune diseases therefore might be attempted by targeting the hCMV virus by vaccination or tolerance-inducing induction strategies.
Acknowledgments
We thank Gaby Duinkerken, Arno van der Slik, and Willemien Benckhuijsen for expert technical assistance, Frits Koning for providing HLA-DR3+ dendritic cells, and Kees Melief, Anneke Brand, and Jeroen van Bergen for critically reading the manuscript. This study was supported by the Royal Netherlands Academy of Arts and Sciences, Diabetes Fonds Nederland, The Juvenile Diabetes Foundation International, and the Macropa Foundation.
Abbreviations
- GAD65
glutamic acid decarboxylase
- CMV
cytomegalovirus
- hCMV
human CMV
- SMS
stiff-man syndrome
References
- 1.Baekkeskov S, Aanstoot H J, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter Olesen H, DeCamilli P. Nature (London) 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 2.Ellis T M, Atkinson M A. Nat Med. 1996;2:148–153. doi: 10.1038/nm0296-148. [DOI] [PubMed] [Google Scholar]
- 3.Roep B O. Diabetes. 1996;45:1147–1156. doi: 10.2337/diab.45.9.1147. [DOI] [PubMed] [Google Scholar]
- 4.Lorish T R, Thorsteinsson G, Howard F M. Mayo Clin Proc. 1989;64:629–636. doi: 10.1016/s0025-6196(12)65339-7. [DOI] [PubMed] [Google Scholar]
- 5.Solimena M, Folli F, Denis-Donini S, Comi G C, Pozza G, DeCamilli P, Vicari A M. N Engl J Med. 1988;318:1012–1020. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- 6.Oldstone M B A. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Herrath M G, Oldstone M B. Curr Opin Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pak C Y, Eun H M, McArthur R G, Yoon J W. Lancet. 1988;2:1–4. doi: 10.1016/s0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- 9.Dalakas M C. Ann Neurol. 1995;37:S2–S13. doi: 10.1002/ana.410370703. [DOI] [PubMed] [Google Scholar]
- 10.Hiemstra H S, Van Veelen P A, Schloot N C, Van Meijgaarden K E, Willemen S J M, Leunissen J A M, Benckhuijsen W E, Amons R, De Vries R R P, Roep B O, et al. J Immunol. 1998;161:4078–4082. [PubMed] [Google Scholar]
- 11.Geluk A, Van Meijgaarden K E, Southwood S, Oseroff C, Drijfhout J W, De Vries R R P, Ottenhoff T H, Sette A. J Immunol. 1994;152:5742–5748. [PubMed] [Google Scholar]
- 12.Pugliese A, Gianani R, Eisenbarth G S, DeCamilli P, Solimena M. Lancet. 1994;344:1027–1028. doi: 10.1016/s0140-6736(94)91691-8. [DOI] [PubMed] [Google Scholar]
- 13.Schloot N C, Batstra M, Duinkerken G, De Vries R R P, Dyrberg T, Chaudhuri A, Behan P O, Roep B O. J Autoimmun. 1999;12:289–296. doi: 10.1006/jaut.1999.0280. [DOI] [PubMed] [Google Scholar]
- 14.Hiemstra H S, Duinkerken G, Benckhuijsen W E, Amons R, De Vries R R P, Roep B O, Drijfhout J W. Proc Natl Acad Sci USA. 1997;94:10313–10318. doi: 10.1073/pnas.94.19.10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann M, Wilm M S. Anal Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- 17.Nagai K, Thogersen H C. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- 18.Hemmer B, Vergell M, Gran B, Ling N, Conlon P, Pinilla C, Houghton R, McFarland H F, Martin R. J Immunol. 1998;160:3631–3636. [PubMed] [Google Scholar]
- 19.Anders D G, McCue L A. Intervirology. 1996;39:378–388. doi: 10.1159/000150508. [DOI] [PubMed] [Google Scholar]
- 20.Banchereau J, Steinman R M. Nature (London) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 21.Vornhagen R, Hinderer W, Sonneborn H H, Bein G, Matter L, The T H, Jahn G, Plachter B. J Clin Microbiol. 1995;33:1927–1930. doi: 10.1128/jcm.33.7.1927-1930.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiemstra H S, Drijfhout J W, Roep B O. Curr Opin Immunol. 2000;12:80–84. doi: 10.1016/s0952-7915(99)00054-0. [DOI] [PubMed] [Google Scholar]
- 23.Santamaria P, Nakhleh R E, Sutherland D E, Barbosa J J. Diabetes. 1992;41:53–61. doi: 10.2337/diab.41.1.53. [DOI] [PubMed] [Google Scholar]
- 24.Pak C Y, Cha C Y, Rajotte R V, McArthur R G, Yoon J W. Diabetologia. 1990;33:569–572. doi: 10.1007/BF00404146. [DOI] [PubMed] [Google Scholar]
- 25.Jenson A B, Rosenberg H S, Notkins A L. Lancet. 1980;2:354–358. [PubMed] [Google Scholar]
- 26.Poland S D, Bambrick L L, Dekaban G A, Rice G P. J Infect Dis. 1994;170:1267–1271. doi: 10.1093/infdis/170.5.1267. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert G L, Hayes K, Hudson I L, James J. Lancet. 1989;i:1228–1231. doi: 10.1016/s0140-6736(89)92330-1. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi P S, Oehen S, Buerki K, Pirchner H, Ohashi C T, Odermatt B, Malissen B, Zinkernagel R M, Hengarter H. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 29.Ridge J P, Di Rosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 30.Ufret-Vincenty R L, Quigley L, Tesser N, Pak S H, Gado A, Hausmann S, Wucherpfennig K W, Brocke S. J Exp Med. 1998;188:1725–1738. doi: 10.1084/jem.188.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkinson M A, Bowman M A, Campbell L, Darrow B L, Kaufman D L, Maclaren N K. J Clin Invest. 1994;94:2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schloot N C, Roep B O, Wegmann D R, Yu L, Wang T, Eisenbarth G S. Diabetologia. 1997;40:332–338. doi: 10.1007/s001250050683. [DOI] [PubMed] [Google Scholar]
- 33.Horwitz M S, Bradley L M, Harbertson J, Krahl T, Lee J, Sarvetnick N. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 34.Bach J M, Otto H, Jung G, Cohen H, Boitard C, Bach J F, van Endert P M. Eur J Immunol. 1998;28:1902–1910. doi: 10.1002/(SICI)1521-4141(199806)28:06<1902::AID-IMMU1902>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]