Abstract
T cells need to cross endothelial barriers during immune surveillance and inflammation. This involves T-cell adhesion to the endothelium followed by polarization and crawling with a lamellipodium at the front and contractile uropod at the back. T cells subsequently extend lamellipodia and filopodia under the endothelium in order to transmigrate. Rho GTPases play key roles in cell migration by regulating cytoskeletal dynamics and cell adhesion. We have found that the Rho GTPase RhoA is required for efficient T-cell polarization and migration on endothelial cells as well as transendothelial migration. RhoA-depleted cells lack both lamellipodia and uropods, and instead have narrow protrusions extending from a rounded cell body. Using a RhoA activity biosensor, we have shown that RhoA is active at the leading edge in lamellipodia and filopodia of crawling and transmigrating T cells, as well as in the uropod. In lamellipodia, its activity correlates with both protrusion and retraction. We predict that RhoA signals via the formin mDIA 1 during lamellipodial protrusion whereas it induces lamellipodial retraction via the kinase ROCK and actomyosin contractility. We propose that different guanine-nucleotide exchange factors (GEFs) are responsible for coordinating RhoA activation and signaling in different regions of transmigrating T cells.
Key words: Rho GTPases, T cells, diapedesis, endothelial cells, actin cytoskeleton, cell migration, uropod, actomyosin
Introduction
During inflammation leukocytes are recruited from the vasculature into tissues using a specialized migratory pathway.1 Following adhesion to the endothelial cells (EC) that line blood vessel walls, leukocytes polarize and then migrate over the EC surface (crawling) to sites where they undergo transendothelial migration (TEM). Leukocytes can either cross the endothelium between EC (paracellular route) or through EC (transcellular route).2
Rho GTPase family proteins are key regulators of cytoskeletal dynamics and cell migration,3 and thus would be expected to contribute to the process of TEM. Most Rho GTPases cycle between an inactive, GDP-bound form and an active, GTP-bound form, which interacts with and activates downstream targets. Rho GTPases are stimulated by guanine nucleotide exchange factors (GEFs), which stimulate exchange of GDP for GTP and inactivated by GTPase-activating proteins (GAPs), which stimulate GTP hydrolysis.
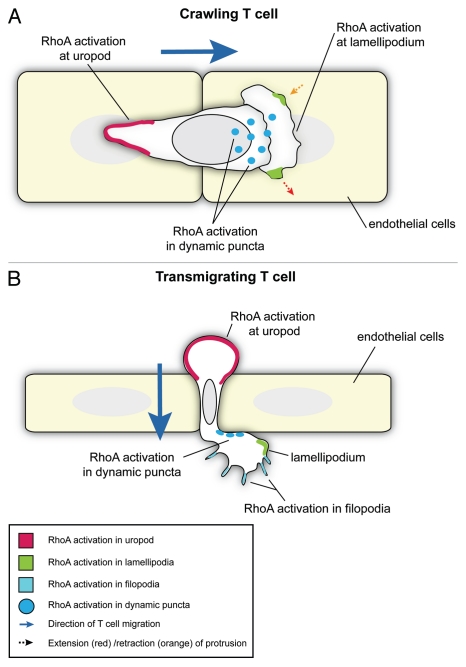
In our recent paper we used an RNAi screen to identify which Rho GTPases are important for T cell TEM.4 We found that knockdown of RhoA induced the strongest inhibition of this process and so subsequently examined the role of RhoA in detail during both T cell crawling on EC and subsequent TEM. Significantly, active RhoA was observed at both the front and back of polarized T cells as they crawled on EC and transmigrated, as well as in dynamic puncta in the basal membrane and filopodia extending beneath the endothelium of transmigrating cells (Fig. 1). Here we discuss the implications of these findings in the context of current understanding of RhoA signaling and the potential function of these cellular processes in T-cell migration.
Figure 1.
Localization of active RhoA in crawling and transmigrating T cells. Schematics show a T cell crawling on the apical surface of EC (viewed from above) (A) or transmigrating between adjacent EC (viewed from the side) (B). RhoA is active in the uropod (red) in both the crawling (A) and transmigrating T cell (B). In the crawling T cell (A), RhoA is also active in lamellipodia (green) where it is associated with both extension and retraction events (dotted arrows), and in dynamic puncta on the basal membrane (dark blue dots). During transmigration (B), RhoA is active at the leading edge of the T cell in filopodia (pale blue regions), lamellipodia (green) and in dynamic puncta (dark blue dots).
RhoA is Required for Lamellipodium and Uropod Formation in T Cells
When T cells attach to EC, they normally assume a polarized morphology with a lamellipodium extending at the front and a uropod at the back4,5 (Fig. 1). Lamellipodial extension is driven by actin polymerization, whereas uropod retraction depends on actomyosin-based contractility.
Using small-interfering RNA (siRNA) to knock down RhoA in T cells resulted in a strikingly different phenotype: the cells attached to EC but were unpolarized and had a rounded cell body with 2 or more narrow protrusions. The membrane dynamics of these protrusions were very different to that of control cells.4 The narrow protrusions extended very slowly, and membrane ruffles sometimes extended laterally from the sides of the protrusions, whereas in control cells membrane ruffling was associated with the broad lamellipodium at the leading edge. This suggests that RhoA is important for both the formation of a single broad lamellipodium at the front of T cells and for the normal actin filament dynamics within the lamellipodium.
Intriguingly we observed two distinct T-cell phenotypes that correlated with the level of RhoA knockdown. High levels of RhoA knockdown led to an unpolarized morphology with multiple protrusions, whereas T cells with intermediate levels of RhoA knockdown were still polarized but had defects in retracting the uropod and often had long tails. Using a RhoA bio-sensor to examine where RhoA is active, we demonstrated that RhoA is activated at both the leading edge and uropod during migration.4 Partial knockdown of RhoA might predominantly affect RhoA signaling in the uropod if more RhoA activity is required for uropod retraction than for protrusion at the leading edge. Indeed, high levels of RhoA activity are reported to be required for tail retraction in leukocytes.6 Thus partial reduction of total RhoA levels in T cells could inhibit signaling at the uropod, but enough active RhoA would remain at the leading edge to permit lamellipodium extension.
The tail retraction phenotype of partial RhoA knockdown is similar to treatment of T cells or other leukocytes with small-molecule inhibitors of Rho-associated coiled-coil forming protein serine/threonine kinases (ROCKs),7 which are activated downstream of RhoA.4,8,9 ROCKs are known to increase the phosphorylation of myosin light chain (p-MLC), leading to increased acto-myosin contractility. This implies that ROCKs act predominantly at the back of migrating leukocytes to induce acto-myosin contraction in the uropod. However, we observed that some ROCK inhibitor-treated T cells did not have broad lamellipodia either, but rather narrow protrusions. We therefore propose that ROCKs also act at the front of cells to regulate lamellipodial dynamics (Fig. 2) but that this function probably requires less ROCK activity than tail retraction.
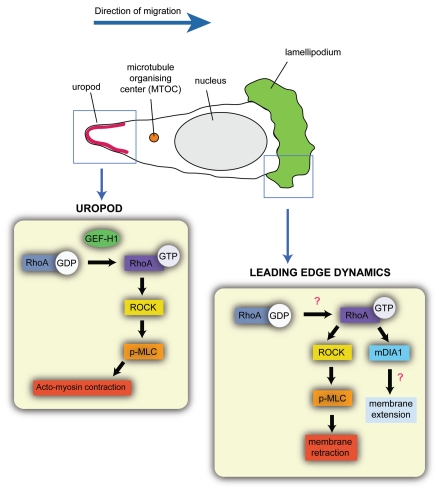
Figure 2.
Potential roles of RhoA at the leading edge and uropod during T cell migration. Schematic showing a T cell migrating on the apical surface of EC. In migrating T cells, the microtubule organizing center (MTOC) is behind the nucleus. Boxed areas (blue) highlight RhoA signaling in the lamellipodium (green) and the uropod (red) of the T cell. In the uropod, RhoA is activated by the RhoGEF GEF-H1 and signals through ROCK and p-MLC to induce acto-myosin contraction. In the lamellipodium, we propose that activation of RhoA by as yet unknown RhoGEFs signals via the formin mDIA1 to induce actin polymerization leading to membrane extension, and via ROCK and phosphorylation of myosin light chain (p-MLC) to induce membrane retraction.
RhoA is closely related to RhoB and RhOC,10 but it is unlikely that either of these contribute to TEM: RhOC was not detectably expressed in T cells, and RhoB depletion did not significantly alter T-cell TEM.4 Previous studies using the Clostridium botulinum exoenzyme C3 transferase, which ADP-ribosylates and thereby inhibits RhoA, RhoB and RhOC, have described a failure of tail retraction at the back of migrating neutrophils, eosinophils and monocytes but not a loss of lamellipodia.6,8 This resembles the phenotype we observe with partial RhoA depletion, suggesting that in these studies C3 transferase did not completely inhibit RhoA function. However treatment of the T cell line HPB-ALL with C3 transferase resulted in the generation of aberrant protrusions11 somewhat similar to the phenotype we observed with RhoA siRNA, and thus the effect of C3 transferase on RhoA could have been stronger in this cell type.
RhoA Signaling at the Rear of Migrating T Cells
The best documented role for Rho/ROCK signaling during leukocyte migration is to increase p-MLC and thereby stimulate acto-myosin contraction in the uropod of migrating cells12 (Fig. 2). Using a RhoA activity biosensor, we found that RhoA is dynamically activated at the rear of cells in association with uropod retraction during T-cell crawling and TEM.4 Similarly, RhoA is active in the uropod of neutrophils migrating on glass.13
RhoA Signaling at the Leading Edge of Migrating T Cells
Initial studies using C3 transferase and dominant negative Rac1 led to a model for cell migration in which Rac1 acted at the front to stimulate actin-driven membrane protrusion, whereas RhoA acted at the back to induce actomyosin-driven tail retraction.14 However, studies examining where Rho GTPases are active in migrating cells demonstrated RhoA activity at the front as well as at the back of a variety of cell types migrating on rigid surfaces.15–18 Our work shows for the first time that RhoA is active at the front of T cells under physiological conditions, migrating on and through the pliable EC surface.
RhoA activity is associated with protrusion at the leading edge of fibroblasts and HeLa cells16,19 and also with membrane ruffle formation.15,16 However, at the leading edge of T cells we found that pulses of RhoA activity were associated with both extension and retraction events4 suggesting that RhoA is likely to act through at least two different effectors to produce these different outcomes.
The RhoA target, mammalian diaphanous 1 (mDIA1),20 localizes to the front of migrating T cells4,21 and is required for actin polymerization and migration.21,22 mDIA1 is a member of the formin family, which can nucleate and extend actin filaments in vitro.23 We hypothesize that RhoA signaling to mDIA1 promotes actin polymerization to drive membrane extension in lamellipodia (Fig. 2).
On the other hand, we propose that RhoA activity acts through ROCK to regulate acto-myosin-mediated retraction events at the leading edge (Fig. 2), since the ROCK target phosphorylated myosin light chain (p-MLC) was enriched at the leading edge in a proportion of T cells. RhoA/ROCK signaling has been previously reported at the leading edge of migrating cells. For example, RhoA/ROCK signaling suppresses Rac signaling at the leading edge of EGF-stimulated carcinoma cells, and inhibition of ROCK increased protrusion but reduced migration.18 Additionally, ROCK/p-MLC is implicated in protrusive force generation at the leading edge of sarcoma cells.24 ROCKs may drive retraction events to allow cells to reorient their direction of migration.
Fluorescence resonance energy transfer (FRET) has been used to show that the Rho GTPase Cdc4225 activates its two effectors, neural Wiskott-Aldrich syndrome protein (N-WASP),26 and p21-activated kinase (PAK),27 in different locations in carcinoma cells.28 A similar approach could be used to examine the spatio-temporal binding of RhoA to its effectors mDIA1 and ROCK at the leading edge of migrating T cells, in order to determine their contributions to extension and retraction.
Coordinating Signaling at the Leading Edge
Our RNAi screen showed that the Rho GTPase Rac2,29 as well as RhoA, contributes to T cell TEM.4 It would therefore be very interesting to examine the relationship between RhoA and Rac2 at the leading edge of migrating T cells. In fibroblasts, RhoA activity is temporarily segregated from Rac and Cdc42 during membrane protrusion,19 but little is known of the molecular interplay between RhoA and Rac and Cdc42 signaling at the leading edge of cells. Rac and Rho are well documented to be antagonistic, each inhibiting the activity of the other.30,31 Furthermore it has been suggested that mDIA1 may suppress Rac1 activity in T cells but promote ROCK activation.21
From recent work, it is becoming clear that different actin nucleators can work in concert to promote actin polymerization.32 For example, nucleation of actin filaments can be initiated by the actin related protein 2/3 (Arp2/3) complex or Spire, and elongation subsequently promoted by formins such as mDIA1.33,34 Interestingly Wiskott-Aldrich syndrome protein (WASP)35 and mDIA1 co-operate at the leading edge during neutrophil migration.36 The balance of activity between N-WASP, WASP family Verprolin homologous (WAVE) proteins and formins has also been shown to regulate the type of actin filament structures that are formed at the leading edge of carcinoma cells,37 suggesting a complex interplay between these different actin regulators. It is thus possible that RhoA and Rac2 activate different actin regulators at the front of T cells that act together to induce lamellipodial protrusion.
A Possible Role for RhoA in Integrin Clusters
RhoA activity was observed in dynamic puncta in the basal membrane under the front lamella of crawling cells (Fig. 1).4 We observed similar puncta in transmigrating cells, although it was not possible to determine if they were at the apical T cell/basal endothelial interface or the basal T cell/extracellular matrix interface.
Assembly and disassembly of integrin-based adhesions are important in cell migration and RhoA plays a key role in this process in many cell types.14 It is possible that the punctate areas of RhoA activity are localized to regions of integrin clustering, where the T-cell integrin lymphocyte function-associated antigen 1 (LFA-1)38 binds its ligand inter-cellular adhesion molecule 1 (ICAM-1)39 on the endothelium. Similar clusters of active LFA-1 have been observed in the lamellipodia of T cells migrating on a rigid substrate coated with ICAM-1.40,41 It has been hypothesized that RhoA and ROCK are involved in switching LFA-1 from a high to low affinity for ICAM-1, thereby disengaging LFA-1 from ICAM-1 and allowing migration forwards.41 Inhibition of Rho or ROCK promoted integrin clustering in T cells42 supporting a role for RhoA in disassembly of integrin clusters. We also observed that RhoA altered the distribution of LFA-1: in control T cells it was predominantly localized behind the leading edge, but following RhoA depletion was distributed throughout the cell. It will therefore be interesting to investigate the contribution of adhesion turnover to the RhoA knockdown phenotype of T cells. Future experiments examining LFA-1 distribution in T cells expressing the RhoA activity biosensor would reveal if RhoA activity is localized to regions of integrin clustering during migration.
Interestingly it has recently been shown that as T cells crawl on the surface of EC, they often extend protrusions downwards, pushing the apical endothelial membrane towards the basal membrane.43,44 These protrusions were associated with LFA-1 clustering and required Cdc42 in part.44 Whether RhoA activity is localized in these protrusions remains to be investigated.
A Possible Role of RhoA in Filopodia during T Cell TEM
During TEM, we observed T cells extending F-actin-rich filopodia under the EC (Fig. 1).4 Filopodia usually act as exploratory sensors of the environment. For example, they have a pathfinding role in nerve growth cones and endothelial tip cells during angiogenesis.45 Leukocytes have been observed to cross the basement membrane at regions where there are lower levels of some matrix proteins46 and it is tempting to speculate that the filopodia extended by T cells are probing the basement membrane for these regions.
Although filopodium formation is normally associated with Cdc42, RhoA has be implicated in filopodia in some contexts via mDIA signaling.45 For example, RhoA is required for mDIA1-induced filopodia at the front of N-WASP/WAVE-depleted carcinoma cells.37 Whilst it is not possible to look at the formation of filopodia during TEM in RhoA-depleted T cells as these cells do not initiate TEM, it would be interesting to examine whether proteins involved in filopodium formation in other cell types, such as vasodilator-stimulated phospho-protein (VASP), fascin, insulin receptor substrate p53 (IRSp53) and myosin X,47 localize to the structures we observe in transmigrating T cells. The mDIA proteins are likely candidates for contributing to filopodium extension downstream of RhoA and thus future experiments will investigate if they are localized to the tips of filopodia in transmigrating T cells.
RhoA Signaling Controlled by the Differential Localization of GEFs
Spatial segregation of RhoA signaling in different regions of T cells could involve different RhoGEFs. There are more than 3 times as many RhoGEFS as Rho GTPases48 and it is speculated that multiple RhoGEFs could mediate the activation of the same Rho GTPase in response to different stimuli. Our work indicates that the RhoGEF GEF-H1,49 is likely to activate RhoA at the rear of T cells (Fig. 2).4 First, it localized specifically to the uropod of crawling and transmigrating cells. Second, knockdown of GEF-H1 by RNAi resulted in a lengthening of the uropod, implying that acto-myosin contraction was reduced.
It will be very interesting to identify the RhoGEFs that activate RhoA in lamellipodia, filopodia and dynamic puncta in T cells and to know whether each GEF activates similar or distinct signaling cascades downstream of RhoA. For example, is ROCK signaling at the leading edge activated by a different GEF to the uropod? The role of each RhoGEF can differ between cell types. For example, in HeLa cells, GEF-H1 is activated following microtubule depolymerization, and activates RhoA at the leading edge.19 Interestingly, microtubules are concentrated in the uropod of migrating T cells,50 and thus it is possible that GEF-H1 function in the uropod is similarly linked to microtubule dynamics. On the other hand, in neutrophils stimulated with the bacterial mimetic peptide N-formyl-methionylleucyl-phenylalanine (fMLP), a RhoGEF called PDZ-RhoGEF51 activates RhoA-mediated acto-myosin contraction in the uropod.52 Which RhoGEF acts at the back of leukocytes to activate RhoA is therefore likely to depend on the stimulus.
Future Perspectives
Our work together with that of others has expanded the role of RhoA during migration from rear retraction to both protrusion and retraction at the leading edge. Future studies should determine how RhoA is activated in different parts of T cells and which of its downstream partners contribute to lamellipodial and filopodial dynamics. It is clear that RhoA plays a major role in T cell polarity during migration and TEM, but other Rho GTPases are also involved in these processes. It will therefore be important to investigate how RhoA acts in concert with these Rho GTPases to coordinate T cell TEM.
Acknowledgements
This work was funded by the Medical Research Council (UK) and the Wellcome Trust.
Extra View to: Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190:553–563. doi: 10.1083/jcb.201002067.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/14724
References
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Carman CV. Mechanisms for transcellular diapedesis: probing and pathfinding by ‘invadosome-like protrusions’. J Cell Sci. 2009;122:3025–3035. doi: 10.1242/jcs.047522. [DOI] [PubMed] [Google Scholar]
- 3.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 4.Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190:553–563. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 6.Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of RhoA and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 8.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhOC and cell motility. Exp Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Woodside DG, Wooten DK, Teague TK, Miyamoto YJ, Caudell EG, Udagawa T, et al. Control of T lymphocyte morphology by the GTPase Rho. BMC Cell Biol. 2003;4:2. doi: 10.1186/1471-2121-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci USA. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa K, Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol Biol Cell. 2005;16:4294–4303. doi: 10.1091/mbc.E04-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 17.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Sibai M, Pertz O, Pang H, Yip SC, Lorenz M, Symons M, et al. RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp Cell Res. 2008;314:1540–1552. doi: 10.1016/j.yexcr.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell. 2009;20:4070–4082. doi: 10.1091/mbc.E09-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicente-Manzanares M, Rey M, Perez-Martinez M, Yanez-Mo M, Sancho D, Cabrero JR, et al. The RhoA effector mDia is induced during T cell activation and regulates actin polymerization and cell migration in T lymphocytes. J Immunol. 2003;171:1023–1034. doi: 10.4049/jimmunol.171.2.1023. [DOI] [PubMed] [Google Scholar]
- 22.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, et al. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosel D, Brabek J, Tolde O, Mierke CT, Zitterbart DP, Raupach C, et al. Upregulation of Rho/ROCK signaling in sarcoma cells drives invasion and increased generation of protrusive forces. Mol Cancer Res. 2008;6:1410–1420. doi: 10.1158/1541-7786.MCR-07-2174. [DOI] [PubMed] [Google Scholar]
- 25.Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 27.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 28.Parsons M, Monypenny J, Ameer-Beg SM, Millard TH, Machesky LM, Peter M, et al. Spatially distinct binding of Cdc42 to PAK1 and N-WASP in breast carcinoma cells. Mol Cell Biol. 2005;25:1680–1695. doi: 10.1128/MCB.25.5.1680-1695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- 30.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 32.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11:62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- 33.Bosch M, Le KH, Bugyi B, Correia JJ, Renault L, Carlier MF. Analysis of the function of Spire in actin assembly and its synergy with formin and profilin. Mol Cell. 2007;28:555–568. doi: 10.1016/j.molcel.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Lee K, Gallop JL, Rambani K, Kirschner MW. Self-assembly of filopodia-like structures on supported lipid bilayers. Science. 2010;329:1341–1345. doi: 10.1126/science.1191710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;79:922. [PubMed] [Google Scholar]
- 36.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182:3837–3845. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 37.Sarmiento C, Wang W, Dovas A, Yamaguchi H, Sidani M, El-Sibai M, et al. WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. J Cell Biol. 2008;180:1245–1260. doi: 10.1083/jcb.200708123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothlein R, Springer TA. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986;163:1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 40.Stanley P, Smith A, McDowall A, Nicol A, Zicha D, Hogg N. Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell. EMBO J. 2008;27:62–75. doi: 10.1038/sj.emboj.7601959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Fernandez JL, Sanchez-Martin L, Rey M, Vicente-Manzanares M, Narumiya S, et al. Rho and Rho-associated kinase modulate the tyrosine kinase PYK2 in T-cells through regulation of the activity of the integrin LFA-1. J Biol Chem. 2001;276:40518–40527. doi: 10.1074/jbc.M102896200. [DOI] [PubMed] [Google Scholar]
- 43.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 44.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellor H. The role of formins in filopodia formation. Biochim Biophys Acta. 2010;1803:191–200. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faix J, Rottner K. The making of filopodia. Curr Opin Cell Biol. 2006;18:18–25. doi: 10.1016/j.ceb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 50.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One. 2010;5:8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274:5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 52.Wong K, Van Keymeulen A, Bourne HR. PDZRhoGEF and myosin II localize RhoA activity to the back of polarizing neutrophil-like cells. J Cell Biol. 2007;179:1141–1148. doi: 10.1083/jcb.200706167. [DOI] [PMC free article] [PubMed] [Google Scholar]