Abstract
Introduction of activated Ras into normal cells leads to senescence, a tumor suppressive mechanism, whereas expression of this oncogene in many immortalized cell lines leads to transformation. Studying the signaling differences in cells that undergo Ras-induced senescence versus Ras transformation may shed light on potential therapeutic targets in the treatment of cancer. C/EBPβ is a transcription factor necessary for both Ras-induced senescence and Ras transformation. Three isoforms of this transcription factor exist due to alternative translation initation at three in frame ATGs. C/EBPβ1 is the isoform responsible for oncogene-induced senescence, and this isoform is degraded by the proteosome during Ras transformation. Phosphorylation of C/EBPβ1 on Thr235 by Cdk2 is necessary, but not sufficient, for degradation of C/EBPβ1. Proteasomal degradation of C/EBPβ1 may represent a mechanism to evade senescence. In contrast, C/EBPβ2 is expressed in breast cancer cells and is involved in proliferation, supporting a role for this isoform in Ras transformation. We propose here that one potential signaling difference in Ras-induced senescence versus Ras transformation is that Ras signals through different C/EBPβ isoforms (C/EBPβ1 versus C/EBPβ2) during these processes.
Key words: C/EBPβ, Ras, senescence, cdk2, IL6
Expression of activated Ras in cells can have very different outcomes depending on the cell type. Introduction of Ras into normal cells frequently leads to senescence, a growth inhibitory response. This phenomenon is called oncogene-induced senescence (OIS) and is a tumor suppressive mechanism inherent to normal cells. In contrast, expression of activated Ras in many immortalized cell lines leads to transformation of these cells. It is not entirely clear why expression of activated Ras in these different cell types leads to very different phenotypic outcomes. OIS protects cells from the initial steps of tumorigenesis caused by a variety of stimuli including activation of oncogenes, DNA damage and oxidative stress. Mechanistically, OIS converges on the pRB and p53 pathways. p16INK4A and pRB are oftentimes activated during OIS, preventing progression through the cell cycle. In addition, the DNA damage response is frequently activated, leading to activation of p53. OIS was first proposed to be a consequence of the artificial growth conditions inherent in cell culture, and thus of questionable relevance in vivo. Nonetheless, markers of senescence have recently been identified in numerous benign, pre- or early neoplastic lesions, such as human melanocytic nevi and prostatic adenomas, and early murine melanomas, murine lymphomas and lung adenomas.1 This suggests that overt tumors are able to subvert OIS; or alternatively, that a few rogue cells were resistant to OIS in the first place. In any case, therapeutic targeting of proteins and/or pathways that promote senescence rather than transformation may be beneficial in the treatment of cancer. Therefore, studying the signaling pathways and their targets in Ras-induced senescence versus Ras-induced transformation may have important clinical implications.
C/EBPβ
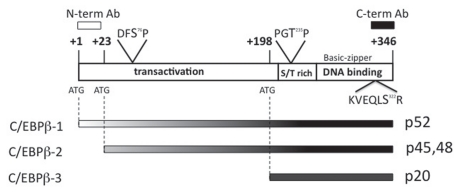
The transcription factor CCAAT/enhancer binding protein beta (C/EBPβ) is essential for both Ras-induced senescence and Ras transformation.2,3 C/EBPβ-/- mouse embryonic fibroblasts (MEFs) expressing Ras(V12) continue to grow, whereas wild type MEFs senesce upon the introduction of Ras(V12).2 C/EBPβ-induced senescence occurs in a pRB-dependent, p53-independent manner.2 In addition, in a skin tumor model, C/EBPβ-/- mice were completely refractory to skin tumor development induced by a variety of carcinogens that produce tumors containing oncogenic Ras mutations.3 Three protein isoforms of this basic leucine zipper transcription factor are alternatively translated due to the presence of three in frame methionines (Fig. 1). Full length C/EBPβ1 begins at the first in frame methionine and has an apparent molecular weight of 52 kDa in humans. C/EBPβ2 is translated from the second in frame ATG and appears on immunoblots as a doublet at 45 kDa and 48 kDa in humans. Translation of C/EBPβ3 begins at the final in frame methionine and has an apparent molecular weight of 20 kDa in humans. C/EBPβ1 and C/EBPβ2 are both activators of transcription because they contain the N-terminal transactivation domain along with the C-terminal DNA binding/dimerization domain. In contrast, C/EBPβ3 lacks the N-terminal transactivation domain, causing it to be a transcriptional repressor.4
Figure 1.
Three proteins are translated from the C/EBPβ mRNA. Schematic of human C/EBPβ mRNA showing locations of the three in-frame methionines used to initiate translation as depicted below. The basic-leucine zipper DNA binding domain and a S/T rich region are common to all three isoforms, whereas the transactivation domain located in the N-terminal half is only found in C/EBPβ-1 and -2. Ras-dependent phosphorylation sites are indicated and include T235 (rat T189) by ERK1/2 or CDKs,18,25,28 S76 (rat S64) catalyzed by CDK2,18 and S322 (rat S273) mediated by p90RSK.18 Additional Ras-dependent modifications not shown include p-T109 and pS-111 by unknown kinases and monomethylation of R114 (numbering for rat protein).18 Additional kinases known to phosphorylate C/EBPβ include PKA,31 PKC,32 p38,33 Ca2+/calmodulin-dependent kinase,34 and GSK3.35
C/EBPβ is expressed in many tissues and cell types and numerous genes are regulated by C/EBPβ.5 The C/EBPβ knockout mouse displays multiple phenotypic alterations in the immune system, liver, adipose tissue, skin and mammary glands,6–12 implicating C/EBPβ as a key regulator in the proliferation and differentiation of a variety of tissues. C/EBPβ has been shown to play a role in very different cell processes ranging from cell growth, survival and transformation to senescence and cell death. One explanation for these contrasting phenotypes is that the three isoforms of C/EBPβ are functionally distinct. While many groups consider C/EBPβ1 and C/EBPβ2 to be functionally alike because of their similarity in size, evidence is accumulating that there are functional differences between the isoforms of C/EBPβ, with C/EBPβ1 playing a role in differentiation and C/EBPβ2 promoting proliferation.
Kowenz-Leutz and Leutz13 demonstrated that C/EBPβ1 but not C/EBPβ2 is able to cooperate with c-Myb to turn on differentiation genes such as mim-1 in myeloid cells. This activation was attributed to the ability of C/EBPβ1, but not −2, to interact with and recruit the SWI/SNF chromatin remodeling complex.13 In addition, C/EBPβ1 is expressed at high levels in the mouse mammary gland during lactation, the stage of mammary gland development that represents terminal differentiation.14 C/EBPβ1 is present at high levels in secretory mammary epithelial cells exfoliated in human breast milk.15 In contrast, C/EBPβ2, but not C/EBPβ1, can transactivate cyclin D1 and PLAC1, two genes whose protein products are involved in proliferation and are commonly upregulated in breast cancer.15,16 Additionally, when C/EBPβ2 is overexpressed in MCF10A mammary epithelial cells, these cells exhibit transformed characteristics such as anchorage independence, the ability to form colonies in soft agar and increased invasive potential.17 Overexpression of C/EBPβ1 in MCF10A mammary epithelial cells does not have this effect. Further expression patterns of the C/EBPβ1 and C/EBPβ2 transcription factors in normal versus transformed cells supports a role for C/EBPβ2 in proliferation and C/EBPβ1 in differentiation. p52C/EBPβ1 is observed in normal human liver, lung, ovary, colon, skin and breast tissue, whereas C/EBPβ2 is not.15,16 Moreover, C/EBPβ2 is expressed in cancer cell lines, while p52C/EBPβ1 is not.15,16
Because the isoforms of C/EBPβ have different functions, it is essential to be able to distinguish the three isoforms of C/EBPβ from each other via immunoblot analysis. As shown in Figure 1, C/EBPβ1 and C/EBPβ2 only differ from each other by 23 amino acids in humans (21 amino acids in mice), and are thus difficult to distinguish on immunoblots when a C-terminal C/EBPβ antibody is used. Additionally, C/EBPβ2 appears as a doublet in anti-C/EBPβ immunoblots. The top band of the doublet is post-translationally modified C/EBPβ2, such as phosphorylated C/EBPβ2,18 although other modifications of C/EBPβ (acetylation,19 arginine methylation,18 and O-GlcNAcylation20) have been reported and could be present. p52C/EBPβ1 is not expressed in transformed cell lines, so many groups confuse the top band of the C/EBPβ2 doublet as being C/EBPβ1. To further compound the confusion in deciphering the two transactivator isoforms of C/EBPβ, the human and mouse proteins are different sizes with the mouse proteins being smaller than the human. Mouse C/EBPβ1 is very close in apparent molecular weight to human C/EBPβ2. Therefore, it is important to utilize a C/EBPβ1-specific antibody raised to the N-terminal amino acids unique to C/EBPβ1 (Fig. 1). This antibody only recognizes the full length isoform of C/EBPβ, and can therefore distinguish C/EBPβ1 from phosphoC/EBPβ2.
C/EBPβ and OIS
As mentioned above, C/EBPβ is critical for Ras-induced senescence.2 A subsequent study demonstrated that C/EBPβ is essential for activated Raf-induced senescence.21 Kuilman and colleagues showed that C/EBPβ induced IL6 expression, which was necessary for OIS. Recently, we examined which transactivator isoform of C/EBPβ was responsible for the induction of IL6 and in turn senescence. C/EBPβ1 expression in normal human fibroblasts more effectively induced senescence than equivalent expression of C/EBPβ2.22 Performing quantitative real time PCR using a primer for IL6 we found that C/EBPβ1 induced IL6 expression 6.6 fold when introduced into the normal human diploid fibroblast cell line WI-38, whereas C/EBPβ2 only induced IL6 2.1 fold in these cells.22 This is consistent with the analysis by Uematsu et al. of C/EBPβ knock-in mice unable to express C/EBPβ2 (due to mutation of its ATG start site) demonstrating that C/EBPβ1 is responsible for IL6 expression. Likewise, Kuilman et al. used a mutant C/EBPβ in which the alternative start sites were mutated so that only C/EBPβ1 could be translated; expression of this construct in normal fibroblasts led to induction of IL6 and cell cycle arrest. Moreover our own expression profiling of MCF10A-C/EBPβ2 cells showed that C/EBPβ2 does not induce IL6, nor can C/EBPβ2 be found at the IL6 promoter using chromatin immunoprecipition (unpublished data). Taken together our data and others indicate that C/EBPβ1 is the primary transactivator isoform of C/EBPβ responsible for the induction of IL6, and thus senescence (Fig. 3).
Figure 3.
Ras signals to C/EBPβ1 during OIS but signals to C/EBPβ2 during transformation. In normal cells that undergo senescence in response to activated Ras expression, Ras(V12) signals through the full length isoform of CEBPβ to induce IL6 and senescence (left). In transformed cells, Ras(V12) activates Cdk2 which phosphorylates C/EBPβ1 on Thr235 leading to its degradation. However, this phosphorylation, while necessary, is not sufficient for proteasomal degradation of C/EBPβ1. Other modifications and/or protein interactions are required for degradation of C/EBPβ1 during Ras transformation. Ras(V12) signals through C/EBPβ2 during transformation, which leads to activation of C/EBPβ2 to transactivate the expression of genes involved in proliferation.
Negative Regulation of C/EBPβ1 in Cells Transformed by Ras
p52C/EBPβ1 is expressed in normal cells but not in breast cancer cell lines, consistent with a role for C/EBPβ1 in OIS, a tumor suppressive mechanism. We therefore examined how p52C/EBPβ1 is being regulated during transformation. To study this we utilized the MCF10A/MCF10A-Ras cell system. MCF10A cells are an immortalized but non-transformed mammary epithelial cell line. Introduction of activated Ras(V12) into these cells leads to their transformation. p52C/EBPβ1 is expressed in MCF10A cells, whereas expression of this full length isoform of C/EBPβ is lost in the transformed MCF10A-Ras cells. We determined that C/EBPβ1 is degraded by the proteasome in the MCF010A-Ras cells, and this degradation is likely ubiquitin-mediated.22 Exogenous expression of C/EBPβ1 in breast cancer cell lines also led to proteasomal degradation of C/EBPβ1. Therefore, p52C/EBPβ1 is likely degraded by the proteasome in breast cancer cells. This may represent a mechanism by which cells escape senescence.
Next we determined that phosphorylation of C/EBPβ1 on Thr235 by Cdk2 was leading to the degradation of C/EBPβ1 in the MCF10A-Ras cells.22 Cdk2 phosphorylates C/EBPβ1 on Thr235, and Cdk2 is activated in MCF10A-Ras cells as well as breast cancer cells. We then wanted to extend and confirm our findings in a different cell system. It is well documented that WI-38 normal fibroblasts can be transformed by the introduction of a small number of oncogenes. Introduction of human telomerase reverse transcriptase (hTERT, the catalytic subunit of telomerase), simian virus 40 large and small T antigens (SV40T/t) and activated Ras(V12) into normal fibroblasts is sufficient for transformation of these cells.24 An advantage of this cell system is that we have the ability to analyze the same cells in a stepwise progression, from normal cells that are capable of senescing (WI-38), to immortalized cells (WI-38-hTERT-SV40T/t), and then transformed cells (WI-38-hTERT-SV40T/t-Ras). Therefore, we decided to examine p52C/EBPβ1 expression in transformed WI-38 cells expressing these three genes to determine if transformation of fibroblasts by Ras would lead to loss of p52C/EBPβ1 expression, similar to what we observe in mammary epithelial cells transformed by Ras.
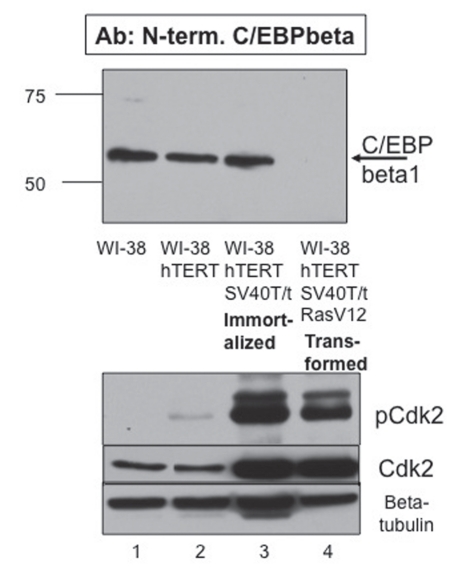
To produce the WI-38-hTERTSV40T/t-Ras cells, serial infections were performed followed by appropriate selection. Immunoblot analysis was performed with our C/EBPβ1-specific antibody raised to the 23 N-terminal amino acids unique to the C/EBPβ1 isoform. p52C/EBPβ1 is expressed in the WI-38, WI-38-hTERT and the immortalized WI-38-hTERT-SV40T/t cells (Fig. 2 and lanes 1–3). Importantly, we do see loss of p52C/EBPβ1 in the transformed WI-38-hTERT-SV40T/t-Ras cells (Fig. 2 and top immunoblot, compare lanes 4 with lanes 1–3). This confirms our observations with the transformed mammary epithelial cells (MCF10A-Ras) that transformation by Ras leads to the loss of p52C/EBPβ1.
Figure 2.
Phosphorylation of C/EBPβ1 by Cdk2 is necessary but not sufficient for proteasomal degradation of C/EBPβ1. Top immunoblot—immunoblot analysis of WI-38, WI-38-hTERT, immortalized WI-38-hTERT-SV40T/t and transformed WI-38-hTERT-SV40T/t-Ras(V12) cells with the N-terminal C/EBPβ1-specific antibody described in Eaton et al. WI-38 cells were engineered via infection and subsequent selection with pBABE-hTERT-hygromycin (Addgene), pBABE-SV40T/t-neomycin (Addgene), and/or pBABE-Ras(V12)-puromycin. Cell lysates were prepared and analyzed via 10% SDS-PAGE. Bars indicate the mobility of standard molecular weight markers, in kilo-Daltons (kDa). Bottom immunoblots—the top part is the same samples with an anti-phosphoT160 Cdk2 antibody (Cell Signaling), the middle part is with an anti-Cdk2 antibody (Santa Cruz) and the bottom part is for β tubulin (Sigma) as a loading control.
We then wanted to confirm that Cdk2 is activated in the transformed WI-38-hTERT-SV40T/t-Ras cells which lack p52-C/EBPβ1. We performed an immunoblot with the anti-phosphoT160 Cdk2 antibody, which detects active Cdk2. As shown in the bottom of Figure 2, Cdk2 is activated in the transformed WI-38-hTERT-SV40T/t-Ras cells (Fig. 2 and bottom, compare lane 4 to lane 1). However, Cdk2 is also activated in the immortalized but non-transformed WI-38-hTERT-SV40T/t cells in which p52-C/EBPβ1 is not degraded (Fig. 2 and bottom, lane 3). This indicates that either C/EBPβ1 is not phosphorylated on Thr235 by Cdk2 in these cells or that phosphorylation of C/EBPβ1 on Thr235 by Cdk2 is necessary but not sufficient for loss of p52-C/EBPβ1. Apparently, C/EBPβ1 is protected from degradation in the WI-38-hTERT-SV40T/t cells, despite Cdk2 being activated.
It is currently unknown why C/EBPβ1 is protected from degradation in the WI-38-hTERT-SV40T/t cells. Particular protein-protein interactions or addition or removal of necessary post-translational modifications may be preventing phosphorylation of C/EBPβ1 on Thr235 by active Cdk2. Alternatively, C/EBPβ1 may be phosphorylated on Thr235 by Cdk2; however, addition or removal of other post-translational modifications of p52-C/EBPβ1, such as deacetylation, may be required for its degradation via the proteosome. Also, expression or appropriate localization of a necessary ubiquitin E3 ligase may be required for ubiquitination and subsequent degradation of phosphoC/EBPβ1. Identifying this additional necessary component(s) for the degradation of C/EBPβ1 (illustrated in Fig. 3) is an area of active research in our laboratory as it may be an important key to understanding how Ras-expressing cells downregulate IL6 expression and bypass OIS.
C/EBPβ in Ras Induced Senescence Versus Ras Transformation
Activated Ras leads to OIS in normal cells yet leads to transformation of many immortalized cell lines. These two very different functional outcomes likely occur because of differences in signaling, the details of which remain unclear at this point. One contributing explanation (see model, Fig. 3) could be that in normal cells where C/EBPβ2 is not expressed, Ras is signaling through C/EBPβ1 to activate IL6, as well as other C/EBPβ-regulated genes including IL8 and p15INK4B, leading to senescence. During transformation Ras may be signaling through C/EBPβ2, as C/EBPβ1 is degraded.
In support of this model, C/EBPβ1 is the predominant isoform of C/EBPβ expressed in normal mammary epithelial cells and C/EBPβ1 is the primary transactivator isoform able to induce IL6 and senescence in normal cells.15,22 Conversely, C/EBPβ2 is the predominant C/EBPβ isoform expressed in breast cancer cells, transformed cells in which the Ras pathway is activated.15 Moreover, overexpression of C/EBPβ2 in MCF10A mammary epithelial cells allows these cells to acquire transforming characteristics.17 Additionally, p52C/EBPβ1 is negatively regulated by activated Ras via ubiquitin-mediated degradation in transformed mammary epithelial cells and transformed WI38 fibroblasts.22 Therefore Ras is likely signaling through the second isoform of C/EBPβ during transformation. Signaling from Ras to C/EBPβ2 is likely through phosphorylation and subsequent activation of C/EBPβ2,18,25 allowing C/EBPβ2 to transcriptionally activate genes involved in proliferation and epithelial to mesenchymal transition.25–30
Summary and Conclusions
Expression of activated Ras in cells leads to either OIS, a tumor suppressive mechanism or transformation. Both of these processes require the transcription factor C/EBPβ. We propose that one possible mechanism by which the introduction of activated Ras into cells leads to two different outcomes in different cells may be the particular isoform of C/EBPβ targeted by Ras signaling (Fig. 3). We show that full length C/EBPβ1 is the primary isoform responsible for the induction of IL6 and senescence in normal fibroblasts. The expression pattern of C/EBPβ1 in normal versus transformed cells is consistent with this isoform playing a role in OIS. Moreover, during transformation by Ras, C/EBPβ1 is negatively regulated by phosphorylation on Thr235 by Cdk2 and subsequent proteasomal degradation, representing a mechanism to bypass OIS. On the contrary, C/EBPβ2 is expressed in breast cancer cells, transactivates the expression of genes involved in proliferation, and promotes transforming characteristics in MCF10A cells. Therefore, it is likely that Ras signals through the C/EBPβ1 isoform during OIS and C/EBPβ2 during transformation (Fig. 3).
Activation of ras oncogenes is frequently encountered in human tumors. Moreover, we have never encountered a cancer cell line of any type (breast, colon, kidney, liver, prostate among others) where p52C/EBPβ1 is present, when examined by immunoblotting with an N-terminal specific antibody to clearly distinguish C/EBPβ1 from phosphoC/EBPβ2 (unpublished data). Loss of p52C/EBPβ1 may be a prerequisite for tumor cells to subvert Ras-induced senescence; and as such, understanding how the stability of p52C/EBPβ1 is regulated upon Cdk2 phosphorylation is likely to reveal important details about how tumor cells escape OIS.
Acknowledgements
Thanks to Maria Abreu, Kim Boelte, Linda Bundy, Alisha Russell and David Vaught for insightful suggestions and Rachel Jerrell for technical assistance. We also thank Hal Moses (Vanderbilt University) for the WI-38 fibroblasts, Scott Lowe (Cold Spring Harbor) for the pBABE-Ras(V12)-puromycin and S. Akira for the CMV-NF-IL6-T235A. This work was funded by NIH GM69634 and the Cell Biology and Molecular Sciences training grant.
Abbreviations
- Cdk2
cyclin dependent kinase 2
- C/EBPβ
CCAAT/enhancer binding protein beta
- C-terminal
carboxy terminal
- DNA
deoxyribonucleic acid
- hTERT
human telomerase reverse transcriptase
- IL6
interleukin 6
- kDa
kilodaltons
- MEFs
mouse embryonic fibroblasts
- N-terminal
amino terminal
- OIS
oncogene-induced senescence
- Phospho
phosphorylated
- PLAC1
placenta-specific 1
- SV40T/t
simian virus 40 large and small T antigens
- SWI/SNF
switch sucrose nonfermentable
- Thr
threonine
Extra View to: Atwood AA, Sealy L. Regulation of C/EBPbeta1 by Ras in mammary epithelial cells and the role of C/EBPbeta1 in oncogene-induced senescence. Oncogene. 2010;29:6004–6015. doi: 10.1038/onc.2010.336.
References
- 1.Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr. Opinions in Cell Biol. 2008;20:150–155. doi: 10.1016/j.ceb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian T, Malik R, Thomas S, Sage J, Johnson PF. C/EBPbeta cooperates with RB:E2F to implement Ras (V12)-induced cellular senescence. EMBO J. 2005;24:3301–3312. doi: 10.1038/sj.emboj.7600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc Natl Acad Sci. 2002;99:207–212. doi: 10.1073/pnas.012437299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Descombes P, Schibler U. A liver enriched transcriptional activator protein, LAP and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;3:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 5.Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Akira S, Yoshida K, Umemtot M, Yoneda Y, Shirafuji N. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 7.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D. Lymophoproliferative disorder and imbalanced T-helper response in C/EBPbeta-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R. CCAAT enhancer-binding protein beat is required fro normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC. C/EBPbeta modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol Cell Biol. 1999;19:7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes and Development. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seagroves T, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington G, et al. C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation and functional differentiation in the mouse mammary gland. Genes and Development. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowenz-Leutz E, Leutz A. A C/EBPbeta isoform recruits the SWI/SNF complex to activate myeloid genes. Mol Cell. 1999;4:735–743. doi: 10.1016/s1097-2765(00)80384-6. [DOI] [PubMed] [Google Scholar]
- 14.Raught B, Liao WS, Rosen J. Developmentally and hormonally reculated CCAAT/Enhancer-binding protein isoforms influence beta-casein gene expression. Mol Endo. 2005;9:1223–1232. doi: 10.1210/me.9.9.1223. [DOI] [PubMed] [Google Scholar]
- 15.Eaton EM, Hanlon M, Bundy L, Sealy L. Characterization of C/EBPbeta isoforms in normal versus neoplastic mammary epithelial cells. J Cell Physiol. 2001;189:91–105. doi: 10.1002/jcp.1139. [DOI] [PubMed] [Google Scholar]
- 16.Koslowski M, Tureci O, Biesterfeld S, Seitz G, Huber C, Sahin U. Selective activation of trophoblast-specific PLAC1 in breast cancer by CCAAT/enhancer binding protein beta (C/EBPbeta) isoform 2*. J Biol Chem. 2009;284:28607–28615. doi: 10.1074/jbc.M109.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bundy LM, Sealy L. CCAAT/enhancer binding protein beta (C/EBPbeta)-2 transforms normal mammary epithelial cells and induces epithelial to mesenchymal transition in culture. Oncogene. 2003;22:869–883. doi: 10.1038/sj.onc.1206216. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Shuman JD, Guszczynski T, Sakchaisri K, Sebastian T, Copeland TD, et al. RSK-mediated phosphorylation in the C/EBP{beta} leucine zipper regulates DNA binding, dimerization and growth arrest activity. Mol Cell Biol. 2010;30:2621–2635. doi: 10.1128/MCB.00782-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceseña TI, Cui TX, Subramanian L, Fulton CT, Iñiguez-Lluhí JA, Kwok RP, Schwartz J. Acetylation and deacetylation regulate CCAAT/enhancer binding protein beta at K39 in mediating gene transcription. Mol Cell Endocrinol. 2008;289:94–101. doi: 10.1016/j.mce.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Molina H, Huang H, Zhang YY, Liu M, Qian SW, et al. O-linked N-acetylglucosamine modification on CCAAT enhancer-binding protein beta: role during adipocyte differentiation. J Biol Chem. 2009;284:19248–19254. doi: 10.1074/jbc.M109.005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuilman T, Michaloglou C, Vredeveld L, Dourma S, van Doom R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Atwood AA, Sealy L. Regulation of C/EBPbeta1 by Ras in mammary epithelial cells and the role of C/EBPbeta1 in oncogene-induced senescence. Oncogene. 2010;29:6004–6015. doi: 10.1038/onc.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uematsu S, Kaisho T, Tanaka T, Matsumoto M, Yamakami M, Omori H, et al. The C/EBPbeta isoform 34 kDa LAP is responsible for NF-IL-6-mediated gene induction in activated macrophages, but is not essential for intracellular bacterial killing. J Immuno. 2007;179:5378–5386. doi: 10.4049/jimmunol.179.8.5378. [DOI] [PubMed] [Google Scholar]
- 24.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanlon M, Sealy L. Ras regulates the association of serum response factor and CCAAT/Enhancer-binding protein beta. J Biol Chem. 1999;274:14224–14228. doi: 10.1074/jbc.274.20.14224. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon M, Bundy LM, Sealy L. C/EBPbeta and Elk-2 synergistically transactivate the c-fos serum response element. BMC Cell Biol. 2000;1:2. doi: 10.1186/1471-2121-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanlon M, Sturgill TW, Sealy L. ERK2- and p90Rsk2-dependent pathways regulate the CCAAT/Enhancer-binding protein-beta interaction with serum response factor. J Biol Chem. 2001;276:38449–38456. doi: 10.1074/jbc.M102165200. [DOI] [PubMed] [Google Scholar]
- 29.Hanlon M, Sealy L. Ras regulates the association of serum response factor and CCAAT/Enhancer-binding protein beta. J Biol Chem. 1999;274:14224–14228. doi: 10.1074/jbc.274.20.14224. [DOI] [PubMed] [Google Scholar]
- 30.Bundy L, Wells S, Sealy L. C/EBPbeta2 confers EGF-independent growth and disrupts the normal acinar architecture of human mammary epithelial cells. Mol Cancer. 2005;4:43. doi: 10.1186/1476-4598-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell A, Boone B, Jiang A, Sealy L. Genomic profiling of C/EBPbeta2 transformed mammary epithelial cells: a role for nuclear IL1beta. Cancer Biol Ther. 2010;10:509–519. doi: 10.4161/cbt.10.5.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metz R, Ziff E. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to trans-locate to the nucleus and induce c-fos transcription. Genes Dev. 1991;5:1754–1766. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- 33.Mahoney CW, Shuman J, McKnight SL, Chen HC, Huang KP. Phosphorylation of CCAAT-enhancer binding protein by protein kinase C attenuates site-selective DNA binding. J Biol Chem. 1992;267:19396–19403. [PubMed] [Google Scholar]
- 34.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 35.Wegner M, Cao Z, Rosenfeld MG. Calcium-regulated phosphorylation within the leucine zipper of C/EBPbeta. Science. 1992;256:370–373. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- 36.Tang QQ, Grønborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]