Abstract
We recently reported that a complex between focal adhesion kianse (FAK) and the molecular scaffold RACK1 controlled nascent integrin adhesion formation and cell polarization, via peripheral recruitment of the cAMP - degrading PDE4D5 isoform. Here we review and extend these studies by demonstrating that the FAK/RACK1/PDE4D5 ‘direction-sensing’ complex likely functions by signaling, via the guanine nucleotide exchange factor EPAC , to its small GTPase target Rap1. Specifically, activating EPAC suppresses polarization of squamous cancer cells, while, in contrast, modulating PKA, the other major cAMP effector, has no effect. Moreover, FAK-deficient malignant keratinocytes re-expressing a FAK mutant that cannot bind to RACK1, namely FAK-E139A,D140A, display elevated Rap1 that is linked to impaired polarization. Thus, it is likely that the FAK/RACK1/PDE4D5 complex signals to keep Rap1 low at appropriate times and in a spatially-regulated manner as cells first sense their environment and make decisions about nascent adhesion stabilization and polarization. RACK1 is abundantly expressed in both normal and malignant keratinocytes, while FAK and PDE4D5 are both elevated in the cancer cells, suggesting that the FAK/RACK1/PDE4D5/Rap1 signaling axis may contribute to FAK's well documented role in tumor progression.
Key words: integrns, adhesion, spreading initiation, focal adhesion kinase, scaffold, RACK1, phosphodiesterase, cAMP, GTPase, Rap1, polarization, direction-sensing, cancer
Cell Polarization and Nascent Adhesion Structures: RACK1
Cell polarization requires the differential localization of specific protein complexes within cells, as well as the correct spatial distribution of structures like Golgi and the microtubule-organizing center. Understanding the signals that regulate spatial events is important, since polarization and directional migration are key to many physiological processes such as embryonic development, inflammatory responses, wound healing and during cancer cell invasion. Although important for directional migration, the mechanisms underlying assembly and spatial distribution of signaling complexes as cells first contact ECM remain to be fully defined. Previous studies have identified protein complexes that regulate polarization in a number of contexts. For example, integrin-induced activation of Cdc42 controls the ability of astrocytes to polarize in response to a wound made in a confluent cell monolayer, by leading to the activation and recruitment of the mPar6/PKC complex.1,2
Cell polarization and directional migration also require the coordinated and dynamic regulation of the actin cytoskeleton, critically at structures close to the protruding edge and cell-substratum interface as cells move forward. Mass spectrometry studies have identified proteins that can bind transiently to focal adhesion components, and demonstrated the existence of nascent adhesion structures, originally called Spreading Initiation Centers (SICs). These are actin-based structures that form transiently as cells first make contact locally with the ExtraCellular Matrix (ECM; reviewed in ref. 3). Exaggerated versions of SICs form in close proximity to the cell-substratum interface as suspended cells are plated onto ECM, and so first make contact with ECM components. Therefore, SICs probably represent nascent or ‘first point of contact’, structures that assemble and disassemble rapidly as cells adhere and move around on ECM components. SICs have been described to contain ribosomal RNA and RNA-binding proteins, which contribute to cell spreading3 and are clearly distinct from mature focal adhesions. In our recent work, we described nascent adhesive structures that may be similar to the previously described SICs, and these are distinguished from other focal adhesion structures by containing the signaling scaffold protein Receptor for Activated C Kinase 1 (RACK1; reviewed in ref. 4), which is not generally found at mature adhesions.3,4
RACK1: A Complex with FAK Important for Nascent Adhesion Assembly
RACK1 is a widely expressed WD-repeat protein that was originally identified as a protein that could bind phorbol ester-activated PKC isoforms, notably PKCβII.5–8 However, RACK1 is now known to regulate diverse cellular processes such as protein translation, cytokinesis, cell adhesion and migration. RACK1 is a multi-functional scaffold protein that binds to a wide variety of proteins, impacting on multiple signal transduction pathways via compartmentalization, so bringing together key signaling components in time and space.8–10 Amongst the increasing repertoire of RACK1 binding partners are Src and Abl tyrosine kinases,11,12 β1-integrin cytoplasmic domains,13 the p85 regulatory sub-unit of PI 3-kinase,14 a number of protein tyrosine phosphatases, including PTP1B13,15–17 and PTPmu,17 PLCγ,18 dynamin,10 p120Ras-GAP,19 HIF1α20 and others (reviewed in ref. 8 and 21). Notable amongst RACK1 binding partners is the cAMP-degrading phosphodiesterase PDE4D5, which binds to RACK1 via two well-defined sites, one within its isoform-specific amino-terminal region and the other within its conserved catalytic unit.22,23 A number of these RACK1 protein interactions influence integrin function, and integrin-dependent cell migration and chemotaxis.13,15,16 RACK1 binds to the integrin effector protein Focal Adhesion Kinase (FAK) and RACK1 tyrosine phosphorylation by c-Abl is proposed to mediate insulin-like growth factor1-dependent regulation of FAK.24
FAK is present and co-localizes with RACK1, at nascent protrusive structures that form as Mouse Embryo Fibroblasts (MEFs) spread on ECM components.4,25 FAK is a pivotal signal integrator operating at focal adhesions (its biological functions are depicted in Fig. 1A) and is required for cell spreading, optimal integrin-dependent cell migration and integrin-induced signaling that promotes proliferation and survival.26–32 FAK's role in assembly of actin-based structures that mediates cell spreading includes the binding and regulation of the Arp2/3 actin nucleation complex.25 Nascent lamellipodia, which originate at the tips of SICs, do not form in FAK-deficient cells or in cells in which FAK cannot be tyrosine phosphorylated after integrin engagement.4,25 Thus, FAK links integrin signaling directly to the actin assembly machinery, and we have implicated the FAK FERM domain in this process.4,25
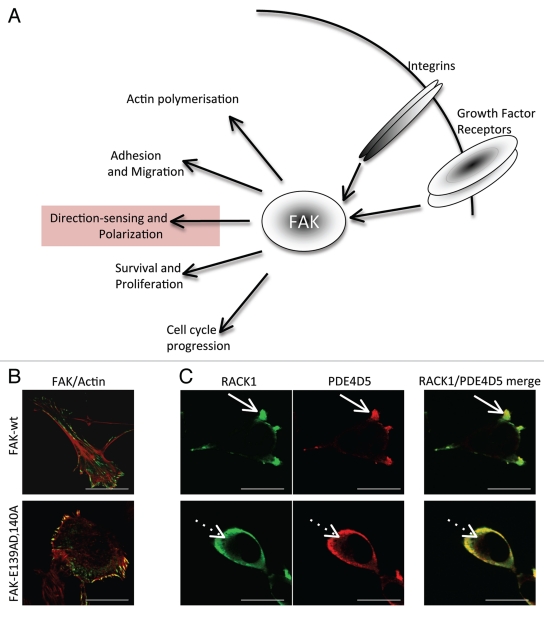
Figure 1.
(A) Schematic showing the recognized cellular functions of FAK, highlighting the direction-sensing and polarization functions we describe here. (B) Images show comparison of cell shape and actin filaments in FAK-deficient SCC cells re-expressing either FAK-wt or the FAK/RACK1 binding impaired mutant FAK-E139A,D140A. (C) The co-staining of RACK1 and PDE4D5 is shown in protrusive nascent adhesions as FAK-wt SCC cells are plated on to FN for 15 min (solid arrows). By comparison, both RACK1 and PDE4D5 are cytoplasmic in FAK-E139A,D140A-expressing cells (broken arrows). Scale bars 20 µm.
In more recent work, we identified that the FAK FERM domain, specifically residues E139 and D140, which is required for direct binding of FAK to RACK1. Unlike the previously identified complex between FAK and Arp3, where binding is regulated by integrin induced FAK-Y397 phosphorylation, the FAK/RACK1 complex does not change under these conditions. Using Squamous Cell Carcinoma (SCC) cells, in which FAK could be genetically deleted by tamoxifen treatment, we found that FAK is required for both cell spreading and RACK1 localization to nascent adhesions close to the cell-substratum interface as imaged by Total Internal Reflection Fluorescence microscopy (TIRF).4 Small patches of FAK/RACK1 co-localization are also evident at nascent adhesive structures in fully spread cells. Disruption of this interaction through expression of an effecter mutant of FAK, namely FAK-E139A,D140A, results in profound changes in cellular morphology reminiscent of non-polarized cells that lack cytoplasmic actin filaments (Fig. 1B and reviewed in ref. 4). Moreover, RACK1 localization is restricted to the cytoplasm and the FAK-E139A,D140A-expressing cells are unable to efficiently form nascent adhesion structures when compared to FAK-wt-expressing SCC cells (Fig. 1C; solid arrows point to nascent adhesions in FAK-wt cells and broken arrows point to cytoplasmic RACK1 in FAK-E139A, D140A cells). These data indicate that the FAK/RACK1 complex is spatially regulated at nascent adhesions.
FAK/RACK1: A “Direction-Sensing Complex”
While typical apical-basolateral epithelial polarity is frequently lost in cancer cells, polarization and directional migration towards extracellular stimuli (and perhaps host cells) likely plays a role in cancer invasion and metastatic spread. However, little is known about protein complexes specifically involved in direction-sensing and how invasive cancer cells turn and polarize, towards extracellular stimuli. We found a role for the FAK/RACK1 complex in cell invasion and wound induced cell polarity. In particular, FAK is needed for wound-induced polarization of SCC cells and chemotactic invasion through 3D matrix gels.4 We believe this represents a novel “direction-sensing complex” that is essential for the formation of nascent adhesion structures which permit cells to sense and polarize towards chemotactic factors. As the FAK/RACK1 complex was also evident in fully spread cells, we used time-lapse microscopy to monitor RACK1 position in live cells. This demonstrated that cells are constantly generating RACK1 containing membrane “blebs”, which can stabilize and these transient structures presumably act as environmental sensors; the pattern of their stabilization and turnover, i.e., their dynamic regulation controls cell polarization and migration in response to directional cues (Movies can be viewed in Sup. Information in ref. 4).
How does FAK/RACK1 Complex Control Direction-Sensing?: Recruitment of PDE4D5
A key question was why inhibiting FAK binding to the enzymatically-inert scaffold protein RACK1 blocked stabilization of transient nascent adhesion structures and caused profound direction sensing and polarization defects. We addressed the nature of the sequestered cargo delivered by FAK-bound RACK1 to nascent adhesions, and found a key role for the RACK1 cargo—a particular PDE4 family isoform, namely PDE4D5,23 known to bind to RACK1 through a well-characterized interaction involving its isoform specific N-terminal region.33,34 Inhibition of the FAK/RACK1 complex blocked the co-recruitment of RACK1 and PDE4D5 to nascent adhesions (also shown here in Fig. 1C) and to the leading edge of polarizing cells.4 Moreover, a complex exists containing FAK, RACK1 and PDE4D5 that requires FAK's association with RACK1 and RACK1's association with PDE4D5, and this complex is necessary for nascent adhesion stabilization and wound-induced polarization.4 Our work showed that when the FAK-RACK1 complex is perturbed, neither RACK1 nor PDE4D5 can localize to nascent protrusions or to the leading edge of wounded cells. However, the question remained as to the nature of PDE4D5-induced or suppressed, signals that are permissive for stabilization of nascent adhesion, cell polarization and directional migration.
FAK/RACK1/PDE4D5: Signaling via PKA or EPAC?
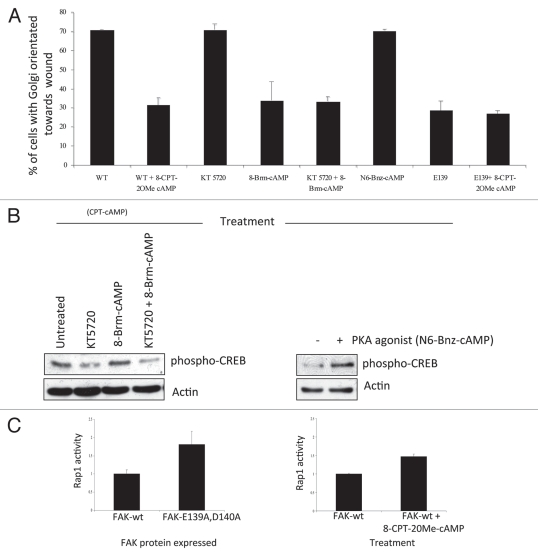
It is now well established that cAMP signaling is compartmentalized in cells with gradients of cAMP interpreted by sequestered populations of the two cAMP effectors, Protein Kinase A (PKA) and EPAC.34 Sub-populations of spatially constrained PDEs form and shape these gradients at defined locales, thereby determining the activation threshold for specific signaling complexes that incorporate either or both, PKA and EPAC.34 The role of EPAC can usefully be inferred by using the selective EPAC agonist, 8-CPT-2OMe-cAMP (CPT-cAMP), which is unable to activate PKA.35 We found that CPT-cAMP treatment of FAK-wt cells elicited a profound loss of wound-induced polarization (∼71% to ∼31%), similar to the polarization displayed by FAK-deficient cells (∼27%, data not shown) or in cells where FAK and RACK1 can no longer interact (∼28%) (FAK-E139A,D140A termed E139 in Fig. 2A), suggesting that the cAMP polarization phentotype regulated by the FAK/RACK1/PDE4D5 complex may be determined by the action of EPAC rather than PKA (Fig. 2A). We also noted that CPTcAMP did not induce any further loss of polarization in FAK-E139 cells, implying the FAK/RACK1 disrupting mutation and CPT-cAMP influence the same signaling effector, namely EPAC. Consistent with this, we now report that the selective PKA agonist, N6-Bnz-cAMP,35 had no effect on polarization, even although it visibly induced phosphorylation of the transcription factor substrate of PKA, i.e., CREB (cAMP-regulatory binding protein; Fig. 2B, right part). Furthermore, loss of polarization in cells challenged with the cAMP analogue, 8-Brm-cAMP, which can activate both EPAC and PKA, was not attenuated by the selective PKA inhibitor, KT5720 (Fig. 2A). This occurred under circumstances where the PKA-mediated phosphorylation of CREB was clearly inhibited by KT5720 (Fig. 2B). Thus, it appears that elevated cAMP exerts its inhibitory effect on polarization through an action mediated by EPAC, rather than by PKA.
Figure 2.
(A) FAK-wt or FAK-E139A,D140A cells (as indicated) were plated on FN for 2 hours and wounded in the presence of 8-CPT-2OMe cAMP (CPT-cAMP; 10 µM), KT 5720 (1 µM), 8-Brm-cAMP (10 µM), KT 5720 + 8-Brm-cAMP or N6-Bnz-cAMP (10 µM). After 1.5 hours cells were fixed and stained with the Golgi marker anti-GM130, TRITC phalloidin and DAPI, to assess polarization in response to the wounded areas. The percentage of each cell type with the Golgi orientated to the wound was calculated by counting 100 cells in 3 experiments and is shown in the graph. (B) FAK-wt cells were treated with KT 5720 (1 µM) and/or N8 = Brm-cAMP (left parts) and with 6-Bnz-cAMP (10 µM) for 30 min then immunoblotted using anti-phospho-CREB and actin antibodies as probes. (C) FAK-wt cells with or without CPT-cAMP (10 µM for 3.5 hrs, time point chosen to maintain consistency with cell polarization experiments, conditions detailed above; left part) or FAK-wt and FAK-E139A,D140A cells (right part), were harvested and a Rap1 assay performed as described in Materials and Methods. Quantification of Rap1-GTP normalized to total Rap1 from three separate experiments is shown.
RACK1 Mediates Signaling from FAK to the Small GTPase Rap1
EPAC exerts actions via its role as a guanine-nucleotide exchange factor for the Rap small GTP binding proteins.35 We found that challenge of FAK-wt cells with CPT-cAMP (the EPAC agonist) increased Rap1 activation, as judged by increased GTP loading, providing evidence that CPT-cAMP does indeed activate EPAC in these SCC cells (Fig. 2C, right part). Importantly, we also found that the activity of Rap1 in FAK-E139A,D140A cells was increased when compared to FAK-wt cells (Fig. 2C, left part), while there was no change in Cdc42 activity that is often associated with polarity (not shown). Quantification shown is a combination of multiple experiments and demonstrated a consistent 1.8 fold increase in Rap1 activity in FAK-E139A,D140A cells vs. FAK-wt cells. As this FAK-E139A,D140A mutant fails to sequester the PDE4D5/RACK1 complex and stabilize nascent adhesions (or SICs) during cell spreading, our data imply that in cells expressing this FAK/RACK1 binding-impaired FAK mutant protein—cAMP levels to rise unchecked in the vicinity of FAK at the periphery of cells as they sense their environment. In this way, EPAC and its downstream target Rap1, would become constitutively activated. This uncontrolled activity of EPAC/Rap1 is linked to the loss of ability to stabilize nascent adhesive structures, and consequentially impaired cell spreading and responses to directional cues that are needed for cells to polarize.
Final Thought
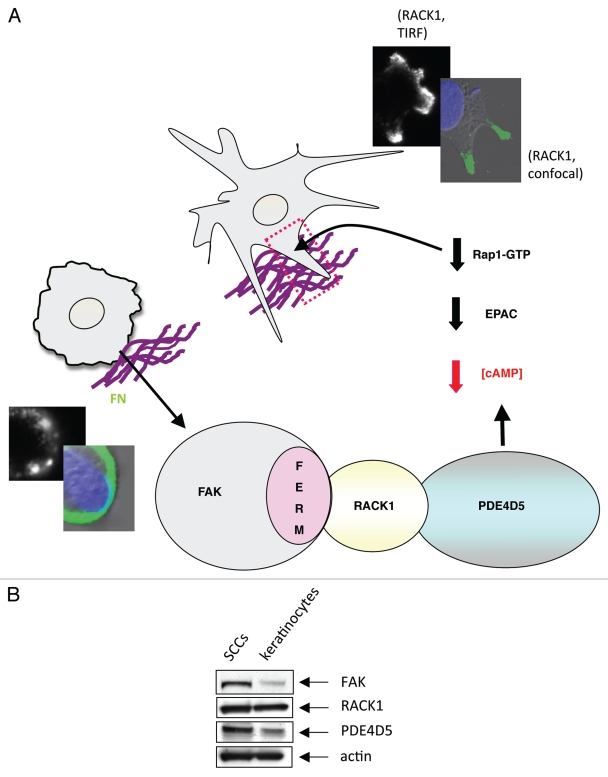
Thus, we previously defined a novel “direction sensing complex” that comprises of FAK, RACK1 and PDE4D5. This is needed for cells to stabilize nascent actin-based adhesive structures and to polarize in response to directional cues. This work identified a new function for the FAK FERM domain, and a physiological function for the enigmatic RACK1-PDE4D5 complex. Indeed, both FAK and RACK1 are molecular scaffolds that can bind independently to a wide range of intracellular proteins, yet these two scaffold proteins also bind to each other, with FAK dictating the spatial distribution of RACK1 and one of its clients, PDE4D5. The assembly of this complex presumably regulates local gradients of cAMP. Here we show that this likely regulates EPAC-dependent activation of the small GTPase Rap1. This new work ‘joins up’ a line of research articles that show the EPAC-Rap1 signaling axis has an important role in integrin adhesion dynamics, migration and polarity.35–37 We interpret our results and these previous findings to imply that Rap1's role in cell polarization requires both GTPase activation and inactivation to be tightly regulated in space and time, and that the FAK/RACK1/PDE4D5 complex serves to keep Rap1 transiently inactive at appropriate times in the vicinity of nascent adhesions as they form and stabilize. The FAK mutant protein that would result in an inability to depress Rap1 activity in the vicinity of nascent adhesions as they form causes lack of stabilization (depicted in model in Fig. 3A).
Figure 3.
(A) Model outlining how our data explains the role of the FAK/RACK1/PDE4D5 complex in keeping cAMP levels low in the vicinity of nascent peripheral adhesion structures as these form and sense their environment. In our model, this is required for a sub-set of nascent adhesions to stabilize, typically along one cell edge, so inducing a polarized phenotype. The FAK/RACK1-mediated recruitment of PDE4D5 signals to Rap1, likely via EPAC, to keep Rap1 appropriately suppressed in space and time so as to permit nascent adhesion stabilization, spreading and polaization. This is in keeping with our earlier data recently published in reference 4. The edges of cells expressing FAK-wt (top images showing stabilized protrusive structures) and FAK-E139A,D140A (lower images) are shown, including TIR F images (black and white) taken at the cell-substratum interface. (B) Expression levels of FAK, RACK1 and PDE4D5 were determined in lysates from normal and malignant keratinocytes (SCCs) which were immunoblotted using anti-FAK, anti-RACK1, anti-PD4D5 and anti-actin antibodies as probes.
The spatially determined FAK/RACK/PDE4D5-EPAC/Rap1 signaling axis may well contribute to FAK's role in promoting the invasive cancer phenotype, that we showed previously by tissue-specific FAK deletion studies in skin and breast cancer models.38,39 Several studies have also implicated release of cAMP gating, PDE enzymes and PDE4 in particular, in cancer cell invasion and migration of a number of cell types.40–42 Hence the direction-sensing FAK/RACK1/PDE4D5 complex, via signaling to EPAC and Rap1 in space and time, is a likely contributor to the invasive cancer phenotype. In this regard, we note that while RACK1 is abundantly and equivalently expressed in both murine normal and malignant keratinocytes (SCC cells) (Fig. 3B), both FAK and PDE4D5 are elevated in SCC cells, showing that the pathway potential is elevated during progression to the malignant phenotype.
Methods
Materials.
Antibodies for immunoblotting and immunocytochemistry were as follows: anti-FAK (Transduction Laboratories, 610088); anti-actin (Sigma, A4700); anti-pCREB (Cell Signaling Technology, 9196); and anti-PDE4D5 (described previously in ref. 4). Antimouse and anti-rabbit IgG-peroxidase conjugated secondary antibodies were from New England Biolabs (7076, 7074), anti-sheep IgG-peroxidase conjugated secondary antibodies were obtained from Invitrogen and TRITC-phalloidin was obtained from Sigma. Human fibronectin (FN) and reduced growth factor Matrigel were obtained from Becton Dickinson, (354230). Triton X100, TRITC-phalloidin and 4-OHT were obtained from Sigma.
Generation of FAK-deficient SCC cell lines.
K14CreERT2/FAKflox/flox mice were subjected to chemical carcinogenesis and cells generated as described previously in reference 4. The cells were harvested by trypsinization and subcultured. To delete fak, cells were treated with 4-hydroxy (OH)-tamoxifen at a concentration of 10 µM for 24 hours. FAK-deficient (-/-) SCC cells expressing FAK mutants (wt or FAK-E139A, D140A) were generated by amaxa nucleofection kit v, programme P20, using the pWZL-FAK expression constructs outlined before.4
Immunocytochemistry and cell polarization assays.
For immunocytochemistry, cells were grown on glass coverslips, rinsed in TBS, fixed in 3.7% formaldehyde-PBS, 100 mM K-Pipes pH 6.8, 10 mM EGTA, 1 mM MgCl2, 0.2% triton x100 (Sigma), washed twice in TBS 0.1% triton x100 and blocked in TBS 0.1% BSA (Sigma, A7906) and incubated with primary antibody overnight. Primary antibody incubation was followed by several washes with TBS containing 0.1% triton x100 and subsequent incubation with FITC-labelled or Texas Red-labelled secondary antibodies. Actin stress fibres were visualized after staining with TRITC-phalloidin. For polarization assays, cells were plated on FN at a density of 2.5 × 106 cells per well on a 12 well dish for 2 hours. Cells were then wounded using a pipette tip, washed twice in PBS and twice in complete medium, left in complete medium for 1.5 hours and fixed, as above. To investigate the role of EPAC and PKA in cell polarity assays, cells were incubated in the presence of either 10 µM 8-CPT-2MeO-cAMP (Sigma, C8988), 10 µM 8-Brm-cAMP (BioLog, B022), 1 µM KT5720 (Sigma, K3761) or 10 µM N6-Bnz-cAMP (BioLog, B009) for the entire duration of the experiment as indicated above.
Immunoblotting.
Cells were washed twice with PBS and lysed in RIPA lysis buffer (50 mM Tris-HCL at pH 7.4, 150 mM sodium chloride, 0.1% SDS and 1% deoxycholate) with inhibitors (10 mM pyrophosphate, 100 mM sodium fluoride, 1 mM PMSF, 10 µg ml−1 aprotinin, 100 mM sodium orthovanadate, 10 mg ml−1 leupeptin and 10 mg ml−1 benzamidine; all purchased from Sigma). Clarification was by high speed centrifugation (16,000x g at 4°C for 15 min). Cell lysate (10–20 mg protein, as measured by Micro BCA Protein Assay Kit, was supplemented with SDS sample buffer (Tris at pH 6.8, 20% glycerol, 5% SDS, β-mercaptoethanol and bromo-phenol blue) separated by SDS-PAGE, transferred to nitrocellulose and immunoblotted with specific antibodies.
Rap1 activity assays.
0.5 × 106 cells were plated on a 90 mm tissue culture dish in the presence of serum and left to grow for 48 hours. Cells were then harvested and Rap1 activity measured using an Ez detect Rap1 activation kit (Perbio, 89872) as per manufacturers instructions. Samples were resolved on a 4–12% Nupage gel (Invitrogen, NPO322BOX) and western blotted for both active and total Rap1. For analysis, densitometry measurements were taken using Image J software and the percentage of activated Rap1 calculated relative to total Rap1.
Acknowledgements
This work was supported by a Cancer Research UK Program Grant to M. Frame (C157/A9148). We thank Miles Houslay for advice on use of inhibitors and agonists of cAMP effectors.
Extra View to: Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, et al. A complex between FAK, RACK1 and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–1092. doi: 10.1016/j.cub.2010.04.042.
References
- 1.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 2.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 3.de Hoog CL, Foster LJ, Mann M. RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell. 2004;117:649–662. doi: 10.1016/s0092-8674(04)00456-8. [DOI] [PubMed] [Google Scholar]
- 4.Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, et al. A complex between FAK, RACK1 and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–1092. doi: 10.1016/j.cub.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 5.McCahill A, McSorley T, Huston E, Hill EV, Lynch MJ, Gall I, et al. In resting COS1 cells a dominant negative approach shows that specific, anchored PDE4 cAMP phosphodiesterase isoforms gate the activation, by basal cyclic AMP production, of AKAP-tethered protein kinase A type II located in the centrosomal region. Cell Signal. 2005;17:1158–1173. doi: 10.1016/j.cellsig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Mochly-Rosen D, Smith BL, Chen CH, Disatnik MH, Ron D. Interaction of protein kinase C with RACK1, a receptor for activated C-kinase: a role in beta protein kinase C mediated signal transduction. Biochem Soc Trans. 1995;23:596–600. doi: 10.1042/bst0230596. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 9.Dorn GW, 2nd, Mochly-Rosen D. Intracellular transport mechanisms of signal transducers. Annu Rev Physiol. 2002;64:407–429. doi: 10.1146/annurev.physiol.64.081501.155903. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez MM, Ron D, Touhara K, Chen CH, Mochly-Rosen D. RACK1, a protein kinase C anchoring protein, coordinates the binding of activated protein kinase C and select pleckstrin homology domains in vitro. Biochemistry. 1999;38:13787–13794. doi: 10.1021/bi991055k. [DOI] [PubMed] [Google Scholar]
- 11.Chang BY, Chiang M, Cartwright CA. The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. J Biol Chem. 2001;276:20346–20356. doi: 10.1074/jbc.M101375200. [DOI] [PubMed] [Google Scholar]
- 12.Huang CC, Liu CH, Chuang NN. An enhanced association of RACK1 with Abl in cells transfected with oncogenic ras. Int J Biochem Cell Biol. 2008;40:423–431. doi: 10.1016/j.biocel.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Liliental J, Chang DD. Rack1, a receptor for activated protein kinase C, interacts with integrin beta subunit. J Biol Chem. 1998;273:2379–2383. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- 14.Kiely PA, Sant A, O'Connor R. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J Biol Chem. 2002;277:22581–22589. doi: 10.1074/jbc.M201758200. [DOI] [PubMed] [Google Scholar]
- 15.Buensuceso CS, Woodside D, Huff JL, Plopper GE, O'Toole TE. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J Cell Sci. 2001;114:1691–1698. doi: 10.1242/jcs.114.9.1691. [DOI] [PubMed] [Google Scholar]
- 16.Cox EA, Bennin D, Doan AT, O'Toole T, Huttenlocher A. RACK1 regulates integrin-mediated adhesion, protrusion and chemotactic cell migration via its Src-binding site. Mol Biol Cell. 2003;14:658–669. doi: 10.1091/mbc.E02-03-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourton T, Hellberg CB, Burden-Gulley SM, Hinman J, Rhee A, Brady-Kalnay SM. The PTPmu protein-tyrosine phosphatase binds and recruits the scaffolding protein RACK1 to cell-cell contacts. J Biol Chem. 2001;276:14896–14901. doi: 10.1074/jbc.M010823200. [DOI] [PubMed] [Google Scholar]
- 18.Disatnik MH, Hernandez-Sotomayor SM, Jones G, Carpenter G, Mochly-Rosen D. Phospholipase C-gamma 1 binding to intracellular receptors for activated protein kinase C. Proc Natl Acad Sci USA. 1994;91:559–563. doi: 10.1073/pnas.91.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehler JA, Moran MF. RACK1, a protein kinase C scaffolding protein, interacts with the PH domain of p120GAP. Biochem Biophys Res Commun. 2001;283:888–895. doi: 10.1006/bbrc.2001.4889. [DOI] [PubMed] [Google Scholar]
- 20.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schechtman D, Mochly-Rosen D. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene. 2001;20:6339–6347. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- 22.Bolger GB, Baillie GS, Li X, Lynch MJ, Herzyk P, Mohamed A, et al. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem J. 2006;398:23–36. doi: 10.1042/BJ20060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarwood SJ, Steele MR, Scotland G, Houslay MD, Bolger GB. The RACK1 signaling scaffold protein selectively interacts with the cAMP-specific phosphodiesterase PDE4D5 isoform. J Biol Chem. 1999;274:14909–14917. doi: 10.1074/jbc.274.21.14909. [DOI] [PubMed] [Google Scholar]
- 24.Kiely PA, Baillie GS, Barrett R, Buckley DA, Adams DR, Houslay MD, et al. Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for insulin-like growth factor I-mediated regulation of focal adhesion kinase. J Biol Chem. 2009;284:20263–20274. doi: 10.1074/jbc.M109.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, et al. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 26.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:982–996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 27.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 28.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 30.Richardson A, Malik RK, Hildebrand JD, Parsons JT. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 32.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 33.Baillie GS, Houslay MD. Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes. Curr Opin Cell Biol. 2005;17:129–134. doi: 10.1016/j.ceb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 2005;579:3264–3270. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 35.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 36.Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol. 2006;80:1542–1552. doi: 10.1189/jlb.0506357. [DOI] [PubMed] [Google Scholar]
- 37.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, et al. Rap1 translates chemokine signals to integrin activation, cell polarization and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, et al. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dua P, Gude RP. Pentoxifylline impedes migration in B16F10 melanoma by modulating Rho GTPase activity and actin organisation. Eur J Cancer. 2008;44:1587–1595. doi: 10.1016/j.ejca.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Murata K, Sudo T, Kameyama M, Fukuoka H, Muka M, Doki Y, et al. Cyclic AMP specific phosphodiesterase activity and colon cancer cell motility. Clin Exp Metastasis. 2000;18:599–604. doi: 10.1023/a:1011926116777. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor KL, Shaw LM, Mercurio AM. Release of cAMP gating by the alpha6beta4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol. 1998;143:1749–1760. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]