Abstract
Background
The time requirements for multiple daily nebulizer treatments are important impediments to the quality of life for most patients with cystic fibrosis (CF). The I-neb Adaptive Aerosol Delivery (AAD) System can be used with a new mode of breathing during inhalation of aerosol, the Target Inhalation Mode (TIM). As a function of the TIM algorithm, the patient is guided to a slow and deep inhalation, which can result in shorter treatment times.
Methods
This study was conducted as a 3-month patient handling study of the I-neb AAD System in 42 patients with CF aged 12–57 years. The I-neb AAD System was supplied in both the standard Tidal Breathing Mode (TBM), and in TIM. Patients were trained to use the I-neb AAD System in TIM for the delivery of all their inhaled medications, but if they were not comfortable with the TIM maneuver they could change to the TBM maneuver. The primary variables were compliance with the correct use of the I-neb AAD System, and treatment times. The secondary variables were based on study questionnaires at the end of the study and covered ease of use, patient confidence, and patient satisfaction with the I-neb AAD System.
Results
There were a total of 10,240 complete treatments and of these, 8979 (88%) were in TIM. Compliance with the correct use of the I-neb AAD System was 97.6%. The mean treatment time for complete treatments in TIM was 4.20 min, compared with 6.83 min when using the I-neb AAD System in TBM. The responses to the questionnaires indicated that over 77% of the patients found the I-neb AAD System in TIM to be either: very easy, easy, or acceptable to use.
Conclusions
The results demonstrated that by using the I-neb AAD System in TIM, a 40–50% reduction of nebulizer treatment times, and a high level of compliance could be achieved. The results also showed that the patients found the I-neb AAD System easy to use.
Key words: I-neb AAD System, Adaptive Aerosol Delivery, ease of use, patient satisfaction, mesh nebulizer
Introduction
The use of a slow inhalation maneuver followed by a breath hold has been the preferred method for administration of aerosol with the pressurized metered dose inhaler (pMDI), the most widely used aerosol delivery system.(1) The aim of the slow inhalation maneuver has been to maximize lung deposition by minimizing particle impaction in the upper airway, and to increase particle penetration into the lower airways.(2–6) The aim of the breath hold has been to increase deposition of the inhaled particles suspended in the airways by allowing these to settle.(2–4) The coordination of inhalation with firing of the bolus of aerosol from the pMDI has been shown to be rather difficult in the domiciliary setting.(7) Patients unable to use the pMDI as per instructions have generally been given a nebulizer with less demands on coordinated use during tidal breathing. Handling studies have, however, shown that domiciliary compliance with the correct use of nebulizers has also been poor.(8–11)
The analysis of the compliance related shortcomings of nebulizers has lead to the development of “intelligent” nebulizer systems such as the I-neb Adaptive Aerosol Delivery (AAD) System.(12) The I-neb AAD System is a portable (150 × 65 × 45 mm, h, w, d; 210 g), virtually silent mesh nebulizer system that can record adherence to treatment regimen, compliance with correct use of the device, and provide the patient with feedback on the correct use of the device. The I-neb AAD System was designed with two different patient breathing pattern algorithms: the Tidal Breathing Mode (TBM) and the Target Inhalation Mode (TIM, not commercially available in the United States).(12)
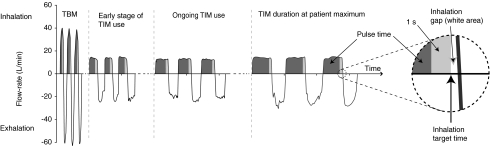
In TBM, the patient inhales the aerosol during tidal breathing, and the aerosol is pulsed during 50–80% of the inhalation. The length of each pulse of aerosol is determined by the patient's breathing pattern (inspiratory time per breath, tidal volume) and is based on the average of the preceding three breaths, a continuous calculation throughout the treatment. In TIM, the patient is guided to a slow and deep inhalation with a maximum length of 8 sec, of which the aerosol pulse is 7 sec long. During the last 1 sec when the patient reaches the end of the slow and deep inhalation, no aerosol is generated. The lack of aerosol delivery at the end of the inhalation minimizes the loss of aerosol during the following exhalation, and thereby enhances lung deposition of the inhaled aerosol. The 1 sec of inhalation without aerosol generation has the same effect as a 1 sec breath hold to achieve particle residence time in the lungs. In TIM, the I-neb AAD System adapts to the patient's lung volume by increasing the inhalation target time until the patient is just able to inhale past the target. If the patient is unable to extend the inhalation up to 8 sec, the I-neb AAD System adapts the inhalation target time to match the patient's preferred length of inhalation. The inhalation target time is thus optimized to each patient's comfort level and lung volume. At the end of the treatment the duration of the final pulse of aerosol is stored in the device memory to set the inhalation target time of the first inhalation of the next treatment.
The first clinical studies with the I-neb AAD System in TIM focused on the acceptability of the slow and deep breathing maneuver and were conducted in patients with cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD).(13,14) The results showed that the patients could be trained to perform the maneuver, and found the maneuver both easy to perform and acceptable. The studies were, however, only designed to be performed during 1 day at a clinic and only evaluated the patients' ability to learn how to perform the TIM maneuver. To evaluate the long-term acceptance of the I-neb AAD System in TIM, a handling study was run in patients with CF being treated with nebulized colistimethate sodium.
Materials and Methods
Patients
Forty-two patients (mean age 29 years, age range 12–57 years) with a diagnosis of CF participated in the handling study. The patients were included in the study if they had been using nebulized colistimethate sodium prior to inclusion, and had a forced vital capacity (FVC) value >1 L measured during the previous three months. All were outpatients and in stable condition. All patients signed an informed written consent form.
Study design
The study was designed as a 3-month handling study during which each patient included was supplied with an I-neb AAD System with two different mouthpieces: one designed for the TBM breathing maneuver and one for the TIM maneuver (Fig. 1). A questionnaire was used to determine the proportion of patients who accepted the TIM maneuver during the 3-month home usage, and the patients' compliance with the training in the use of the I-neb AAD System in TIM.
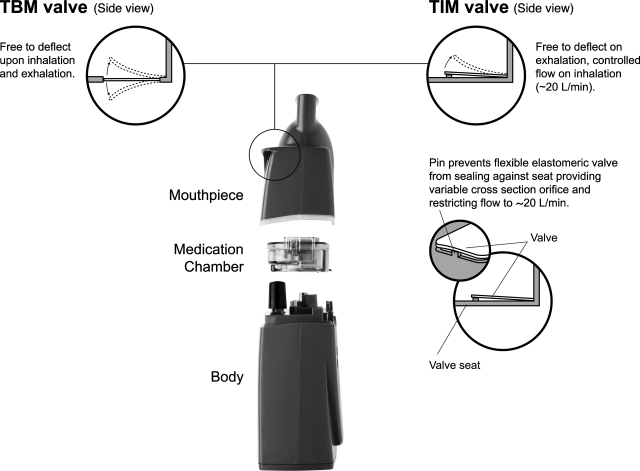
FIG. 1.
The I-neb Adaptive Aerosol Delivery (AAD) System. The main components of the device are the mouthpiece, the medication chamber assembly, and the body. The I-neb AAD System has been designed to deliver aerosol with two different breathing pattern algorithms: the Tidal Breathing Mode (TBM) and the Target Inhalation Mode (TIM). In TIM the inspiratory flow through the valve in the mouthpiece is limited to ∼20 L/min.
Patients were trained during inclusion in the use of the I-neb AAD System in both breathing maneuvers. At study start patients were initiated on the I-neb AAD System with the TIM mouthpiece, but were also instructed that if they were unable to adapt to the TIM maneuver they could change to the TBM mouthpiece and maneuver. In addition to colistimethate sodium, patients were able to nebulize albuterol, dornase alpha, tobramycin, and hypertonic saline with the I-neb AAD System during the study. Study procedures were checked after 2 days, 2 weeks, 1 month, 2 months, and 3 months. Patients were also given access to a daytime telephone support service by the study sponsor.
The patients were asked to return the I-neb AAD System after 3 months and to complete the questionnaire at the end of the study on their experience with the device. Data regarding the patients' compliance with the use of the I-neb AAD System was downloaded from the patient logging system (PLS) function for further analysis.
Study device
The I-neb AAD System is a portable, virtually silent mesh nebulizer.(12) The main parts are the nebulizer body, the medication chamber assembly including the mesh and the drug guide, and the mouthpiece (Fig. 1). Built into the body are the microprocessor that runs the AAD algorithm, the electronic aerosol generation circuit, the piezo element connected to the horn, the pressure sensor, the LCD screen, the radio frequency antenna for the AAD Disc, the PLS, the battery, buzzer, and the vibration device for tactile feedback. The piezo element connected to the horn has a variable power range for the optimisation of the aerosol output rate. The AAD Disc is programmed with operational parameters such as power level. The I-neb AAD System has been designed with the capability to record adherence to treatment regimen and compliance with the correct use of the device via a built in PLS. The patient can take pauses during an ongoing treatment, but these are not recorded.
When the I-neb AAD System is used in TBM, the patient inhales spontaneously during tidal breathing, and aerosol is pulsed into 50–80% of each inhaled breath. The I-neb AAD System delivers a preset dose of drug to the patient, and when the preset dose has been delivered the patient gets feedback indicating that the treatment is complete. In TBM, the I-neb AAD System adapts to changes in the patient's tidal breathing pattern. When the I-neb AAD System is used in TIM, the patient is guided to a slow, deep inhalation (Fig. 2). The configuration of the I-neb AAD System to the TIM maneuver is achieved via a magnet in the TIM mouthpiece through which the TIM algorithm is activated. A restriction in the mouthpiece guides the peak inspiratory flow (PIF) to ∼20 L/min. There is, however, no resistance on exhalation. At the beginning of the first treatment the device is set to an inhalation target time of 2 sec (Fig. 2; Early stage of TIM use). The TIM algorithm adapts to the patient's inspiratory capacity by increasing the inhalation target time after each inhalation the patient is able to inhale past the target. Inhalation to the maximum 8 sec results in an aerosol pulse of up to 7 sec. During the last 1 sec when the patient reaches the end of the slow and deep inhalation, no aerosol is generated. The lack of aerosol delivery at the end of the inhalation minimizes the loss of aerosol during the following exhalation, and thereby enhances lung deposition of the inhaled aerosol. The 1 sec of inhalation without aerosol generation has the same effect as a 1-sec breath hold to achieve particle residence time in the lungs (Fig. 2; TIM duration at patient maximum). If the patient is unable to extend the inhalation up to 8 sec, the I-neb AAD System gradually shortens the inhalation target time to match the patient's preferred length of inhalation. At the end of the treatment when the preset dose of drug has been delivered, the duration of the final pulse of aerosol is stored in the device memory, to set the inhalation target time of the first inhalation of the next treatment.
FIG. 2.
Graphical presentation of the two breathing patterns used with the I-neb AAD System. The first part of the graph shows the patient's tidal breathing when using the I-neb AAD System in Tidal Breathing Mode (TBM). The second, third, and last parts of the graph show the process to extend the patient's slow and deep inhalations from a 3-sec inhalation (2-sec aerosol pulse) to an 8-sec inhalation (7-sec aerosol pulse) when using the I-neb AAD System in Target Inhalation Mode (TIM).
The I-neb AAD System provided for the study was delivered with a 0.3 mL metered medication chamber for administration of albuterol, colistimethate sodium, and dornase alpha. A 0.5 mL metered medication chamber was used for administration of hypertonic saline and tobramycin. Patients were provided with two power level 15 AAD discs: one for the administration of all the above drugs, and a backup.
Outcome measures—PLS function and questionnaires
The primary variables were compliance with the correct use of the I-neb AAD System, and treatment times. The secondary variables included ease of use, patient confidence, and patient satisfaction with the I-neb AAD System.
The PLS function incorporated in the I-neb AAD System consists of a memory chip that can record information on up to 4000 treatments, and an infrared interface for transmission of the information. During the study information on each treatment was recorded as an individual line of data including the date and time of the treatment, the dose delivered (complete or noncomplete), the drug, whether the TBM or TIM mouthpiece was used, and the treatment time. To ensure the data was accurately recorded and retrieved every line of data was accompanied by a checksum and the number of data lines recorded was logged. Compliance with the correct use of the I-neb AAD System was calculated through the PLS data. Correct use was defined as the patient's ability to complete each started treatment with verification through the screen that the complete dose had been delivered. Laboratory diagnostic software was used to read the memory to determine the duration of the final aerosol pulse for each patient. The duration of the final aerosol pulse was used to define subgroups of patients that were able to extend their inhalation times from 2 sec to >2 sec, >3 sec, >5 sec and up to 8 sec. The mean treatment times of complete treatments in each subgroup were then calculated to determine the effect of the increase in inhalation times on the treatment times. The means of the treatment times were weighed according to the number of treatments recorded.
The study questionnaires were designed by the investigators and contained 24 questions (Table 1) concerning the use of the I-neb AAD System in TIM. Eighteen of the 24 questions were Likert-style questions in which each item of the question was designed to have a similar psychological “weight.”(15) Six of the 24 questions were paired to investigate patient opinion regarding the same parameter at the beginning and end of the study. The 18 Likert-style nonpaired questions covered descriptive questions, and categories: ease of use, confidence, and satisfaction related to the I-neb AAD System used in TIM. The paired questions covered length of inhalation and number of inhalations during the TIM maneuver.
Table 1.
End of Study Questionnaire with 18 Likert-Style Questions (1–13, 20–24), and Six Paired Questions (14–19)
| 1 |
Did you use the I-neb in TIM mode during the handling study? |
| 2 | If you did not use I-neb in TIM mode during the handling study, why not? |
| 3 | How confident were you that you could use I-neb in TIM mode following the training provided? |
| 4 | How easy did you actually find using the I-neb in TIM mode following the training provided? |
| 5 | Did you use TIM throughout the whole study? |
| 6 | Did you change to TBM permanently or intermittently during the study? |
| 7 | How may weeks had you been using TIM before the permanent change? |
| 8 | On average how many times did you use I-neb in TIM mode each week? |
| 9 | If you changed to TBM permanently or intermittently during the study, why did you do this? |
| 10 | What did you think about the vibration that indicated you had breathed in long enough? |
| 11 | How confident were you that you had received some medication following each vibration? |
| 12 | How difficult did you find it to breathe in through the TIM mouthpiece? |
| 13 | How difficult did you find it to breathe out through the TIM mouthpiece? |
| 14 | How did you find the length of breath needed to reach the vibration at the beginning of the handling study? |
| 15 | How did you find the length of breath needed to reach the vibration at the end of the handling study? |
| 16 | How did you find the treatment times at the beginning of the handling study? |
| 17 | How did you find the treatment times at the end of the handling study? |
| 18 | This question concerns the first week you used I-neb in TIM. When you took your first treatment of each day, on average how many breaths did you take before you were able to inhale to the vibration? |
| 19 | This question concerns the last week you used I-neb in TIM. When you took your first treatment of each day, on average how many breaths did you take before you were able to inhale to the vibration? |
| 20 | On average, during how many treatments were you unable to inhale to the first vibration? |
| 21 | What did you do on these occasions? |
| 22 | How confident were you that you were receiving your medication when using the I-neb in TIM mode? |
| 23 | How easy did you find I-neb to use in TIM mode? |
| 24 | How satisfied were you with I-neb in TIM mode? |
Statistics
The data on patient compliance downloaded from the PLS function was analyzed descriptively to determine the number of complete (full dose of drug inhaled) and noncomplete treatments. The treatment (nebulization) times, pulse times and inhalation times were also analyzed descriptively. The 18 nonpaired questions were analyzed descriptively. The relationship between the three sets of questions comparing the beginning and end of the study in five categories was examined using tests for marginal homogeneity. Whether the two questions were similar or not in terms of how often the patients used each of the five categories were tested. Bhapkar's method, which allowed for a natural ordering in categories, was used.(16) Data has been presented in k × k tables (k ≥ 2) in which the diagonals on each table indicate perfect agreement. The distribution of the raw data was chi-squared. In a square table (5 × 5, etc.) the number of degrees of freedom (df ) was determined as the number of categories −1. The direction of change was assessed using McNemar's test on 1 df.(17) An arbitrary level of 5% significance (two-tailed) was assumed.
Results
PLS data—compliance
PLS data from all 42 patients were available for analysis. The number of days the patients spent in the study ranged from 6 to 210 days.
A total of 10,492 treatments with the I-neb AAD System were recorded during the study. The majority of the treatments or 9197 (∼88%) of these were taken in TIM, and only 1295 (∼12%) in TBM. There were 10,240 complete treatments, and of these, 8979 were in TIM and 1261 in TBM, showing a 97.6% compliance with the correct use of the I-neb AAD System.
Impact of final pulse time on treatment time
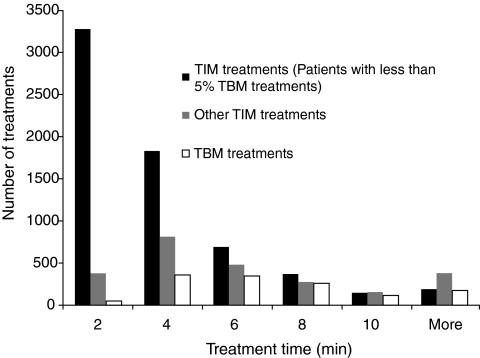
The mean treatment time for complete treatments in TIM was 4.20 min, compared with 6.83 min when using the I-neb AAD System in TBM. The mean treatment time in TIM during the first 10 treatments was 5.33 min and 4.83 min during the last 10 treatments, and in TBM the corresponding results were 6.65 min and 6.50 min. The treatment times were also analyzed separately in 2-min increments (Fig. 3). For this purpose the treatment time data was divided into three groups: TIM treatment times belonging to patients with less than 5% of treatments taken in TBM, treatment times for the remaining TIM treatments, and treatment times for TBM treatments. The majority of the treatments in TIM had treatment times ranging from 2 to 4 min, whereas the rest of the TIM treatments from patients with more than 5% of treatments taken in TBM had treatment times ranging from 2 to 8 min. The majority of the treatments in TBM had treatment times ranging from 4 to 8 min.
FIG. 3.
The histogram presents the treatment times analyzed separately in 2-min increments. For this purpose the treatment time data was divided into three groups: Target Inhalation Mode (TIM) treatment times belonging to patients with less than 5% of treatments taken in Tidal Breathing Mode (TBM), treatment times for the remaining TIM treatments, and treatment times for TBM treatments.
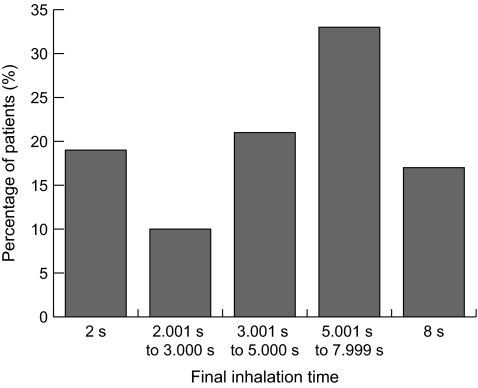
An analysis of the final aerosol pulse times in TIM for the last treatment per patient indicated that >80% of the patients were able to extend the TIM inhalation time above the initial 2-sec inhalation time, that 50% were able to extend the inhalation time up to and above 5 sec, and that 17% were able to reach the upper limit of 8 sec (Fig. 4).
FIG. 4.
An analysis of the final aerosol pulse times in Target Inhalation Mode (TIM) for the last treatment per patient indicated that >80% of the patients were able to extend the TIM inhalation time above the initial 2-sec inhalation time, that 50% were able to extend the inhalation time up to and above 5 sec, and that 17% were able to reach the upper limit of 8 sec.
An analysis of the aerosol pulse times in TIM versus the mean treatment times showed that an increase in pulse times coincided with a decrease in mean treatment times (Table 2). The mean treatment times for the TIM subgroups ranged from 4.20 min to 3.37 min and were between 39 and 51% shorter than the mean treatment time of 6.83 min in TBM.
Table 2.
Subgroup Analysis of the Final Pulse Times When Using the I-neb AAD System in TIM in Comparison with Treatment Times in TBM
| Equivalent inhalation time (s) | Percentage of patients (%) | Mean treatment time (min) | Percentage reduction in treatment time compared with TBM (%) |
|---|---|---|---|
| ≥2 | 100 | 4.20 | 38.5 |
| >2 | 81 | 4.13 | 39.5 |
| >3 | 71 | 3.97 | 41.9 |
| >5 | 50 | 3.66 | 46.4 |
| =8 | 17 | 3.37 | 50.7 |
AAD, Adaptive Aerosol Delivery; TIM, Target Inhalation Mode; TBM, Tidal Breathing Mode.
Questionnaires—Likert-style questions
Twenty-eight patients returned questionnaires at the end of the study and of these 14 reported that they had used the I-neb AAD System in TIM throughout the whole study. The responses to the 18 nonpaired Likert-style questions covered three main catagories: ease of use, confidence, and satisfaction in relation to the use of the I-neb AAD System in TIM (Table 3). Likert questions 7 and 8 had response rates below 50% and have not been reported.
Table 3.
Responses to the Likert-Style Questions Related to Categories: Ease of Use, Confidence, and Satisfaction
| Very confident | Confident | Acceptable | Not confident | Not very confident | Total | |
|---|---|---|---|---|---|---|
| How confident were you that you could use I-neb in TIM mode following the training provided? | 14 | 10 | 3 | 0 | 1 | 28 |
| How confident were you that you had received some medication following each vibration? | 9 | 14 | 1 | 3 | 1 | 28 |
| How confident were you that you were receiving your medication when using the I-neb in TIM mode? | 7 | 14 | 2 | 3 | 2 | 28 |
| Very easy | Easy | Acceptable | Difficult | Very difficult | Total | |
| How easy did you actually find using the I-neb in TIM mode following the training provided? | 11 | 7 | 7 | 2 | 1 | 28 |
| How difficult did you find it to breathe IN through the TIM mouthpiece? | 1 | 11 | 8 | 3 | 4 | 27 |
| How difficult did you find it to breathe OUT through the TIM mouthpiece? | 5 | 12 | 10 | 0 | 0 | 27 |
| How easy did you find I-neb to use in TIM mode? | 5 | 8 | 8 | 3 | 3 | 27 |
| 0 | 1–3 | 4–6 | 7–9 | 9–12 | Total | |
| On average, during how many treatments were you unable to reach the first vibration? | 10 | 6 | 3 | 1 | 1 | 21 |
| Very satisfied | Satisfied | Acceptable | Not satisfied | Not very satisfied | Total | |
| How satisfied were you with I-neb in TIM mode? | 10 | 5 | 6 | 3 | 3 | 27 |
| Very pleasant | Pleasant | Acceptable | Unpleasant | Very unpleasant | Total | |
| What did you think about the vibration that indicated you had breathed in long enough? | 3 | 9 | 16 | 0 | 0 | 28 |
In response to the ease of use-related questions, over 77% of the patients found the I-neb AAD System in TIM to be either: very easy, easy, or acceptable to use. Ease of use responses were least favourable when the patients were asked: “How difficult did you find it to breathe IN through the TIM mouthpiece?” for which seven patients responded either “difficult” or “very difficult.” On the final question regarding ease of use: “How easy did you find I-neb to use in TIM mode,” five out of the seven patients also responded either “difficult” or “very difficult” on this overall question regarding the use of the I-neb AAD System. None of the patients had any difficulties in exhaling through the mouthpiece.
In response to the confidence-related questions, 24 out of the 28 patients reported that they were confident that they could use the I-neb AAD System in TIM after the training. Over 80% of the patients were confident that they were receiving their medication. In response to the satisfaction-related questions, 21 of 27 responding patients rated their experience with the I-neb AAD System in TIM as either: very satisfied, satisfied, or acceptable. All 28 patients found the vibration that indicated that they had inhaled long enough either: acceptable, pleasant, or very pleasant.
Questionnaires—paired questions
There was no statistically significant (p = 0.69; Table 4) difference in opinion regarding the length of the inhalation needed to reach the vibration at the start and end of the study. The majority of the patients found the length of inhalation to reach the vibration “acceptable” during the first (13 patients) and last week (16 patients). The number of patients that found the inhalation “long” or “too long” dropped from 12 patients to 10 patients.
Table 4.
Responses to Paired Questions
| χ2(4) = 14.4, p = 0.006 |
|
During the first week you used I-neb in TIM. When you took your first treatment of each day, on average how many breaths did you take before you were able to inhale to the vibration? |
|||||
|---|---|---|---|---|---|---|---|
| 9+ | 7 to 9 | 4 to 6 | 1 to 3 | Zero | |||
| During the last week you used I-neb in TIM. When you took your first treatment of each day, on average how many breaths did you take before you were able to inhale to the vibration? | 9+ | 1 | 0 | 0 | 0 | 0 | 1 (4.5%) |
| 7 to 9 | 0 | 0 | 0 | 0 | 0 | 0 (0%) | |
| 4 to 6 | 0 | 1 | 4 | 0 | 0 | 5 (22.7%) | |
| 1 to 3 | 0 | 1 | 5 | 6 | 0 | 12 (54.5%) | |
| Zero | 0 | 0 | 0 | 2 | 2 | 4 (18.2%) | |
| 1 (4.50%) | 2 (9.10%) | 9 (40.90%) | 8 (36.40%) | 2 (9.10%) | 22 | ||
| χ2(4) = 2.2, p = 0.69 |
How did you find the length of breath needed to reach the vibration at the beginning of the handling study? |
|
|||||
| |
|
Too long |
Long |
Acceptable |
Short |
Too short |
|
| How did you find the length of breath needed to reach the vibration at the end of the handling study? | Too long | 3 | 1 | 0 | 0 | 0 | 4 (14.8%) |
| Long | 1 | 1 | 2 | 1 | 1 | 6 (22.2%) | |
| Acceptable | 0 | 6 | 10 | 0 | 0 | 16 (59.3%) | |
| Short | 0 | 0 | 1 | 0 | 0 | 1 (3.7%) | |
| Too short | 0 | 0 | 0 | 0 | 0 | 0 (0%) | |
| 4 (14.80%) | 8 (29.60%) | 13 (48.10%) | 1 (3.70%) | 1 (3.70%) | 27 | ||
| χ2(4) = 13.6, p = 0.008 |
How did you find the treatment times at the beginning of the handling study? |
|
|||||
| |
|
Too long |
Long |
Acceptable |
Short |
Too short |
|
| How did you find the treatment times at the end of the handling study? |
Too long |
2 |
0 |
0 |
0 |
0 |
2 (7.4%) |
| Long | 0 | 1 | 0 | 0 | 0 | 1 (3.7%) | |
| Acceptable | 1 | 3 | 10 | 0 | 0 | 14 (51.9%) | |
| Short | 0 | 2 | 4 | 4 | 0 | 10 (37.0%) | |
| Too short | 0 | 0 | 0 | 0 | 0 | 0 (0%) | |
| 3 (11.10%) | 6 (22.20%) | 14 (51.90%) | 4 (14.80%) | 0 (0%) | 27 | ||
There was a statistically significant (p < 0.008; Table 4) difference in opinion regarding the treatment time at the start and end of the study. Fourteen patients found the length of the treatment time “acceptable” during the first and last week. The main difference was found in the number of patients responding “short” during the first week (four patients) and during the last week (10 patients).
There was also a statistically significant difference (p = 0.006; Table 4) in the number of breaths required before the patients were able to inhale up to the vibration at the beginning of each treatment. The majority of the patients required four to six breaths during the first week and only one to three breaths during the last week to reach the vibration.
Discussion
This handling study was designed to evaluate the long-term acceptance of the I-neb AAD System when used in TIM by patients with CF. Overall, the analyses showed that ∼88% of the treatments during the study were taken in TIM, that the treatment times were shorter during the treatments taken in TIM, and that the longer the inhalation the shorter the treatment time in TIM. The patients' compliance with the correct use of the I-neb AAD System was high at 97.6%. Questionnaires with Likert-style questions were returned by 28 patients and showed that the majority found the I-neb AAD System in TIM easy to use, were confident that they could use the I-neb AAD System in TIM and that the device delivered the medication, and were satisfied with their experience with the I-neb AAD System in TIM. The results of the paired questions matching the first and the last week of the study showed that the treatments at the end of the study were shorter with fewer inhalations required to reach the vibration feedback signal.
The use of a slow and deep inhalation maneuver with or without a breath hold has been shown to be optimal for effective lung delivery of inhaled medications.(2–6) Newman et al.(2–3) showed that when using pMDIs, slow inhalations (25–30 L/min) with long breath holds was the optimal inhalation technique in terms of lung deposition in comparison with fast inhalations (80–90 L/min) with long breath holds. When using jet nebulizers, slow and deep inhalations without breath holds have been shown to be superior to tidal breathing in terms of lung deposition.(4–6) In a study of 12 healthy subjects we evaluated lung deposition following administration of a radiolabeled aerosol from the I-neb AAD System with the TBM and TIM breathing patterns.(18) The mean lung deposition was significantly higher with the TIM breathing pattern (73.3%) than with the TBM breathing pattern (62.8%). The mean PIF values were ∼24 L/min in TBM and ∼13 L/min in TIM, whereas the mean minute volumes were 7.19 L in TBM and 7.77 L in TIM. Both mean minute volumes were typical for healthy adults.
The studies showing a higher degree of lung deposition have, however, been laboratory studies and information on the patient's ability to perform the slow and deep inhalation in the domiciliary setting have been lacking. One of the main reasons for the lack of information on the use of inhalation devices in the domiciliary setting has been the lack of suitable hardware and software components built into the devices. The design of the I-neb AAD System with the PLS function has therefore been a critical tool for objective studies of patients' breathing patterns and use of the device in the domiciliary setting. The analysis of the pulse times from the PLS data has added important information on patients' ability to perform the slow and deep inhalation maneuver. In the present study >80% of the patients were able to extend the TIM inhalation time above the initial 2-sec inhalation time, ∼50% were able to extend their inhalation up to 5 sec, and 17% for up to 8 sec. These results indicate that the large majority of the patients found the TIM maneuver easy to use. We can only speculate regarding the reasons for why some of the patients were not able to extend the inhalation time. Data from a previous study of the TIM maneuver in patients with CF indicated that in these patients a forced vital capacity of <1.75 L correlated with a lack of ability to comply with repeated TIM maneuvers.(14) Panting and hyperventilation might be reasons for noncompliance with the TIM maneuver, and has been found to occur in patients with pulmonary arterial hypertension (PAH) that were new to nebulizer therapy and were started on the I-neb AAD System in TBM.(19) The analysis of PLS data showing very long treatment times enabled the healthcare professionals to retrain these patients with PAH. There is, however, no evidence of these problems in the studies of the TIM maneuver in patients with CF or COPD.(13,14)
Aside from improving lung deposition, the slow and deep inhalation maneuver reduces the treatment times. The analysis of the impact of the length of the inhalation in the TIM maneuver on treatment times showed that the longer the inhalation the shorter the treatment time. The mechanism behind the shorter treatment times can best be explained by some hypothetical examples. For a patient with a tidal breathing frequency of 20 breaths per minute (bpm) and a duty cycle of 0.33 (length of inhalation/length of total breath), each inhalation would be of ∼1-sec duration. With a breath hold time of 0.5 sec included at the end the aerosol could only be delivered for the first 0.5 sec of each inhalation. This equates to an inhalation efficiency of only ∼17% as the total aerosol inhalation time would be 10 sec for every minute of inhalation. For a patient using a slow and deep inhalation maneuver of 8 sec followed by an equally long exhalation, the breathing frequency would be ∼4 bpm and the duty cycle 0.5. If we included a breath hold time of 1 sec at the end of each inhalation, then aerosol could be delivered for the first 7 sec of each inhalation. This equates to an inhalation efficiency of ∼47% as the total aerosol inhalation time would be 28 sec for every minute of inhalation. This second example is clinically relevant as the results of the first patient acceptance study in the clinic of the TIM maneuver showed that the patients adapted well to the slow and deep inhalation maneuver with a duty cycle of ∼0.55.(14) However, if the patient could only manage a slow and deep inhalation of 2 sec followed by an equally long exhalation, the breathing frequency would be 15 bpm and the duty cycle 0.5. Including a breath hold time of 1 sec at the end of each inhalation would result in an inhalation efficiency of ∼25%. This means that the higher the inhalation efficiency, the shorter the treatment time. This also means that to almost double the inhalation efficiency from 25 to 47%, the length of the inhalation has to be extended from 1 to 7 sec.
Adherence to treatment and compliance with the correct use of the nebulizer are two of the keystones in a successful domiciliary nebulizer therapy. Motivational factors such as feedback on the use of the device have been shown to be critical for good adherence with the use of the pMDI.(20) Adherence was not measured in the present study, but compliance with the correct use of the I-neb AAD System was recorded through the PLS function. Correct use was defined as the patient's ability to complete each started treatment with verification through feedback—audible signal, vibration, information on the screen—that the complete dose had been delivered. The almost complete compliance of 97.6% was surprisingly high considering the uncontrolled nature of the study in a domiciliary setting. Similar feedback techniques in two previous studies with AAD System devices have shown compliance levels at ∼90%.(21,22)
Feedback on performance therefore seems to be one of the key features to build into new inhalation devices to achieve both good adherence to treatment and good compliance with the correct use of the inhalation device.
In the questionnaires the ease of use with the I-neb AAD System in TIM was overall rated very positively by ∼80% of the patients. The remaining ∼20% responded to the question: “How difficult did you find it to breathe IN through the TIM mouthpiece?” with either “difficult” or “very difficult.” The response pattern matched that for the question: “How easy did you find I-neb to use in TIM mode?” These responses indicated that the level of resistance built into the TIM mouthpiece was perceived as problematic by some of the patients. As there was no information on the clinical status of these patients due to the open handling study design, it was impossible to determine whether the difficulty in inhaling against the resistance was related to disease severity, too high inspiratory flow to sustain a long inhalation maneuver, or any other factors.
The responses to the paired questions showed that the patients adapted to the use of the I-neb AAD System in TIM during the first week of treatment. Almost all (85.2%) of the patients found the treatment time in the first week to be short or acceptable, compared with 88.9% of patients who thought the treatment time at the end of the study to be short or acceptable. The majority of the patients required four to six breaths during the first week and only one to three breaths during the last week to reach the vibration at the end of treatment. These results, although confounded by the fact that the questionnaire was completed at the end of the study, indicate that there was a significant increase in the patients' acceptability of the TIM maneuver over time.
Conclusions
The time requirements for multiple daily nebulizer treatments are important impediments to the quality of life for most patients with CF. A reduction of nebulizer treatment times and a high level of compliance with the correct use of the nebulizer are important steps towards improved quality of life. The present study has shown that a 40–50% reduction in nebulizer treatment times can be achieved in a domiciliary setting with the use of the I-neb AAD System in TIM. The patients' responses to the questionnaire showed that the large majority found the I-neb AAD System in TIM easy to use, and were confident that the aerosol was delivered as intended. This study indicates that the I-neb AAD System in TIM would be an acceptable, user-friendly means of providing inhaled therapy to patients with CF.
Acknowledgments
The authors acknowledge Carly Smart (Philips Respironics, Chichester, UK) for her role in coordinating the study, Nicholas Smith (PS Writing Ltd, Portsmouth, UK) for his assistance with data analysis and graphics, and Allan Rigby (Academic Cardiology, University of Hull, Hull, UK) for the statistical analyses. The study was sponsored by Philips Respironics, Respiratory Drug Delivery, Chichester, UK.
Author Disclosure Statement
John Denyer, Kurt Nikander, and Tony Dyche are employees of Philips Respironics. Alex Black is an employee of Profile Pharma. Ivan Prince was an employee of Philips Respironics during the study.
References
- 1.Fink J. Metered-dose inhalers, dry powder inhalers, and transitions. Respir Care. 2000;45:623–635. [PubMed] [Google Scholar]
- 2.Newman SP. Pavia D. Garland N. Clarke SW. Effects of various inhalation modes on the deposition of radioactive pressurized aerosols. Eur J Respir Dis. 1982;63(Suppl):57–65. [PubMed] [Google Scholar]
- 3.Newman SP. Pavia D. Clarke SW. How should pressurized beta-adrenergic bronchodilators be inhaled? Eur J Respir Dis. 1981;62:3–21. [PubMed] [Google Scholar]
- 4.Bennett WD. Smaldone GC. Human variation in the peripheral air-space deposition of inhaled particles. J Appl Physiol. 1987;62:1603–1610. doi: 10.1152/jappl.1987.62.4.1603. [DOI] [PubMed] [Google Scholar]
- 5.Smaldone GC. Advances in aerosols: adult respiratory disease. J Aerosol Med. 2006;19:36–46. doi: 10.1089/jam.2006.19.36. [DOI] [PubMed] [Google Scholar]
- 6.Newman SP. Woodman G. Clarke SW. Deposition of carbenicillin aerosols in cystic fibrosis: effects of nebuliser system and breathing pattern. Thorax. 1988;43:318–322. doi: 10.1136/thx.43.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DC. Robart P. Inhaler technique of outpatients in the home. Respir Care. 2000;45(10):1182–1187. [PubMed] [Google Scholar]
- 8.Cochrane GM. Horne R. Chanez P. Compliance in asthma. Respir Med. 1999;93:763–769. doi: 10.1016/s0954-6111(99)90260-3. [DOI] [PubMed] [Google Scholar]
- 9.Conway SP. Dodd ME. Marsden RJ. Paul EA. Weller PH. Comparison of compliance in cystic fibrosis patients using either Halolite Adaptive Aerosol Delivery (AAD) system or a conventional high output nebulizer system. J Cyst Fibros. 2002;1:S67. [Google Scholar]
- 10.Denyer J. Dyche T. Proceedings of Drug Delivery to the Lung IX. The Aerosol Society; London, UK: 1998. Patient compliance with inhaled treatment using a novel aerosol delivery system; pp. 124–127. [Google Scholar]
- 11.Corden ZM. Bosley CM. Rees PJ. Cochrane GM. Home nebulised therapy with COPD. Chest. 1997;112:1278–1282. doi: 10.1378/chest.112.5.1278. [DOI] [PubMed] [Google Scholar]
- 12.Nikander K. Denyer J. Adaptive Aerosol Delivery (AAD) Technology. In: Rathbone MJ, editor. Modified Release Drug Delivery Technology. 2nd. Informa Healthcare USA, Inc.; New York, NY: 2008. pp. 603–612. [Google Scholar]
- 13.Prince I. Seamark D. Pinnuck M. Hinch S. Denyer J. Conway J. Evaluation of a guided breathing manoeuvre for nebulised inhaled therapy. In: Byron PR, editor; Dalby RN, editor; Peart J, editor; Suman JD, editor; Farr SJ, editor. Respiratory Drug Delivery IX. Virginia Commonwealth University; Richmond, VA: 2004. pp. 315–318. [Google Scholar]
- 14.Denyer J. Prince I. Dixon E. Agent P. Pryor J. Hodson M. Evaluation of the Target Inhalation Mode (TIM) breathing maneuver in simulated nebulizer therapy in patients with cystic fibrosis. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 1):S29–S36. doi: 10.1089/jamp.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22:1–55. [Google Scholar]
- 16.Bhapkar VP. A note on the equivalence of two test criteria for hypothesis in categorical data. J Am Stat Assoc. 1966;61:228–235. [Google Scholar]
- 17.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 18.Nikander K. Prince I. Coughlin S. Warren S. Taylor G. Mode of breathing—tidal or slow and deep—through the I-neb Adaptive Aerosol Delivery (AAD) System affects lung deposition of 99mTc-DTPA. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 1):S37–S44. doi: 10.1089/jamp.2009.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickerson C. Nikander K. Use of I-neb Insight for training and follow up of patients with pulmonary arterial hypertension. Respir Care. 2007;52:1623. [Google Scholar]
- 20.Ivanovich M. Kreamer JW. Gritzalis D. Moses J. Evaluation of an auditory feedback equipped metered dose inhaler. Am J Ther. 1996;3:818–820. doi: 10.1097/00045391-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Nikander K. Arheden L. Denyer J. Cobos N. Parents' adherence with nebulizer treatment of their children when using an Adaptive Aerosol Delivery (AAD) System. J Aerosol Med. 2003;16:273–281. doi: 10.1089/089426803769017640. [DOI] [PubMed] [Google Scholar]