Abstract
The in vitro characterization of device-related parameters such as the rate of aerosol output, total aerosol output, particle size, and fine particle fraction, is essential when assessing the potential performance of a nebulizer or making comparisons with other nebulizers as they are indicative of potential clinical performance. This article reviews a number of in vitro studies designed to characterize the I-neb Adaptive Aerosol Delivery (AAD) System in terms of drug delivery (particle size, residual, reproducibility, precise dose delivery, dose equivalence), in terms of drug-related performance (osmolality, surface tension, viscosity), and in terms of nebulizer orientation during operation. The results of the in vitro tests of drug delivery indicate that the I-neb AAD System is suitable for delivery of aqueous solutions by nebulization. The evaluation of equivalent doses between the I-neb AAD System (metered dose) and a conventional jet nebulizer (delivered dose), demonstrates that the amount of drug required to deliver the same dose is up to five times less with the I-neb AAD System due to the low residual and controlled drug delivery. The lack of change in osmolality during nebulization might be of importance as it presents an opportunity for delivery of drugs to patients with hyperreactive airways, or where a specific tonicity of the formulation is required. The physicochemical characteristics (surface tension, viscosity) of a number of drugs delivered with the I-neb AAD System highlights some of the demands created by existing and new drug formulations. Finally, the study of the impact of nebulizer orientation shows how important it is to also consider how the nebulizer will actually be physically used by the patient rather than solely under standard conditions used within the laboratory.
Key words: nebulizer, in vitro testing, I-neb Adaptive Aerosol Delivery System, AAD, particle size, precise dose, residual, reproducibility, osmolality, surface tension, viscosity, nebulizer orientation
Introduction
Inhaled aerosols are used in the treatment of a variety of respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis.(1) As many new drugs for inhalation are initially formulated only as either aqueous liquid solutions or suspensions, the use of nebulizers for the delivery of these aerosolized medications is the only option. Nebulizers also have the potential to deliver a variety of drug formulations and drug combinations at high doses in comparison with pressurized metered dose inhalers (pMDIs) and dry powder inhalers (DPIs).(2,3) The recent development of the vibrating mesh technology has made it possible to design portable nebulizers without the drawbacks of conventional jet and ultrasonic nebulizers such as size, noise, and heating of the liquid formulation.(4,5) The combination of the Adaptive Aerosol Delivery (AAD) technology and the vibrating mesh technology has created a small, portable aerosol delivery device, the I-neb AAD System, that adapts the delivery of aerosol to the patient's breathing pattern and delivers aerosol only during inhalation. The I-neb AAD System delivers aerosol using a breathing pattern algorithm, Tidal Breathing Mode (TBM), where a patient inhales spontaneously during tidal breathing while the I-neb AAD System monitors inspiratory flow rate and the length of the inhalation. Aerosol is then pulsed during the first 50–80% of the inhalation. The duration of each pulse of aerosol is determined by the patient's breathing pattern and varies for each subsequent breath depending on the average of the preceding three breaths. These features eliminate waste during exhalation, provide precise dose delivery, and give the patient feedback on performance.(6) The I-neb AAD System is approved as a general purpose nebulizer in the European Union, and for the delivery of drugs that are approved for use with the I-neb AAD System in the United States.
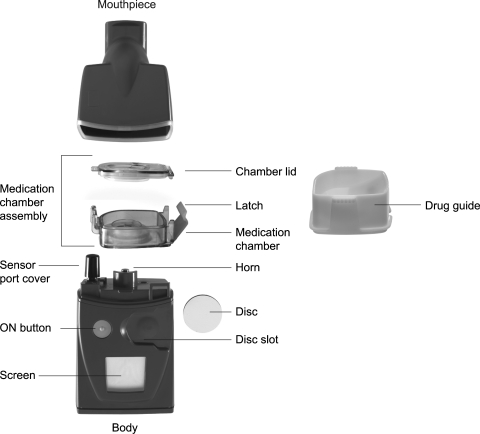
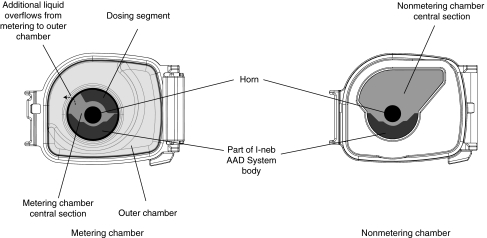
The I-neb AAD System (Fig. 1) is a small, lightweight, and almost silent aerosol delivery system.(6) The main parts of the system are the body, the medication chamber assembly, and the mouthpiece. The body incorporates an LCD screen, a pressure sensor, an AAD Disc and disc slot, and a vibrating horn. The medication chamber assembly includes the metering chamber with optional volumes ranging from 0.25 to 0.75 mL for different drug applications, the vibrating (passive) platinum mesh, and the latch, which keeps the mesh positioned on top of the metering chamber and seals liquid in the chamber. The medication chamber consists of two parts, the metering chamber (Fig. 2) and the outer overflow chamber (Fig. 2). When liquid drug formulation is poured into the metering chamber, excess liquid flows into the overflow chamber. There is a nonmetering medication chamber that has been designed for use when delivered volumes up to 1.7 mL are required. When using these nonmetering medication chambers, the dose to be delivered is metered by the patient when filling the chamber or by using premetered ampoules or vials. The metering chamber volumes may appear small, but with minimal waste during delivery and a small residual, the 1.7 mL volume is similar to the volume delivered with a conventional jet nebulizer filled with ∼6 mL of drug, as will be discussed later in this article. The metered dose as such is defined as the dose emitted ex-mouthpiece, that is, the delivered dose. The metered dose therefore depends on the volume of the metering chamber, the residual, and the small amount of aerosol lost within the mouthpiece. The mouthpiece incorporates an exhalation port and flap valve.
FIG. 1.
The I-neb Adaptive Aerosol Delivery (AAD) System. The main components of the device are the mouthpiece, the medication chamber assembly, and the body. The I-neb AAD System has been designed to deliver aerosol with two different breathing pattern algorithms, the Tidal Breathing Mode (TBM) and the Target Inhalation Mode (TIM). In TIM the inspiratory flow through the valve in the mouthpiece is limited to ∼20 L/min.
FIG. 2.
The medication chamber assemblies of the I-neb Adaptive Aerosol Delivery (AAD) System. The metering chamber with the dosing segment is shown to the left, and the nonmetering chamber to the right.
An in vitro characterization of technology such as the I-neb AAD System is of significant importance as it differs quite substantially from conventional nebulizers. This article presents a number of in vitro studies that evaluate the I-neb AAD System, as follows: (1) particle size, (2) precise dose delivery, (3) residual, (4) reproducibility of metered dose, (5) dose equivalence, (6) change in osmolality during nebulization, (7) surface tension and viscosity, and (8) impact of nebulizer orientation on aerosol delivery.
Materials and Methods
Particle size
The particle size distributions of albuterol and budesonide aerosol produced by the I-neb AAD System was evaluated in terms of mass median aerodynamic diameter (MMAD). The MMAD was determined using the Next Generation Impactor (NGI; MSP Corporation, St. Paul, MN, USA). Albuterol solution (3 mL of 2.5 mg/3 mL DEY, L.P., Napa, CA, USA) was delivered using an I-neb AAD System configured with a 0.25 mL metering chamber and programmed to operate in a laboratory test mode (continuous mode) to facilitate continuous aerosol production at power level 10. Budesonide suspension (3 mL of 0.5 mg/2 mL, Pulmicort, Astrazeneca, Pontevedra, Spain) was delivered using the 0.75 mL metering chamber. The NGI was operated as per EN 13544-1:2007, with a 15 L/min air flow through the system generated by a Copley HCP5 vacuum pump (Copley Scientific Ltd, Nottingham, UK).(7) The airflow was set using a TSI mass flowmeter (TSI Inc., Shoreview, MN, USA) sealed in line between the I-neb AAD System, held in a horizontal position, and the USP throat. The NGI was operated at controlled ambient (23 °C ± 2) conditions. The ambient laboratory environmental relative humidity ranged from 46 to 58% Relative Humidity (RH) throughout all particle size analysis.
Particle size distribution data was represented as per EN 13544-1:2007 by log-normal probability plots of cumulative mass percent of drug on cups versus effective cut-off diameter (ECD). The MMAD was determined by interpolation, Geometric Standard Deviation (GSD) was determined as the square root of the ratio of the 84.1 to 15.9 percentile ECD and fine particle fraction (FPF) was calculated by interpolation as the percent of total mass collected from cups less than 5 μm aerodynamic diameter.(8,9)
Precise dose delivery
The capability of the I-neb AAD System to deliver accurate doses of the five different commonly used inhaled formulations by patients with cystic fibrosis was evaluated using three I-neb AAD System nebulizers with each drug. The drug formulations tested were: tobramycin (300 mg/5 mL TOBI, Chiron Corporation Ltd, Hounslow, UK), dornase alfa (2500 U/2.5 mL Pulmozyme, Roche Products Ltd, Welwyn Garden City, UK), colistimethate sodium (1 MIU/mL Promixin, Profile Pharma, Chichester, UK), albuterol (2 mg/mL Salamol, IVAX, London, UK), and hypertonic saline (sodium chloride, Sigma-Aldrich Inc., St. Louis, MO, USA). Each I-neb AAD System was connected to a Harvard respirator (Harvard Apparatus, Harvard College, Holliston, MA, USA) set to produce the EN 13544-1 CEN adult tidal breathing pattern (Vt = 500 mL, f = 15 breaths/min, I:E ratio = 1:1). Aerosol output was collected in triplicate for each nebulizer/drug combination using a low deadspace filter placed between the I-neb AAD System and the Harvard respirator. A pipette was used to fill 1.5 mL of tobramycin inhalation solution (tobramycin) into three nonmetering medication chambers, whereas a 0.5 mL metering chamber was used for hypertonic saline. The other drugs: albuterol, colistimethate sodium, and dornase alfa, were delivered using 0.3 mL metering chambers. The expected (nominal) delivered dose was calculated as the product of the metered volume and the drug concentration. Drug delivered to filters was quantified by High Performance Liquid Chromatography (HPLC) for tobramycin and dornase alfa, by bioassay for colistimethate sodium, by a spectrophotometric method for albuterol, and by ion analysis for hypertonic saline.
Residual
The gravimetric residual mass of liquid drug formulation remaining in the I-neb AAD System postnebulization was evaluated during a standard output to filter analysis. Twenty-five nonmetering chambers were tested in triplicate using a single I-neb AAD System body. The I-neb AAD System including the mouthpiece was weighed using an analytical balance (ME614S Sartorius AG, Goettingen, Germany), then filled with 0.4 mL albuterol (2 mg/mL, Salamol) via a pipette, and weighed again. A Harvard respirator was used to reproduce an adult tidal breathing pattern as per the EN 13544-1 CEN guidance and a filter with a low dead space filter holder was used in line to collect aerosol emitted from the I-neb AAD System. The I-neb AAD System was weighed again postnebulization, and the gravimetric residual mass remaining in the I-neb AAD System was determined as the weight postnebulization minus the empty nebulizer weight. The nonmetering chamber is ideal for this test as there is no overflow chamber incorporated in the design and therefore any residual liquid remaining in the nonmetering chamber is not obscured by this.
Reproducibility of metered dose
The reproducibility of the delivered dose of albuterol (2 mg/mL, Salamol) was assessed using fifty 0.3 mL metering chambers with a single I-neb AAD System in TBM and a power level 10 AAD Disc. The 0.3 mL metering chamber is designed to deliver 300 μL of albuterol (600 μg albuterol) ex-mouthpiece, that is, the fill volume is designed to compensate for any residual in the metering chamber and mouthpiece to deliver the required metered dose. A Harvard respirator was configured to reproduce the CEN adult tidal breathing pattern, and a filter was used in line to collect aerosol emitted from the I-neb AAD System. On activation of the Harvard respirator, the I-neb AAD System delivered a pulse of aerosol into each inspiration after the first three breaths and continued until the end of treatment. At the end of treatment, filters were collected and eluted for quantification of albuterol by HPLC.
Dose equivalence
The comparison of the delivered doses of drug from the I-neb AAD System (metered dose) and from a conventional jet nebulizer (delivered dose) is complicated by the differences in definitions of dose, and by the differences in dosages (controlled versus continuous) and by the differences in drug delivery mechanisms. To predict the metered dose that may be used in the I-neb AAD System that can be equated to the delivered dose of a jet nebulizer, a hypothetical in vitro model was generated and tested for accuracy. Three jet nebulizers (LC Plus; Pari GmbH, Starnberg, Germany) were each filled by pipette with 2 mL of albuterol (2 mg/mL, Salamol). Each nebulizer was connected to a Harvard respirator set to produce the CEN adult tidal breathing pattern. The jet nebulizers were driven using 6 L/min compressed medical grade air, and aerosol output was collected till sputter plus 60 sec using a filter placed between the nebulizer and the Harvard respirator. Drug was eluted from filters and quantified by HPLC. The above was repeated for jet nebulizer fill volumes of 2.5, 3, 4, and 5 mL of albuterol. A lung deposition factor for the LC Plus jet nebulizer was obtained by dividing the lung deposition with the delivered dose (where delivered dose = sum of the upper airways plus whole lung deposition data) reported in Newman et al.(10) This gave a factor of 0.47. The delivered doses for the different fill volumes were then used to calculate a “predicted lung dose” by multiplying the delivered doses by the lung deposition factor of 0.47.
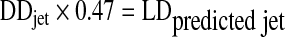 |
where DDjet is the delivered dose from the jet nebulizer, 0.47 is the lung deposition factor for the jet nebulizer, and LDpredicted jet is the predicted lung dose for the jet nebulizer.
The predicted lung doses for the different jet nebulizer fill volumes were then divided by a factor of 0.63, based on lung deposition data for the I-neb AAD System.(11) The result was predicted emitted doses ex-mouthpiece for the I-neb AAD System that would achieve the same predicted lung doses as with the jet nebulizer.
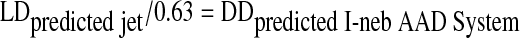 |
where 0.63 is the lung deposition factor for the I-neb AAD System and DDpredicted I-neb AAD System is the predicted delivered (metered) dose from the I-neb AAD System.
To calculate the I-neb AAD System medication chamber fill volumes equivalent to each jet nebulizer fill volume, 0.1 mL was added to allow for residual volume.
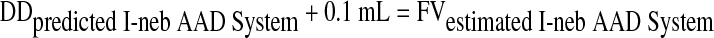 |
where 0.1 mL is the I-neb AAD System residual volume, and FVestimated I-neb AAD System estimated the I-neb AAD System fill volume to give an equivalent dose to the jet nebulizer.
To test whether the model hypothesized did indeed predict similar lung doses of albuterol for both nebulizer brands, three I-neb AAD System nebulizers configured with nonmetering chambers were filled with the estimated medication chamber fill volumes using pipettes, and the in vitro test arrangement used for the jet nebulizer was repeated. Predicted lung doses for each medication chamber volume fill were calculated by multiplying the emitted doses from the I-neb AAD System by the in vivo obtained lung deposition factor of the I-neb AAD System.
Change in osmolality during nebulization
Due to the design and function of the I-neb AAD System medication chamber assembly and the vibrating mesh, osmolality of the liquid drug formulation in the medication chamber should not change during nebulization. To test this assumption, one I-neb AAD System and one jet nebulizer (LC Plus; Pari GmbH) were used to examine any change in formulation concentration during nebulization. The I-neb AAD System was configured with a nonmetering chamber and power level 10, then filled with 1.5 mL of 0.45% saline and then weighed. The jet nebulizer was filled with 4 mL of 0.45% saline (maximum fill volume for use with 0.5 MIU colistin), and then weighed. Compressed medical grade air at 6 L/min was used to run the jet nebulizer. Each nebulizer was connected to a Harvard respirator set with the CEN adult breathing pattern. Each nebulizer was operated for a specified period of time, specifically 3 min for the I-neb AAD System and 1 min for the jet nebulizer. An osmometer sampler was used to remove a 20 μL aliquot of solution remaining in the medication chamber or nebulizer cup for analysis. The nebulizers were then washed and dried. Continuing testing, the nebulizers were filled and run in the same way for a further specified period of time, specifically 6 min for the I-neb AAD System and 2 min for the jet nebulizer, after which the nebulizers were stopped and the solution remaining was sampled for analysis. This method was repeated in increments of 3 min for the I-neb AAD System and in increments of 1 min for the jet nebulizer, until the end of treatment, defined as the end of the treatment buzzer for the I-neb AAD System and sputter plus 60 sec for the jet nebulizer. When approaching the end of the treatment with the I-neb AAD System, the sample volumes were limited. Volumes less than 20 μL of saline were therefore taken from the medication chamber, and diluted with a known volume of deionized water and then analyzed. At end of the treatment, 1.5 mL deionized water was added to the medication chamber and shaken before sampling. Samples from the jet nebulizer were not diluted. For each sample taken, osmolality was quantified using an Advanced Micro Osmometer Model 3300 (Advanced Instruments, Norwood, MA, USA), which measured solution osmolality by freezing point depression. Each test was performed in triplicate.
Surface tension and viscosity
The physicochemical characteristics of a number of liquid drug formulations for inhalation currently on the market and new drug formulations were evaluated in terms of viscosity and surface tension. The drug formulations examined encompassed small molecules, suspensions, proteins, and liposomes. Surface tension was quantified using a SITA t60 Science Line handheld tensiometer (SITA Messtechnic GmbH, Dresden, Germany) which measures the dynamic surface tension of fluids, in the range 10 to 100 mN/m and with a 0.1 mN/m resolution, in terms of bubble pressure. Viscosity was quantified using an AMVn Microviscometer (Anton Paar GmbH, Graz, Austria) based on a “rolling ball” principle in which the fluid to be measured was filled into a glass capillary in which a steel sphere rolls at a predetermined angle. The viscous properties of the fluid could be determined by measuring the rolling time of the sphere. The I-neb AAD System nebulizers used in the tests were programmed to run in continuous mode at power level 10, and production of aerosol from the vibrating mesh was determined by visual inspection of the presence of aerosol without the mouthpiece in place.
Impact of nebulizer orientation on aerosol delivery
Four different examples of vibrating mesh technology nebulizer were used to investigate the impact of nebulizer orientation on performance: the I-neb AAD System, the Aeroneb Go (Aerogen, Inc., Mountain View, CA, USA), the eFlow rapid (Pari GmbH, Starnberg, Germany), and the Micro Air NE-U22 (Omron Healthcare Co. Ltd, Kyoto, Japan). The delivered volume, residual volume, and mass median diameter (MMD) of each brand was examined in three orientations; 0° pitch (orientation as per instructions for use), +45° pitch (base of nebulizer pointing upward), and −45° pitch (base of nebulizer pointing downward). Roll and yaw were not examined in this study. Angle was determined using a protractor. The delivered volume and residual volume were determined in triplicate for each brand using a gravimetric method. The nebulizers were weighed, and filled with 1 mL of albuterol (2 mg/mL, Salamol) and weighed again. A 2 mL volume was used for the eFlow rapid as per the manufacturer's instructions. The nebulizers were connected to a Harvard respirator set with the CEN adult tidal breathing pattern, and a filter was used to collect emitted aerosol. A complete treatment from each nebulizer was delivered onto the collection filter, after which the nebulizer was removed from the test setup and weighed again. The I-neb AAD System features an “out of angle” sensor, which provides feedback to the patient during the treatment and prevents the patient from beginning a treatment if the nebulizer is tilted past 45° pitch. Consequently, during testing it was necessary to tilt the I-neb AAD System to just within 45° to start the test. A laser diffraction method was used to establish any change in MMD (% change from 0° pitch) with each orientation for each nebulizer brand. Each nebulizer was used in continuous mode (I-neb AAD System at power level 10) and connected to a Malvern Mastersizer S (Malvern Instruments Ltd, Worchester, UK) laser diffractor particle size analyzer. Where necessary, additional air was introduced into the mouthpieces to facilitate entrainment of aerosol into the measurement cell of the laser diffractor.
Results
Particle size
The particle size distributions of aerosols characterized by cascade impaction show that the I-neb AAD System consistently produces respirable aerosols with albuterol and budesonide. Specifically, at 15 L/min in controlled ambient laboratory conditions, albuterol aerosol was characterized as: MMAD 3.9 μm (Standard Deviation [SD] 0.04); GSD 1.7 (0.03); FPF 67% (1.1), and budesonide aerosol was characterized as: MMAD 5.7 μm (0.1); GSD 1.75 (0.0); FPF 41% (1.5).
Precise dose delivery
The calculated (nominal) metered doses of five commonly used liquid drug formulations delivered by the I-neb AAD System are comparable to the measured (actual) doses delivered (Table 1). The variability between the gravimetrically determined and nominal metered drug volumes range from −0.8 to 10.2%, with the largest variability being for hypertonic saline.
Table 1.
The Nominal Metered Volumes, Drug Concentrations, Gravimetrically Defined Metered Volumes, Nominal Delivered Doses, and Actual Delivered Doses for Five Drugs Used in the Tests for Precise Dose Delivery with the I-neb Adaptive Aerosol Delivery (AAD) System
| Drug | Nominal metered volume | Drug concentration | Gravimetric metered volume | Nominal delivered dose | Actual delivered dose |
|---|---|---|---|---|---|
| Tobramycin | 1400 μL | 60 mg/1 mL | 1388.4 μL | 84 mg | 75.9 mg |
| Colistimethate sodium | 300 μL | 1 MIU/1 mL | 311.2 μL | 300 kIU | 324.9 kIU |
| Dornase alfa | 300 μL | 1 mg/1 mL | 318.3 μL | 300 μg | 283.3 μg |
| Albuterol | 300 μL | 2 mg/1 mL | 317.2 μL | 600 μg | 605.1 μg |
| Hypertonic saline | 500 μL | 70 mg/1 mL | 551.0 μL | 35 mg | 35.9 mg |
Residual
The mean gravimetrically measured residual mass of albuterol remaining in the nonmetering chambers of the I-neb AAD System after nebulization was 84 mg (SD 13) with a range of 57 to 142 mg. As the concentration of albuterol was 2 mg/mL, the mean is equivalent to an 84 μL residual volume.
Reproducibility of the metered dose
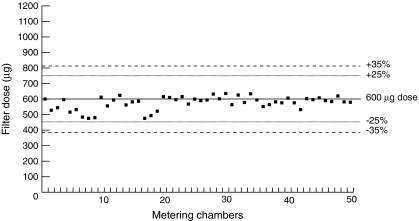
The reproducibility of the metered dose of albuterol, when delivered from fifty 0.3 mL metering chambers with a single I-neb AAD System, is within ±25% of the 600 μg dose (Fig. 3). The mean metered dose of albuterol was 575.4 μg [95% confidence interval (CI) 563.3–587.5 μg]. This equates to 288 μL albuterol solution (95% CI 282–294 μL) in metered volume.
FIG. 3.
Reproducibility of the metered dose of albuterol from fifty 0.3 mL I-neb Adaptive Aerosol Delivery (AAD) System chambers.
Dose equivalence
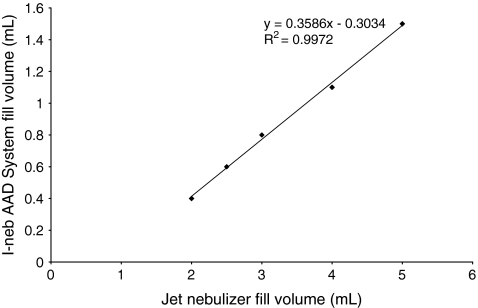
Table 2 shows the measured metered (delivered) doses and the predicted lung doses found for the I-neb AAD System and the jet nebulizer (LC Plus; Pari GmbH) for each of the equivalent nebulizer albuterol fill volumes. Although delivered doses for the jet nebulizer are higher than the delivered doses metered from the I-neb AAD System, the predicted lung doses are similar for all fill volumes. The relationship between the delivered doses and the metered doses is linear (R2 = 0.9972) as shown in Figure 4.
Table 2.
The Fill Volumes of Albuterol for a Jet Nebulizer and the I-neb Adaptive Aerosol Delivery (AAD) System Required to Achieve Similar Predicted Lung Doses
| Jet nebulizer fill volume | 2.0 mL | 2.5 mL | 3.0 mL | 4.0 mL | 5.0 mL |
|---|---|---|---|---|---|
| Jet nebulizer output to filter | 429 μL | 732 μL | 892 μL | 1370 μL | 1836 μL |
| Predicted lung dose of albuterol from jet nebulizer |
404 μg |
688 μg |
838 μg |
1288 μg |
1726 μg |
| Equivalent I-neb AAD System fill volume |
0.42 mL |
0.65 mL |
0.77 mL |
1.12 mL |
1.47 mL |
| I-neb AAD System output to filter | 333 μL | 577 μL | 691 μL | 1034 μL | 1359 μL |
| Predicted lung dose of albuterol from I-neb AAD System | 420 μg | 728 μg | 870 μg | 1302 μg | 1712 μg |
FIG. 4.
The relationship between the nebulizer fill volumes for the I-neb Adaptive Aerosol Delivery (AAD) System and an LC Plus jet nebulizer required to achieve equivalent predicted lung doses.
Change in osmolality during nebulization
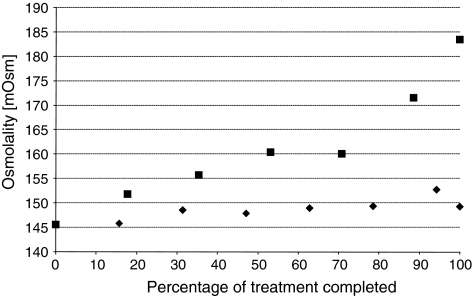
There is a 10-fold difference in change in osmolality of liquid drug formulation remaining in the jet nebulizer compared to the I-neb AAD System. Osmolality of saline in the jet nebulizer increases significantly by 26% during delivery of aerosol from nebulizers compared to the I-neb AAD System (Fig. 5), whereas saline osmolality in the I-neb AAD System increases by 2.5% over the course of a treatment.
FIG. 5.
Change in osmolality during nebulization shown for the I-neb Adaptive Aerosol Delivery (AAD) System (♦) and an LC Plus jet nebulizer (▪).
Surface tension and viscosity
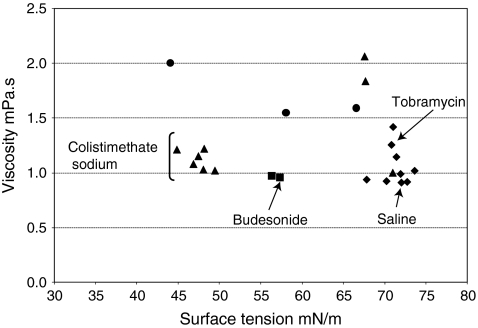
These results show that a number of formulations with a relatively wide range of surface tensions and viscosities are successfully aerosolized by the I-neb AAD System. The measured surface tensions and viscosities of the different small molecule, suspension, protein, and liposome formulations have been plotted in Figure 6. The range of physicochemical properties of formulations delivered by the I-neb AAD System were: surface tensions of 44.0 to 73.6 mN/m and viscosities of 0.91 to 2.06 mPa.s. The surface tensions and dynamic viscosities of some of the marketed drug formulations were: 0.9% saline 72.0 mN/m and 0.91 mPa.s, budesonide (Pulmicort, AstraZeneca AB, Sodertalje, Sweden) 57.3 mN/m and 0.95 mPa.s, colistimethate sodium (Promixin, Profile Pharma, Chichester, UK) 44.8 to 49.4 mN/m and 1.02–1.22 mPa.s, and tobramycin (TOBI, Chiron Corporation Ltd, Hounslow, UK) 70.8 mN/m and 1.26 mPa.s.
FIG. 6.
Surface tensions and viscosities of a number of formulations including small molecules (♦), proteins (▴), suspensions (▪) and liposomes (•).
Impact of nebulizer orientation on aerosol delivery
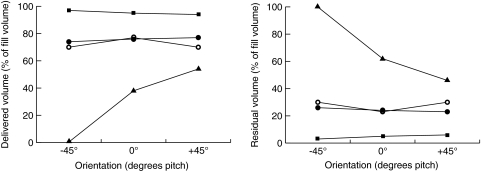
The volumes of albuterol delivered by the I-neb AAD System, the Aeroneb Go and the Micro Air NE-U22, are not significantly affected by orientation angle. However, the volume of albuterol delivered by the eFlow rapid is severely affected as shown in Figure 7. Specifically, less than 1% of the nebulizer fill volume was delivered at −45° pitch, and at +45° pitch the delivered volume is approximately 16% greater than at 0° pitch. For residual volume, the I-neb AAD System, the Aeroneb Go, and the Micro Air NE-U22 nebulizers are relatively unaffected by orientation angle, but again, the eFlow rapid shows large residuals at +45° and −45° pitch angles. The aerosol particle size (MMD) for all four nebulizers is consistent at all orientation angles.
FIG. 7.
Impact of nebulizer orientation on aerosol delivery shown for the I-neb Adaptive Aerosol Delivery (AAD) System (▪), the Aeroneb Go (○), the eFlow rapid (▴), and the Micro Air NE-U22 (•) nebulizers.
Discussion
Methods for in vitro testing of nebulizers have evolved as a result of development by manufacturers, and have been incorporated into guidelines set by different official bodies.(2,5,7,8,9,12) Standardization of the testing methods within the guidelines ensures that performance data for different nebulizers can be directly compared and related to their expected performance in vivo. With the advent of the new high efficiency nebulizers, such as the I-neb AAD System characterized here, this ability to equate relative performance, through standardized testing of the device related parameters that influence the drug delivered to the patient, is even more valuable. These device related parameters include the rate of aerosol output, total aerosol output, particle size, and fine particle fraction, all of which are indicative of clinical performance.(12) The data included in this article (particle size, residual, reproducibility, precise dose delivery, dose equivalence, osmolality, surface tension, and viscosity) covers a broad spectrum of performance characteristics of the I-neb AAD System.
Particle size
The particle size distributions of albuterol and budesonide characterized by cascade impaction show a difference in both particle size and FPF between the drugs. The difference in particle size and FPF between the albuterol solution and the budesonide suspension is in agreement with recently published data.(13) Akapo and colleagues measured an MMAD of 2.9 μm for a formoterol solution, and an MMAD of 4.9 μm for the budesonide suspension.(14) The FPFs were 73.6% for formoterol and 51.0% for budesonide. The results are likely to reflect a difference between the solution, and the suspension with a primary particle size of 2.4 μm.(14) The present results with an MMAD of 3.9 μm and FPFs of 67% for albuterol are in the range that is typically found for nebulizers.(15,16) It is also of note that the in vitro fine particle fraction reported here is close to the mean 63% lung deposition of radiolabeled saline in healthy subjects when delivered by the I-neb AAD System.(11)
Precise dose delivery
The I-neb AAD System is a dosimetric drug delivery system designed to deliver precise metered doses of aerosol,(6) in contrast to typical volumetric nebulizers that continuously generate aerosol with fill volumes determined by vial volume and doses to patients determined by breathing maneuver. This precision in dosing is demonstrated in the results here where the maximum variability in delivered dose was within ∼10% of the expected (metered) dose. This dosimetric feature broadens the possibilities for inhaled treatments to include drugs with narrow therapeutic windows. An example is iloprost (Ventavis, Actelion Pharmaceuticals, South San Francisco, CA, USA) which has been approved by the Food and Drug Administration (FDA) for delivery with the I-neb AAD System in the treatment of pulmonary arterial hypertension in doses of 2.5 μg and 5.0 μg. Van Dyke et al.(13) tested the ability of the I-neb AAD System to deliver precise doses of iloprost in vitro using a breathing simulator and a filter technique to collect emitted aerosol. The metered doses of aerosolized iloprost tested were 2.5 and 5.0 μg, and the measured delivered doses were 2.8 and 4.9 μg. The small variability between the metered and the delivered doses of iloprost were similar to the variability in our in vitro data on five drugs typically used in the treatment of patients with cystic fibrosis.
Residual
Data on evaluating I-neb AAD System nonmetering chamber residual with albuterol indicates that a mean of 84 μL of the metered volume remains inside the chamber at the end of treatment. This residual is extremely low when compared to conventional nebulizers, which can typically have residuals up to, if not more than, 1 mL. When the low residual is combined with the ability of the I-neb AAD System to deliver drug only into inhalation, it has the potential to allow the delivery by nebulization of some of the high cost of manufacture new biotech drugs. Currently conventional nebulizers are not economic for these applications due to high wastage in residual and delivery into exhalation.
Reproducibility of metered dose
The results show that for the I-neb AAD System, metered doses can be delivered in vitro with low variability. This low variability is confirmed in another study where the variability of twenty-three prototype I-neb AAD System nebulizers was evaluated in vitro during the development of the I-neb AAD System.(6) The nebulizers in this latter study were set to deliver a metered volume of 400 μL of albuterol during simulated breathing. The mean gravimetric metered volume delivered from the I-neb AAD System was 407.1 μL, with a 95% CI of 401.2 to 412.8 μL. In the present study, metering chamber variability was examined using fifty 0.3 mL metering chambers with a single I-neb AAD System to deliver a metered dose of 600 μg albuterol (metered volume of 300 μL). The mean metered dose delivered was 575.4 μg albuterol, with a 95% CI of 563.3 to 587.5 μg, or 288 μL equivalent volume and a 95% CI of 282 to 294 μL.
Dose equivalence
Dose equivalence was determined using hypothetical calculations of the metered dose of the I-neb AAD System versus the delivered dose of the jet nebulizer to predict an equivalent dose. The I-neb AAD System can deliver an equivalent dose to a jet nebulizer using less than a third of the volume of that required in the jet nebulizer. The subsequent in vitro evaluation confirms the hypothetical calculations to be broadly correct. The linear relationship in Figure 4 between the fill volumes used in the two nebulizer brands show that for 2 mL fill volume in the jet nebulizer, the comparable fill volume in the I-neb AAD System is 0.4 mL. This is a five times reduction in the amount required. For a 5 mL fill volume in the jet nebulizer, the comparable fill volume in the I-neb AAD System is approximately 1.5 mL, that is, less than a third of the volume. There was a greater dose uniformity with the I-neb AAD System (Residual Standard Deviation [RSD] on dose output 2–4%) compared to the jet nebulizer (RSD on dose output 5–10%). This difference in the precision of uniformity of dose may appear small, but the testing was performed using a single standard CEN (I:E 1:1) breathing pattern. In use, aerosol delivery devices are subject to a wide range of different breathing patterns, and the I-neb AAD System will adapt to these patterns and thus maintain a similar level of dose uniformity across the breathing patterns. Conversely, a jet nebulizer that continually produces aerosol will have minimal adaption capabilities, and consequently, the dose variability across breathing patterns will be substantial. This will particularly be the case where a patient breathes with longer periods of exhalation resulting in more of the drug being expelled from the nebulizer in exhalation rather than being inhaled.
Change in osmolality during nebulization
The I-neb AAD System had a minimal effect on the osmolality of saline solution during nebulization, whereas the osmolality of saline in the conventional jet nebulizer increased significantly over the course of one treatment. The difference in outcome was likely due to the fundamental differences in the mechanism of nebulization and flow of air through the two types of nebulizer. In the I-neb AAD System the medication chamber is isolated from entrained air by the medication chamber lid, and nebulization only occurs during inhalation when the liquid passes through the mesh and is emitted. Conversely, for a conventional jet nebulizer the liquid drug in the nebulizer cup is constantly exposed to entrained air, and nebulization occurs throughout the treatment. Larger aerosol droplets created within the nebulizer impact on inner surfaces and are recycled back in to the nebulizer cup, whereas smaller droplets are entrained in the airflow and emitted from the nebulizer. The constant recycling of larger aerosol droplets results in continuous evaporation and subsequent concentrating of the drug formulation within the reservoir. Increased osmolality has the potential to cause adverse effects in patients with hyperreactive airways such as bronchoconstriction.(17)
Surface tension and viscosity
The study to measure surface tensions and viscosities of different drug formulations aerosolized using the I-neb AAD System was designed to create a database on typical formulation types that could be nebulized through the I-neb vibrating mesh and relate these to surface tension and viscosity. The study presented does not measure quantitatively the impact of surface tension and dynamic viscosity on particle size, output rate or nebulization time.
Surface tension and viscosity are known to affect the ability of nebulizer systems to deliver different formulations. Ghazanfari et al.(18) have studied the effects of these physicochemical properties on nebulizer performance using model formulations with a range of surface tensions and viscosities and two vibrating mesh nebulizers, the Omron Micro Air NE-U22 (Omron Healthcare, UK), and the Aeroneb Pro (Nektar, San Carlos, CA, USA). Results were reported by Ghazanfari et al. as cP for viscosity and dyne/cm for surface tension; however, we have converted them into mPa.s for viscosity and mN/m for surface tension (viscosity: 1 mPa.s = 1 cP, surface tension: 1 mN/m = 1 dyne/cm). The surface tensions tested ranged from 15.6 to 72.80 mN/m, and the viscosities from 0.49 to 2.45 mPa.s. The commercially available drug formulations tested in our study had narrower ranges in terms of surface tension (44.0 to 73.6 mN/m), and viscosity (0.91 to 2.06 mPa.s). Ghazanfari et al. conclude that the two vibrating mesh nebulizers tested were highly dependent on formulation characteristics, were unsuitable for delivery of formulations with viscosities greater than 1.92 mPa.s, and that different surface tensions had no clear effect on delivery. This is in contrast to jet nebulizers where surface tension and viscosity (among other parameters) affects the amount of residual in the nebulizer and thus the delivered dose.(19)
Impact of nebulizer orientation on aerosol delivery
The volume of albuterol delivered by three of the examples of vibrating mesh nebulizers examined is not influenced by angle of orientation, whereas the volume delivered by the eFlow rapid is severely affected. The impact of orientation angle (horizontal versus vertical) on the performance of a vibrating mesh nebulizer was first reported by Geidel and colleagues in 2006.(20) Seven patients with cystic fibrosis used eFlow rapid nebulizers in either a horizontal or a vertical operating orientation to administer tobramycin solution (320 mg/4 mL). Blood was sampled 4 h after nebulization, and plasma levels of tobramycin were determined by HPLC. Mean tobramycin plasma levels were found to be 0.75 μg/mL when the eFlow rapid was operated by the patient in a horizontal orientation and 1.45 μg/mL when operated in a vertical orientation. There was no impact of orientation on particle size. The authors concluded that the vertical position allowed better utilization of the eFlow rapid nebulizer. Our results when nebulizing albuterol with the eFlow rapid show only a 16% increase in the dose from horizontal (0°) to vertical (+45°). The difference in results on vertical orientation might be due to the lack of specification of the “vertical” position in the previous study. Interestingly, the downward position (−45°) prevented delivery of any aerosol from the eFlow rapid. This indicates that it might be of clinical value to measure orientation angle dependent aerosol delivery both in vitro and in vivo to minimize unintentional fluctuations in drug delivery.
Conclusions
This article has reported a number of in vitro studies designed to characterize the I-neb AAD System. The particle size and fine particle fraction of aerosol delivered from the I-neb AAD System is suitable for delivery to the lung by nebulization. The measurement of equivalent doses (metered dose versus delivered dose) between the I-neb AAD System and a conventional jet nebulizer demonstrates that the amount of drug required to deliver the same dose is up to five times less due to the low residual and controlled delivery of the I-neb AAD System. The lack of change in osmolality during nebulization may be of importance, as it presents an opportunity for delivery of drugs to patients with hyperreactive airways, or where a specific formulation osmolality is required. The physicochemical characteristics (surface tension and viscosity) of a number of drugs delivered by the I-neb AAD System highlights some of the demands created by existing and new drug formulations. The study of the impact of nebulizer orientation shows how important it is to consider user related factors in designing appropriate in vitro studies, in addition to regulatory guidances. The amount of aerosol delivered to a patient using a nebulizer has been a vague concept in clinical practice as the prescriber has usually only defined the amount of drug in the vial. The performance of the conventional nebulizer, the patient's breathing pattern, and the patient's ability to use the nebulizer have determined the rather variable amount of aerosol delivered. This is clearly less than satisfactory, especially when using drugs with a narrow therapeutic window, or very expensive drugs.
The aerosol characteristics and precise dosing of the I-neb AAD System combined with its ability to adapt to a patient's breathing pattern and to provide a controlled delivery of aerosol into inhalation allows much of this variability to be reduced and drugs to be delivered with much greater uniformity and precision.
Acknowledgments
The authors acknowledge numerous colleagues and former colleagues at Philips Respironics, Respiratory Drug Delivery, Chichester, UK, for the conduct of the studies; Sarah Byrne, Zena Demissie, Yulia Degtyareva, Tony Dyche, Antony Hurren, Florence Lai, Adam Metcalf, Richard Potter, Katja Williams, and Jeremy Young. The authors also acknowledge Kurt Nikander (Philips Respironics, Respiratory Drug Delivery, Parsippany, NJ) for guidance and input on the manuscript. The studies were sponsored by Philips Respironics, Respiratory Drug Delivery (UK) Ltd, Chichester, UK.
Author Disclosure Statement
Lucy Hardaker and Ross Hatley are employees of Philips Respironics.
References
- 1.Fiel SB. History and evolution of aerosolized therapeutics: overview and introduction. Chest. 2001;120(Suppl):87S–88S. doi: 10.1378/chest.120.3_suppl.87s. [DOI] [PubMed] [Google Scholar]
- 2.Dolovich MB. Ahrens RC. Hess DR. Anderson P. Dhand R. Rau JL. Smaldone GC. Guyatt G. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 3.Geller DE. Comparing clinical features of the nebulizer, metered-dose inhalers, and dry powder inhaler. Respir Care. 2005;50:1313–1321. [PubMed] [Google Scholar]
- 4.Rau JL. Design principles of liquid nebulization devices currently in use. Respir Care. 2002;47:1257–1275. [PubMed] [Google Scholar]
- 5.O'Callaghan C. Barry PW. The science of nebulised drug delivery. Thorax. 1997;52(Suppl):S31–S44. doi: 10.1136/thx.52.2008.s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikander K. Denyer J. Adaptive Aerosol Delivery (AAD) Technology. In: Rathbone MJ, editor. Modified Release Drug Delivery Technology. 2nd. Informa Healthcare USA, Inc.; New York, NY: 2008. pp. 603–612. [Google Scholar]
- 7.Comité Européen de Normalisation. Respiratory Therapy Equipment—Part 1: Nebulizing Systems and Their Components. CEN Brussels; Belgium: 2007. EN 13544-1. [Google Scholar]
- 8.FDA (CDRH) Reviewer Guidance for Nebulizers, Metered Dose Inhalers, Spacers, Actuators. Oct, 1993. http://www.fda.gov/cdrh/ode/784.pdf http://www.fda.gov/cdrh/ode/784.pdf
- 9.FDA (CDER) Guidance for Industry: Nasal Spray and Inhalation Solution, Suspension Spray Drug Products—Chemistry, Manufacturing and Controls Documentation. Jul, 2002. http://www.fda.gov/cder/guidance/4234fnl.pdf http://www.fda.gov/cder/guidance/4234fnl.pdf
- 10.Newman SP. Pitcairn GR. Hooper G. Knoch M. Efficient drug delivery to the lungs from a continuously operated open-vent nebulizer and low pressure compressor system. Eur Respir J. 1994;7:1177–1181. [PubMed] [Google Scholar]
- 11.Nikander K. Prince I. Coughlin S. Warren S. Taylor G. Mode of Breathing—Tidal or Slow and Deep—through the I-neb Adaptive Aerosol Delivery (AAD) System Affects Lung Deposition of 99mTc-DTPA. J Aerosol Med Pulm Drug Del. 2010;23(Suppl 1):S37–S44. doi: 10.1089/jamp.2009.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boe J. Dennis JH. O'Driscoll BR. European Respiratory Society guidelines on the use of nebulizers. Eur Respir J. 2001;18:228–242. doi: 10.1183/09031936.01.00220001. [DOI] [PubMed] [Google Scholar]
- 13.Van Dyke R. Nikander K. Delivery of iloprost inhalation solution with HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery (AAD) systems—an in vitro study. Respir Care. 2007;52:184–190. [PubMed] [Google Scholar]
- 14.Akapo S. Gupta J. Martinez E. McCrea C. Ye L. Roach M. Compatibility and aerosol characteristics of formoterol fumarate mixed with other nebulizing solutions. Ann. Pharmacothers. 2008;42:1416–1424. doi: 10.1345/aph.1L273. [DOI] [PubMed] [Google Scholar]
- 15.Ferron GA. The scientific basis for aerosol therapy using nebulizers. Eur Respir Rev. 1994;4(18):95–98. [Google Scholar]
- 16.Devadason SG. Everard ML. Linto JM. Le Souef PN. Comparison of drug delivery from conventional versus venturi nebulizers. Eur Respir J. 1997;10:2479–2483. doi: 10.1183/09031936.97.10112479. [DOI] [PubMed] [Google Scholar]
- 17.Beasley R. Rafferty P. Holgate ST. Adverse reactions to the non-drug constituents of nebulizer solutions. Br J Clin Pharmacol. 1988;25:283–287. doi: 10.1111/j.1365-2125.1988.tb03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghazanfari T. Elhissi AMA. Ding Z. Taylor KMG. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int J Pharmacol. 2007;339:103–111. doi: 10.1016/j.ijpharm.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 19.McCallion ONM. Taylor KMG. Thomas M. Taylor AJ. Nebulization of fluids of different physicochemical properties with air-jet and ultrasonic nebulizers. Pharmaceut Res. 1995;12:1682–1688. doi: 10.1023/a:1016205520044. [DOI] [PubMed] [Google Scholar]
- 20.Geidel C. Bittner-Dersch P. Schüler D. Weber K. Lindemann H. Tobramycin-spiegel im im serum bei unterschiedlichen positionen des eFlow rapid. www.uniklinikum-giessen.de/pneumologie/tobramycin_eflowrapid www.uniklinikum-giessen.de/pneumologie/tobramycin_eflowrapid