Abstract
In the present study, we investigated the relationship between polymorphisms in the estrogen-metabolizing genes CYP17, CYP1B1, CYP1A1, and COMT and genomic instability in the peripheral blood lymphocytes of 62 BC patients and 62 controls considering that increased or prolonged exposure to estrogen can damage the DNA molecule and increase the genomic instability process in breast tissue. Our data demonstrated increased genomic instability in BC patients and that individuals with higher frequencies of MN exhibited higher risk to BC when belonging Val/Met genotype of the COMT gene. We also observed that CYP17 and CYP1A1 polymorphisms can modify the risk to BC depending on the menopause status. We can conclude that the genetic background in estrogen metabolism pathway can modulate chromosome damage in healthy controls and patients and thereby influence the risk to BC. These findings suggest the importance to ally biomarkers of susceptibility and effects to estimate risk groups.
1. Introduction
Breast cancer (BC) is a prevalent cancer among women worldwide, and it has been estimated that there are 71 cases of BC per year for every 100 000 women in Brazil. BC shows some distinctive features concerning age-specific incidence rates [1]. It is one of the most common causes of death among women, and epidemiological studies indicate that the age of onset is slowly, but steadily, becoming lower. This suggests that there may be changes in environmental factors that are affecting BC risk [2].
The risk factors for BC include early age of menarche, delayed menopause, contraceptive use, hormonal replacement therapy, above-average body mass index, exposure to environmental pollutants, smoking, and alcohol use [1–3]. However, it is generally believed that the initiation of BC is a consequence of cumulative genetic damage, which leads to genetic alterations that result in the activation of proto-oncogenes and the inactivation of tumor suppressor genes [4]. A large number of genetic variants that are associated with BC risk have been identified in genes involved in a wide variety of functions, including steroid hormone metabolism, detoxification of environmental carcinogens, DNA damage repair, and tumor suppression [5].
As observed in drug and chemical metabolism, there is considerable interindividual variability (i.e., polymorphisms) in the conjugation pathways of both estrogen and catechol estrogens. These person-to-person differences, which are attributed to polymorphisms in the genes encoding for the respective enzymes, may define subpopulations of women with higher lifetime exposure to hormone-dependent growth promotion or to cellular damage from particular estrogens and/or estrogen metabolites [4].
Current evidence suggests that the metabolic by-products of estrogens in the body may act as initiators of cellular alterations [6]. Estrogen metabolism products such as quinone-catecholestrogen can bind to DNA and form DNA adducts [7]. The generation of free radicals by metabolic redox cycling between quinone and hydroquinone can damage DNA by causing strand breaks, 8-hydroxylation of purines, and lipid hydroperoxide-mediated DNA modifications [8].
Although still controversial, a number of genes involved in biogenesis (CYP17), bioavailability (CYP1B1 and CYP1A1), and degradation (COMT) of estrogen compounds contain polymorphisms that could affect susceptibility to BC [9]. They are called low penetrance genes (or sometimes modifier genes) and are, in this instance, defined as genes in which subtle sequence variations or polymorphisms may be associated with a slightly to moderately increased relative risk for BC [4].
The hypothesis of the present study is that polymorphic variants of the estrogen metabolizing genes CYP17, CYP1B1, CYP1A1, and COMT may affect the spontaneous levels of chromosome damage in lymphocytes of BC patients and subsequently modulate BC risk.
Therefore, the objective of the present work was to correlate the genotypes of polymorphic variants of the above-mentioned genes with the basal levels of chromosome damage in lymphocytes of untreated BC patients and healthy individuals. Micronucleus assay was employed to determine the extent of baseline chromosome damage in BC patients and controls, and PCR-RFLP was used for genotype analysis.
2. Patients and Methods
2.1. Subjects
The BC patient group consisted of 62 untreated women diagnosed with in situ or invasive ductal breast carcinoma, ranging in age from 25 to 60 years old (mean age, 50.5 years old) and free of any pathology associated with the use of medication that is known to cause DNA damage. The control group consisted of 62 women with ages ranging from 25 to 50 years (mean age, 46.7-years old). They were enrolled in the control group after a detailed investigation in order to ensure that they were free from any breast pathology. They came from the same geographical location, their dietary habits were not appreciably different, and they were not occupationally exposed to genotoxic chemicals. None of the subjects reported alcohol consumption, the use of genotoxic medicine, presence of known inherited genetic disorders or chronic diseases or exposure to ionizing or nonionizing radiation, even for diagnostic or therapeutic purposes, for at least one month prior to enrolling in the study. Patients and controls enrolled in the present study did not report family history of breast and/or ovarian cancer. Characteristics of patients and controls are presented in Table 1. This investigation was approved by the National Ethics Committee (CONEP: 1217/2004) and was performed in accordance with ethical standards. Informed consent of patients and controls was obtained before inclusion in the study and sample collection.
Table 1.
Characteristics of breast cancer patients and controls.
| Patients N (%) | Controls N (%) | P | |
|---|---|---|---|
| Smoking | |||
| Yes | 14 (22.5) | 22 (35.4) | .1 |
| No | 48 (77.5) | 40 (64.6) | |
| Menopause status | |||
| Post | 32 (51.6) | 23 (37) | .1 |
| Pre | 30 (48.4) | 39 (63) | |
| HRT | |||
| Yes | 20 (32.2) | 28 (45.1) | .2 |
| No | 42 (67.8) | 34 (54.9) | |
| Full-term pregnancy | |||
| Yes | 44 (70.9) | 42 (68.4) | .8 |
| No | 18 (29.1) | 20 (32.2) |
HRT: hormone replacement therapy.
2.2. Blood Sampling
Samples of venous blood (10 mL) were collected in heparinized and EDTA Vacutainer tubes (Becton Dickinson, NJ, USA) by venepuncture under sterile conditions. The samples were coded, immediately protected from direct light, and processed for genotyping and micronucleus assay.
2.3. Genotype Determination
Genomic DNA samples were obtained from blood lymphocytes using a Wizard Genomic DNA Purification Kit (Promega, Madison, WI). Isolated DNA was resuspended in Tris-EDTA buffer (pH 8.0) and stored at −20°C until use.
The T1931C polymorphism of the CYP17 gene was determined by PCR-RFLP with the following primers: sense, 5′-CAAGGTGAAGATCAGGGTAG-3′ and antisense, 5′-GCTAGGGTAAGCAGCAAGAG-3′. The 145 bp product was digested overnight with 8 U of the restriction enzyme MspA1I. The T allele remained intact, but the C allele was digested into 75 and 70 bp fragments. The V432L polymorphism of the CYP1B1 gene was genotyped with the following primers: sense, 5′-TCACTTGCTTTTCTCTCTCC-3′ and antisense, 5′-AATTTCAGCTTGCCTCTTG-3′. The 650 bp product was digested overnight with 8 U of the restriction enzyme AcuI. The Leu allele was digested into 350 and 300 bp fragments. The genotypes of the T3205C polymorphism of the CYP1A1 gene were determined with the following primers: sense, 5′-TAGGAGTCTTGTCTCATGCCT-3′ and antisense, 5′-CAGTGAAGAGGTGTAGCCGCT-3′. The 340 bp PCR product was digested for 3 h with 5 U of the restriction enzyme MspI. The C allele was digested into 200 and 140 bp fragments, whereas the T allele remained intact. The genotypes of the V158M polymorphism of the gene COMT were determined with the following primers: sense, 5′-TACTGTGGCTACTCAGCTGT-3′ and antisense, 5′-TGAAGCTGGTGTGAACACCT-3′. The 114 bp PCR product was digested with 5 U of the restriction enzyme NlaIII. The Met allele was digested into fragments of 96, 54, 39, and 27 bp, whereas the Val allele was digested into fragments of 54 and 39 bp only.
2.4. Cell Culture and Cytokinesis-Block Micronucleus Assay
Lymphocyte cultures were prepared combining 0.5 mL of isolated lymphocytes in plasma with 5 mL of complete medium containing 78% of RPMI (Sigma-Aldrich Co., USA), 20% inactivated fetal bovine serum (Gibco-Invitrogen, Denmark), the antibiotics penicillin (5 μg/mL, Sigma-Aldrich Co., USA) and streptomycin (10 μg/mL, Sigma-Aldrich Co., USA), and 2% phytohemagglutinin (Life Technologies, Grand Island, NY, USA) to stimulate cell proliferation. Cultures were incubated at 37°C. After 44 hours of incubation, cytochalasin B (Sigma-Aldrich Co., USA) was added to the cultures to a final concentration of 4 μg/mL, according to the method of Fenech and Morley [10]. One thousand binucleated cells were analyzed per individual and the frequency of binucleated cells micronucleated (BCMN) was determined according to the criteria described by Fenech [11].
2.5. Statistical Analysis
The Mann-Whitney statistical test was used to compare BCMN between patients and controls. The frequency of BCMN in different genotypes was analyzed with one-way ANOVA and the statistical differences between groups for BC risk was calculated using Fisher's exact test (two-tailed). Crude odds ratios (ORs) were calculated and are given with 95% confidence intervals (CIs). Results were considered significant when P < .05.
3. Results and Discussion
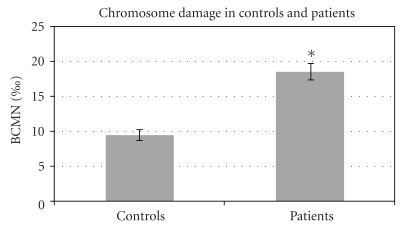
Table 1 compares and shows the homogeneity of BC patients and control groups according to their smoking habits, menopause status, hormone replacement therapy, and full-term pregnancy. Basal level of chromosome damage was measured by micronucleus (MN) assay in lymphocytes from 62 BC patients and 62 age-matched controls, as shown in Figure 1. BC patients exhibited higher levels of chromosome damage than controls according to BCMN (P < .05).
Figure 1.
Frequency of BCMN in untreated BC women and healthy controls. A total of one thousand binucleated cells were analyzed for MN count in each of 62 BC patients and 62 age-matched controls. Bars represent standard error of mean; *P < .001 significantly different from control group.
Table 2 shows the genotype distributions in patients and controls according to menopause status. The genotype distributions for all cases were in agreement with those predicted by the Hardy-Weinberg equilibrium. An association between BC occurrence and the C allele of the T1931C polymorphism in the CYP17 gene was observed in the postmenopause group (OR 4.3; 95% CI 1.3–13.7). CYP1B1 V432L polymorphism had no association with BC occurrence in pre- and postmenopause women, however, in the premenopause women we observed increased risk to BC when the C allele of CYP1A1 gene (T3205C polymorphism) was present (OR 10.5; 95% CI 2.6–41.7). COMT Met allele was also associated with BC occurrence in premenopause women (OR 3.2; 95% CI 1.1–8.6).
Table 2.
The genotype frequencies of the CYP17, CYP1B1, CYP1A1, and COMT gene variants in breast cancer patients and controls, and after menopause.
| Genotype | Breast cancer patients (n = 62) | Controls (n = 62) | OR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Number | Frequency | Number | Frequency | |||
| CYP17 | ||||||
| Premenopause | ||||||
| TT | 16 | 0.25 | 22 | 0.35 | 1.0 (reference) | .81 |
| TC+CC | 14 | 0.23 | 17 | 0.27 | 1.1 (0.4-2.9) | |
| Postmenopause | ||||||
| TT | 11 | 0.18 | 16 | 0.26 | 1.0 (reference) | .01 |
| TC+CC | 21 | 0.34 | 7 | 0.12 | 4.3 (1.3–13.7) | |
| CYP1B1 | ||||||
| Premenopause | ||||||
| Val/Val | 6 | 0.1 | 15 | 0.24 | 1.0 (reference) | .11 |
| Val/Leu+Leu/Leu | 24 | 0.39 | 24 | 0.39 | 2.5 (0.8–7.5) | |
| Postmenopause | ||||||
| Val/Val | 11 | 0.18 | 6 | 0.1 | 1.0 (reference) | .56 |
| Val/Leu+Leu/Leu | 21 | 0.33 | 17 | 0.27 | 0.6 (0.2–2.1) | |
| CYP1A1 | ||||||
| Premenopause | ||||||
| TT | 16 | 0.25 | 36 | 0.58 | 1.0 (reference) | .0004 |
| TC+CC | 14 | 0.23 | 3 | 0.05 | 10.5 (2.6–41.7) | |
| Postmenopause | ||||||
| TT | 24 | 0.39 | 17 | 0.27 | 1.0 (reference) | 1.0 |
| TC+CC | 8 | 0.13 | 6 | 0.1 | 0.9 (0.2–3.2) | |
| COMT | ||||||
| Premenopause | ||||||
| Val/Val | 10 | 0.16 | 24 | 0.38 | 1.0 (reference) | .02 |
| Val/Met+Met/Met | 20 | 0.32 | 15 | 0.24 | 3.2 (1.1–8.6) | |
| Postmenopause | ||||||
| Val/Val | 11 | 0.18 | 12 | 0.2 | 1.0 (reference) | .2 |
| Val/Met+Met/Met | 21 | 0.34 | 11 | 0.18 | 2.0 (0.6–6.2) | |
The basal levels of chromosome damage in BC patients and controls among the different genotypes are presented in Table 3. BCMN frequencies were higher in BC than in control group in all genotypes except for Val/Val of COMT gene where differences between patients and controls were not significant (P = .07).
Table 3.
Frequency of micronucleated cells in untreated breast cancer women and controls with different genotypes.
| Genotype | Breast cancer patients (n = 62) | Controls (n = 62) | P value | ||
|---|---|---|---|---|---|
| Number | CBMN (‰) | Number | CBMN (‰) | ||
| CYP17 | |||||
| TT | 27 | 19 ± 8.9 | 38 | 9.3 ± 6.3 | <.0001 |
| TC+CC | 35 | 18.2 ± 10.5 | 24 | 9.6 ± 5.4 | <.0001 |
| CYP1B1 | |||||
| Val/Val | 17 | 18.7 ± 10.5 | 21 | 6.7 ± 4.1 | <.0001 |
| Val/Leu+Leu/Leu | 45 | 18.5 ± 9.6 | 41 | 10.3 ± 5.9 | <.0001 |
| CYP1A1 | |||||
| TT | 40 | 19.5 ± 10.2 | 53 | 9.9 ± 5.5 | <.0001 |
| TC+CC | 22 | 16.7 ± 9 | 9 | 9.1 ± 5.3 | .001 |
| COMT | |||||
| Val/Val | 21 | 15.2 ± 8.7 | 34 | 11.3 ± 6.7 | .07 |
| Val/Met+Met/Met | 41 | 20.3 ± 10 | 28 | 8.5 ± 4.9 | <.0001 |
MNF: micronucleus frequency; M: mean; SE: standard error; patients compared to controls.
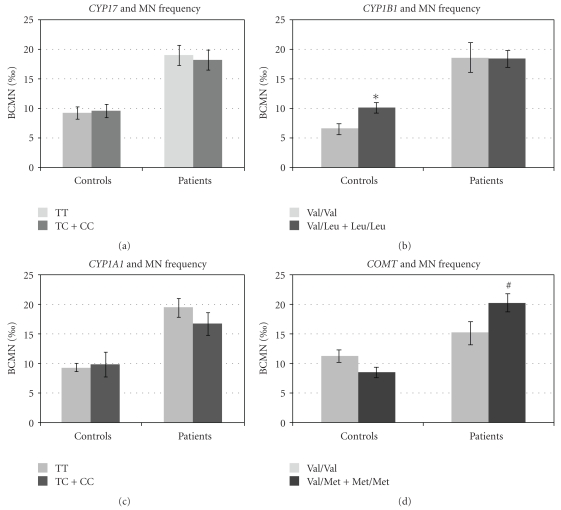
We also evaluated the influence of different genotypes considering the levels of chromosome damage in patient and control groups individually (Figure 2). CYP17 T1931C and CYP1A1 T3205C polymorphisms had no influence in the frequency of BCMN in patients as well as in controls. However, in control group, CYP1B1 432L allele was related to increased frequencies of BCMN (P = .006). Differently, patients with V158M polymorphism of COMT gene presented higher frequencies of BCMN when compared to the wild-type counterparts (P = .04).
Figure 2.
Frequency of BCMN in untreated BC women and healthy controls considering the presence of estrogen-metabolizing genes polymorphisms (a) CYP17, (b) CYP1B1, (c) CYP1A1, and (d) COMT. A total of one thousand binucleated cells were analyzed for MN count in each of 62 BC patients and 62 age-matched controls. Bars represent standard error of mean; *P < .005 significantly different from the wild type in the control group; #P < .005 significantly different from the wild type in the patients group.
Finally, we tested the risk to BC in the group of patients and controls that presented frequencies of BCMN higher than the mean (higher than 18.5 BCMN for patients and higher than 9.5 BCMN for controls) (Table 4). CYP17, CYP1B1, and CYP1A1 polymorphisms did not modify the occurrence of BC, however, COMT V158M polymorphism was more frequent in BC than in control group and resulted in significant OR increasing (OR 3.5; 95% CI 1–11.4).
Table 4.
Estrogen-metabolizing gene polymorphisms in breast cancer patients and controls with frequency of micronucleated cells higher than the mean of each group.
| Genotype | Breast cancer patients (n = 27) | Controls (n = 26) | OR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Number | Frequency | Number | Frequency | |||
| CYP17 | ||||||
| TT | 12 | 0.44 | 9 | 0.35 | 1.0 (reference) | .57 |
| TC+CC | 15 | 0.56 | 17 | 0.65 | 0.6 (0.2–2.0) | |
| CYP1B1 | ||||||
| Val/Val | 6 | 0.22 | 3 | 0.12 | 1.0 (reference) | .46 |
| Val/Leu+Leu/Leu | 21 | 0.78 | 23 | 0.88 | 0.46 (0.1–2.0) | |
| CYP1A1 | ||||||
| TT | 21 | 0.78 | 16 | 0.62 | 1.0 (reference) | .2 |
| TC+CC | 6 | 0.22 | 10 | 0.38 | 0.4 (0.1–1.5) | |
| COMT | ||||||
| Val/Val | 6 | 0.22 | 13 | 0.5 | 1.0 (reference) | .04 |
| Val/Met+Met/Met | 21 | 0.78 | 13 | 0.5 | 3.5 (1–11.4)↑ | |
DNA damage can occur spontaneously or as consequence of exposure to chemical or physical genotoxins. There are modulators that can affect the levels of spontaneous DNA damage, including DNA repair genes, antioxidant defense genes, and estrogen-metabolizing gene polymorphisms that can lead to the accumulation of genotoxic estrogen subproducts. High serum estrogen levels are thought to be a major risk factor for BC [12]. In vitro and in vivo animal and patient-based studies suggest that estrogens, their metabolic compounds, and the entire biochemical metabolic machinery may play a role in BC carcinogenesis [13]. We can look forward into two different processes by which hormones are related to BC: (i) one involves the binding of estradiol to estrogen receptor (ER) alpha with the stimulation of cell proliferation. Errors in DNA occurring during replication result in fixed mutations when not well repaired; (ii) the other process results from the formation of genotoxic metabolites of estradiol, which can bind to DNA, cause depurination, and also result in mutations. Herein, we focused on the second process. Therefore, in the present study, the relationship between the SNPs in four estrogen-metabolizing genes and basal levels of chromosome damage was examined using lymphocytes of untreated BC women and healthy controls once lymphocytes can circulate for years or even decades, accumulating mutations in their DNA [14].
Results presented here show that the basal levels of chromosome damage detected by MN assay and observed in the control group are in accordance with those previously reported in other control populations of similar age [15, 16]. It is well known that MN are formed by the condensation of acentric chromosomal fragments or by whole chromosomes lagging behind during cell division and that this is the only biomarker that allows the evaluation of both clastogenic and aneuploidogenic effects in a vast range of cells, as they are detected in interphase [17]. As we did not observe any statistical difference between smoking and nonsmoking patients or controls (data not shown), we grouped these two subsets together for the analysis of the basal levels of chromosome damage in patients and controls.
The investigation of basal levels of chromosome damage in peripheral lymphocytes of untreated cancer subjects has been previously reported. Baseline levels of DNA damage were significantly higher in bladder cancer patients than in controls [18], and the frequency of MN in stimulated peripheral blood cells from an untreated leukemia population was significantly greater than that in the control group [19]. Similar results were found by Lou et al. [14], who simultaneously investigated both baseline and ionizing radiation-induced (IR) genetic damage in peripheral lymphocytes from 36 cancer patients using MN and comet assays. They found that both spontaneous and IR-induced genetic damages were higher in patients than in controls.
In the present study, the primary objective was to investigate the relationship between the genotypes of polymorphisms in estrogen-metabolizing genes and the extent of endogenous chromosome damage. Although this is not an epidemiological study, the odds ratio for the different genotypes was also examined and displayed interesting findings.
The results presented here show that there is a slight significant relationship between the CYP17 polymorphism, and BC risk is postmenopausal women, however, we found no correlation between spontaneous chromosome damage and the T1931C polymorphism of CYP17 gene, which encodes for cytochrome p450C17α, a 17α-hydroxylase that catalyzes the conversion of pregnenolone and progesterone to 17-OH-pregnenolone and 17-OH-progesterone, respectively [20]. Cytochrome p450C17α also has a 17,20-lyase activity that converts hydroxylase products to dehydroepiandrosterone (DHEAS) and androstenedione, respectively, which can be further converted to estrogens and testosterone [20]. It has been hypothesized that certain genotype variants in the CYP17 gene result in higher hormone levels, leading to an increased risk of BC. However, non-Hispanic white women who were heterozygous or homozygous for the variant allele of CYP17 had lower estrone, total testosterone, free testosterone, and DHEAS concentrations compared to women homozygous for the wild-type allele [21]. Chen and Pei [22] applied both traditional meta-analysis and Bayesian approach to determine the overall effect of CYP17 T1931C polymorphism on risk of BC and detected that carriers of C allele were positively associated with risk of BC in postmenopausal women and were inversely related to BC in pre-menopausal women.
The V432L polymorphism of the CYP1B1 gene is located within a catalytic heme-binding domain in exon 3 [23] and has been analyzed in several independent studies that present controversial results. Chinese women with the Leu/Leu genotype at the L432V polymorphism had a higher risk of BC (OR = 2.0, 95%; CI = 1.0–3.7) [24]. A positive association was also seen in Turkish women but was limited to those with a high body mass index (OR = 2.3, 95%; CI = 1.3–4.2) [25]. In contrast, results presented here showed no association between V432L polymorphism and BC occurrence in pre- or postmenopause women (OR 2.5, 95% CI 0.8–7.5 and OR 0.6, 95% CI 0.2–2.1, resp.). A significant inverse association between the Val allele and BC risk was previously observed in studies of African American or mixed populations [26]. It is interesting to note that in the control group, carriers of the Leu allele exhibited higher levels of DNA damage compared to homozygous wild-type individuals while this was not observed in the patients group. We believe that this difference between patients and controls is mostly due to the increased spontaneous levels of chromosome damage in BC which makes it difficult to identify suitable differences in BCMN between polymorphic and wild-type individuals. It has been described that CYP1B1 catalyzes C4 hydroxylation of estradiol (4-hydroxyestradiol (4-OHE2)). This metabolite can undergo metabolic redox cycling to generate free radicals such as superoxide and chemically reactive estrogen semiquinone/quinone intermediates, which can form DNA adducts [27, 28]. Anyway, these results suggest further investigation of this polymorphism combined with other low-penetrance gene polymorphisms such as DNA repair in the modulation of spontaneous chromosome damage in healthy women.
Cytochrome P450 1A1 (CYP1A1) is one of the most important phase I enzymes expressed in breast tissue. It catalyzes estrogen 2-hydroxylation, generating the 2-OH estrogen metabolite, which can form DNA adducts [28, 29]. The 3205T→C polymorphism in CYP1A1 is located in the 3′ noncoding region and can affect the modulation of enzymatic activity and thereby the susceptibility risk to BC [30]. The results presented here showed that the frequency of this polymorphism was higher in premenopause BC women than in controls and resulted in association to BC risk (OR 10.5, 95% CI 2.6–41.7), however, it had no effect on the frequency of BCMN in patients or in control group. The rare CT and CC genotypes were detected in 14 patients and three controls, and it, therefore, resulted in an extensive confidence interval (CI). Similar results were observed by Taioli et al. [30] that demonstrated an association between this polymorphism and BC cancer risk in African American women (OR = 9.7 95%, CI 2.0–47.9), and Huang et al. [31] demonstrated the same association in Taiwanese women. Nevertheless, the association observed here must be interpreted with caution considering the number of individuals included in the present study and the fact that C allele is rare.
The catechol-O-methyltransferase (COMT) catalyzes the addition of a methyl group to reactive catechol estrogens, converting them into stable methyloxyestrogen conjugates [32]. In patients group, carriers of Met allele of the COMT gene exhibited higher levels of chromosome damage relative to those homozygous for the wild-type allele. Besides, when the analysis considered the association of BC occurrence in individuals that exhibited levels of chromosome damage higher than the mean, Met allele was strongly associated with BC occurrence. This was an interesting finding especially if we consider that biomarkers of susceptibility and risk contribute to the identification of high-risk subgroups of the population, independent of whether they are associated with previous exposure or they are involved in a defined pathway or mechanism [33]. It is known that G to A polymorphism at codon 108 in the COMT gene leads to a substitution of methionine to valine, resulting in decreased enzymatic activity and increased thermolability [34, 35]. As metabolites of catechol estrogens have the potential to induce oxidative DNA damage and form DNA adducts, the conversion of catechol estrogens into stable conjugates may be important in preventing BC [32]. In a previous study of a Taiwanese population, a strong association between the COMT polymorphism and BC risk was observed (OR = 3.5 95%, CI 1.15–13.3) [31].
The correlation between genetic polymorphisms and specific phenotypes was evaluated by Synowiec et al. [36]. They correlated a polymorphism in a DNA repair gene with oxidative DNA damage in 41 BC patients and 48 controls. They demonstrated a strong association between BC occurrence and the C/C genotype of the RAD51-135G/C polymorphism, the Ser/Ser genotype of the OGG1-Ser326Cys polymorphism and the Lys/Gln genotype of the XPD-Lys751Gln polymorphism. In contrast, the G/C genotype of the RAD-135G/C polymorphism and the Lys/Lys genotype of the XPD-Lys751Gln polymorphism exhibited a protective effect against BC. In this study, the risk of BC was calculated in patients and controls that had higher levels of endogenous oxidative DNA damage.
We previously reported that Brazilian untreated BC patients exhibited higher levels of chromosome damage than healthy controls [37] and that XRCC3 polymorphism was weakly associated with the risk to BC in those individuals that presented higher levels of chromosome damage [38]. It is well known that DNA damage as detected in peripheral blood represents a surrogate for what would be observed in breast tissue. While the polymorphisms are obviously expressed in both breast tissue and lymphocytes, the level of expression of the genes and in particular, the level of substrate that in our present hypothesis is estradiol, may be and is likely very different within breast tissue and the lymphocytes, however, genome damage in lymphocytes may be correlated with cancer-initiating events in target tissues via a common genetic, dietary, or environmental factor [39]. Therefore, the manifestation of effects of the polymorphisms on levels of the reactive quinones may be quite different in breast tissue and lymphocytes, making extrapolation of the findings being reported to implications for BC quite tenuous. As mentioned above, this is not an epidemiological study and although the limited number of participants, we consider that our results point that polymorphisms in the enzymes involved in the metabolism of estradiol/estrone to reactive quinones that cause DNA damage may represent low penetrance risk factors susceptible of further detailed investigations.
4. Conclusions
It is unknown whether the healthy controls and patients with BC had equal levels of DNA damage before the appearance of clinical symptoms in the patients. It is also unclear whether MN enhancement is a consequence or causative agent of the disease process [19]. However, the followup of those controls who are carriers of Met allele of CYP1B1 gene that exhibited higher frequencies of BNMN than wild-type counterparts could help to further answer this question.
In conclusion, according to the data presented here both the genetic background of genes involved estrogen metabolism and DNA damage in healthy controls and patients can modulate BC risk reflected by higher chromosomal damage in lymphocytes.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
The authors gratefully acknowledge the cooperation of all volunteers who participated in this study. This investigation was supported in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Grant no. 400887/2005 and 3473493/2004-7) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES for fellowship to RA Santos and MB Mayorano.
References
- 1.Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2001;38(1):103–116. doi: 10.1016/s0378-5122(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen VN, Borresen-Dale AL. Molecular epidemiology of breast cancer: genetic variation in steroid hormone metabolism. Mutation Research. 2000;462(2-3):323–333. doi: 10.1016/s1383-5742(00)00018-1. [DOI] [PubMed] [Google Scholar]
- 3.Kang HJ, Kim SW, Kim HJ, et al. Polymorphisms in the estrogen receptor-alpha gene and breast cancer risk. Cancer Letters. 2002;178(2):175–180. doi: 10.1016/s0304-3835(01)00861-8. [DOI] [PubMed] [Google Scholar]
- 4.Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer: the role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutation Research. 2003;544(1):9–41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 5.Dunning AM, Healey CS, Pharoah PDP, Teare MD, Ponder BAJ, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiology Biomarkers and Prevention. 1999;8(10):843–854. [PubMed] [Google Scholar]
- 6.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. New England Journal of Medicine. 2006;354(3):228–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 7.Bianco NR, Perry G, Smith MA, Templeton DJ, Montano MM. Functional implications of antiestrogen induction of quinone reductase: inhibition of estrogen-induced deoxyribonucleic acid damage. Molecular Endocrinology. 2003;17(7):1344–1355. doi: 10.1210/me.2002-0382. [DOI] [PubMed] [Google Scholar]
- 8.Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutation Research. 1999;424(1-2):107–115. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 9.Yuan X, Zhou G, Zhai Y, et al. Lack of association between the functional polymorphisms in the estrogen-metabolizing genes and risk for hepatocellular carcinoma. Cancer Epidemiology Biomarkers and Prevention. 1999;17(12):3621–3627. doi: 10.1158/1055-9965.EPI-08-0742. [DOI] [PubMed] [Google Scholar]
- 10.Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutation Research. 1985;147(1-2):29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 11.Fenech M. The in vitro micronucleus technique. Mutation Research. 2000;455(1-2):81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 12.Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360(9336):861–868. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- 13.Zumoff B. Does postmenopausal estrogen administration increase the risk of breast cancer? Contributions of animal, biochemical, and clinical investigative studies to a resolution of the controversy. Proceedings of the Society for Experimental Biology and Medicine. 1998;217(1):30–37. doi: 10.3181/00379727-217-44202. [DOI] [PubMed] [Google Scholar]
- 14.Lou J, He J, Zheng W, et al. Investigating the genetic instability in the peripheral lymphocytes of 36 untreated lung cancer patients with comet assay and micronucleus assay. Mutation Research. 2007;617(1-2):104–110. doi: 10.1016/j.mrfmmm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Bolognesi C, Lando C, Forni A, et al. Chromosomal damage and ageing: effect on micronuclei frequency in peripheral blood lymphocytes. Age and Ageing. 1999;28(4):393–397. doi: 10.1093/ageing/28.4.393. [DOI] [PubMed] [Google Scholar]
- 16.Varga D, Hoegel J, Maier C, et al. On the difference of micronucleus frequencies in peripheral blood lymphocytes between breast cancer patients and controls. Mutagenesis. 2006;21(5):313–320. doi: 10.1093/mutage/gel035. [DOI] [PubMed] [Google Scholar]
- 17.Pastor S, Creus A, Parrón T, et al. Biomonitoring of four European populations occupationally exposed to pesticides: use of micronuclei as biomarkers. Mutagenesis. 2003;18(3):249–258. doi: 10.1093/mutage/18.3.249. [DOI] [PubMed] [Google Scholar]
- 18.Schabath MB, Spitz MR, Grossman HB, et al. Genetic instability in bladder cancer assessed by the comet assay. Journal of the National Cancer Institute. 2003;95(7):540–547. doi: 10.1093/jnci/95.7.540. [DOI] [PubMed] [Google Scholar]
- 19.Hamurcu Z, Dönmez-Altuntas H, Patiroglu T. Basal level micronucleus frequency in stimulated lymphocytes of untreated patients with leukemia. Cancer Genetics and Cytogenetics. 2008;180(2):140–144. doi: 10.1016/j.cancergencyto.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Sharp L, Cardy AH, Cotton SC, Little J. CYP17 gene polymorphisms: prevalence and associations with hormone levels and related factors. A HuGE review. American Journal of Epidemiology. 2004;160(8):729–740. doi: 10.1093/aje/kwh287. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamson PE, Tworoger SS, Aiello EJ, et al. Associations between the CYP17, CYPIB1, COMT and SHBG polymorphisms and serum sex hormones in post-menopausal breast cancer survivors. Breast Cancer Research and Treatment. 2007;105(1):45–54. doi: 10.1007/s10549-006-9426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Pei J. Factors influencing the association between CYP17 T34C polymorphism and the risk of breast cancer: meta-regression and subgroup analysis. Breast Cancer Research and Treatment. 2010;122(2):471–481. doi: 10.1007/s10549-009-0690-9. [DOI] [PubMed] [Google Scholar]
- 23.Bailey LR, Roodi N, Dupont WD, Parl FF. Association of cytochrome P450 1B1 (CYP1B1) polymorphism with steroid receptor status in breast cancer. Cancer Research. 1998;58(22):5038–5041. [PubMed] [Google Scholar]
- 24.Zheng W, Xie DW, Jin F, et al. Genetic polymorphism of cytochrome P450-1B1 and risk of breast cancer. Cancer Epidemiology Biomarkers and Prevention. 2000;9(2):147–150. [PubMed] [Google Scholar]
- 25.Kocabas NA, Sardas S, Cholerton S, Daly AK, Karakaya AE. Cytochrome P450 CYP1B1 and catechol O-methyltransferase (COMT) genetic polymorphisms and breast cancer susceptibility in a Turkish population. Archives of Toxicology. 2002;76(11):643–649. doi: 10.1007/s00204-002-0387-x. [DOI] [PubMed] [Google Scholar]
- 26.Paracchini V, Raimondi S, Gram IT, et al. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432Leu polymorphism and breast cancer: a HuGE-GSEC review. American Journal of Epidemiology. 2007;165(2):115–125. doi: 10.1093/aje/kwj365. [DOI] [PubMed] [Google Scholar]
- 27.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi Y, Noguchi S. Polymorphisms of estrogen synthesizing and metabolizing genes and breast cancer risk in Japanese women. Biomedicine and Pharmacotherapy. 2003;57(10):471–481. doi: 10.1016/j.biopha.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Huang Y, Li Y, Mao Y, Xie Y. Cytochrome P450 1A1 (CYP1A1) T3801C and A2455G polymorphisms in breast cancer risk: a meta-analysis. Journal of Human Genetics. 2007;52(5):423–435. doi: 10.1007/s10038-007-0131-8. [DOI] [PubMed] [Google Scholar]
- 30.Taioli E, Bradlow HL, Garbers SV, et al. Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detection and Prevention. 1999;23(3):232–237. doi: 10.1046/j.1525-1500.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 31.Huang CS, Chern HD, Chang KJ, Cheng CW, Hsu SM, Shen CY. Breast cancer risk associated with genotype polymorphism of the estrogen-metabolizing genes CYP17, CYP1A1, and COMT: A multigenic study on cancer susceptibility. Cancer Research. 1999;59(19):4870–4875. [PubMed] [Google Scholar]
- 32.Gaudet MM, Chanock S, Lissowska J, et al. Comprehensive assessment of genetic variation of catechol-O- methyltransferase and breast cancer risk. Cancer Research. 2006;66(19):9781–9785. doi: 10.1158/0008-5472.CAN-06-1294. [DOI] [PubMed] [Google Scholar]
- 33.Boffetta P. Biomarkers in cancer epidemiology: an integrative approach. Carcinogenesis. 2009;31(1):121–126. doi: 10.1093/carcin/bgp269. [DOI] [PubMed] [Google Scholar]
- 34.Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O- methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 35.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Synowiec E, Stefanska J, Morawiec Z, Blasiak J, Wozniak K. Association between DNA damage, DNA repair genes variability and clinical characteristics in breast cancer patients. Mutation Research. 2008;648(1-2):65–72. doi: 10.1016/j.mrfmmm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Santos RA, Teixeira AC, Mayorano MB, Carrara HHA, Andrade JM, Takahashi CS. Basal levels of DNA damage detected by micronuclei and comet assays in untreated breast cancer patients and healthy women. Clinical and Experimental Medicine. 2010;10:87–92. doi: 10.1007/s10238-009-0079-4. [DOI] [PubMed] [Google Scholar]
- 38.Santos RA, Teixeira AC, Mayorano MB, Carrara HHA, Andrade JM, Takahashi CS. DNA repair genes XRCC1 and XRCC3 polymorphisms and their relationship with the level of micronuclei in breast cancer patients. Genetics and Molecular Biology. 2010;33(4):637–640. doi: 10.1590/S1415-47572010005000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonassi S, Znaor A, Ceppi M, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28(3):625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]