Abstract
Background
Atrial stretch is thought to play a role in the development of atrial fibrillation (AF). However, the precise mechanism by which stretch contributes to AF maintenance in humans is unknown.
Methods
The subjects of this study were 58 consecutive patients with persistent AF (n=40) or paroxysmal AF (n=18) undergoing left atrial (LA) ablation. LA pressure was measured before ablation. Both atria and the coronary sinus were mapped, and regional dominant frequency (DF) was determined.
Results
The mean LA pressure in the persistent AF group was significantly higher than in the paroxysmal AF group (18±5 vs. 10±4 mm Hg, p<0.0001). The mean DF in the persistent AF group was also higher than in the paroxysmal AF group (6.36±0.51 and 5.83±0.54 Hz, p=0.0006). In patients with persistent AF, there was a significant correlation between LA pressure and the DF at the LA appendage (r=0.55, p=0.0002). DFmax was found at the LA appendage region in 24 of the 40 patients (60%) with persistent AF (p=0.0006). In multivariate analysis, LA pressure was the only independent predictor of DFmax in the LA appendage (p=0.04, OR 1.41, 95% CI, 1.02 to 1.94).
Conclusions
Higher LA pressure in patients with persistent AF implies that these patients are more vulnerable to stretch-related remodeling than patients with paroxysmal AF. The DF of AF was directly related to LA pressure in patients with persistent AF. This suggests that atrial stretch may contribute to the maintenance of AF in humans by stabilizing high frequency sources.
Keywords: atrial fibrillation, atrial pressure, dominant frequency, effective refractory period, atrial stretch, electrical remodeling
Introduction
Atrial stretch has long been implicated in the pathogenesis of atrial fibrillation (AF).1 A prior study showed that atrial stretch, imparted by elevated left atrial (LA) pressure, increased the rate of AF drivers in an animal model.2 In humans, stretch is thought to play a role in the development of AF in patients with hypertension, mitral valve disease, and heart failure.3 However, the electrophysiologic mechanism by which stretch contributes to the fibrillatory process in humans has not been clarified. The aim of the study was to determine the impact of LA pressure on AF frequency in patients undergoing catheter ablation of AF.
Methods
Study subjects
The subjects of this study were 40 consecutive patients with persistent AF4 undergoing left atrial (LA) ablation. Thirty of the 40 patients (75%) had longstanding, persistent AF.4 An estimate of the duration of continuous AF was obtained from a combination of records from the referring cardiologist and history obtained from the patient. If a patient underwent transthoracic cardioversion and AF recurred thereafter, the duration of continuous AF was defined as the time period extending from the date of recurrence to the date of the ablation procedure.
Patients with paroxysmal AF4 who presented to the laboratory in AF served as a comparison group (N=18). Patients who had undergone a prior ablation procedure, and those with structural heart disease, history of heart failure, or those currently taking diuretic medications were excluded from the study. These patients were excluded since these conditions may be associated with increased LA pressure. The clinical characteristics of the study subjects are described in table 1.
Table 1.
Patient characteristics
| Paroxysmal AF group (N=18) | Persistent AF group (N=40) | P | |
|---|---|---|---|
| Age | 58±8 | 59±10 | 0.71 |
|
| |||
| Gender (M/F) | 14/5 | 33/7 | 0.44 |
|
| |||
| Body mass index (kg/m2) | 27±3 | 32±5 | 0.0001 |
|
| |||
| Sleep apnea syndrome | 3 (17%) | 7 (18%) | 0.94 |
|
| |||
| Hypertension | 6 (32%) | 25 (63%) | 0.03 |
|
| |||
| Diabetes | 1 (6%) | 2 (5%) | 0.93 |
|
| |||
| Period from first diagnosis of AF (months) | 54±38 | 53±47 | 0.96 |
|
| |||
| Duration of continuous AF (months) | N/A | 26±19 | - |
|
| |||
| LA pressure (mmHg) | 10±4 | 18±5 | <0.0001 |
|
| |||
| LA diameter (mm) | 38±4 | 48±6 | <0.0001 |
|
| |||
| LA volume indexed (ml/m2) | 43±10 | 68±20 | <0.0001 |
|
| |||
| Ejection Fraction (%) | 64±7 | 58±7 | - |
|
| |||
| Medication before the procedure | |||
| Amiodarone | 1 | 3 | 0.74 |
| Sotalol | 1 | 2 | 0.97 |
| Class I AADs | 9 | 2 | <0.0001 |
| Ca blocker | 1 | 14 | 0.01 |
| Beta blocker | 12 | 25 | 0.76 |
| ACE inhibitor/ARB | 2 | 14 | 0.06 |
| Digitalis | 1 | 4 | 0.54 |
| Diuretics | 0 | 0 | - |
Data are shown as mean±SD. AF: atrial fibrillation, M: male, F: female, LA: left atrium, AADs: antiarrhythmic drugs, ACE: angiotensin converting enzyme, ARB: angiotensin II receptor blocker.
Transthoracic echocardiography was performed before the ablation procedure and LA volume was measured off-line using a prolate ellipsoid model: V = πD2L/6, where D is the minor axis (width) and L is the major axis (length) of the LA as measured in the apical 4-chamber view. All patients with persistent AF underwent transesophageal echocardiography (Phillips iE33, Andover, MD) to rule out the presence of thrombus prior to the ablation procedure.
Measurements of LA pressure and electroanatomical mapping
The study protocol was approved by the Institutional Review Board and all patients provided informed written consent. Rhythm- and rate-controlling medications were discontinued 4–5 half-lives before the procedure, except for amiodarone, which was discontinued at least 8 weeks beforehand. Vascular access was obtained through a femoral vein. A steerable decapolar catheter (Biosense-Webster, Diamond Bar, CA) was positioned in the coronary sinus. LA pressure was defined as the height of ‘v’ wave during AF (normal range; 6 to 21 mmHg)5 and measured just after transseptal puncture using a long sheath (SL0, St. Jude Medical, Inc., Minnetonka, MN) connected to a pressure transducer (Transpac, Hospira, Lake Forest, Illinois). After the transseptal puncture, systemic anticoagulation was achieved with intravenous heparin to maintain an activated clotting time of 300–350 seconds. An open-irrigation, 3.5-mm-tip deflectable catheter (Thermocool, Biosense-Webster) was used for mapping and ablation. Bipolar electrograms were displayed and recorded at filter settings of 30 to 500 Hz during the procedure (EPMed Systems, West Berlin, NJ).
All patients underwent electroanatomical mapping during AF before ablation. Endocardial contact was ensured by fluoroscopy, electrogram stability, and the 3-D navigation system. Electrograms were recorded from the following 16 bi-atrial regions inpatients with persistent AF, and 12 left atrial regions in patients with paroxysmal AF: (1) right pulmonary vein (PV) antrum, (2) left PV antrum, (3) posterior wall (4) anterior wall, (5) roof, (6) septum, (7) mitral isthmus, (8) inferior wall, (9) LA appendage, (10) base of the appendage, (11) ridge between left PV and LA appendage, (12) coronary sinus, (13) right atrial (RA) appendage, (14) RA septum, (15) cavotricuspid isthmus, and (16) RA lateral wall. Three sites per region were sampled for ≥5 seconds in order to obtain the mean DF and atrial voltage for each region.
Digital signal processing and data analysis
The details regarding spectral analysis have been described previously.6 Briefly, bipolar electrograms recorded for 5 seconds were processed off-line in the MatLab environment (MathWorks, Inc., Natick, Massachusetts) during AF. Electrogram voltage was defined as the mean of 10 the largest electrograms in a sampling window of 5000 ms and measured using custom software (Figures 1A and 1B). In the spectral analysis, the pre-processing steps included bandpass filtering with cutoffs at 40 and 250 Hz, rectification, and low-pass filtering with a 20-Hz cutoff.7 The DF was defined as the frequency of the highest peak of the periodogram in the interval of 0.5 to 20 Hz using Fast Fourier Transformation. Points demonstrating an organization index (OI) < 0.2 were excluded (4%) in subsequent analysis to control for ambiguity in DF detection related to poor signal-to-noise ratio. OI was defined as the ratio of the total area of the spectrum under the first five harmonic peaks to the total area of the spectrum from 0 to 60 Hz.8
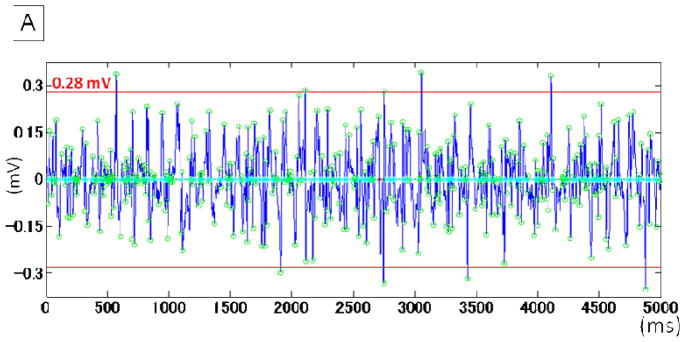
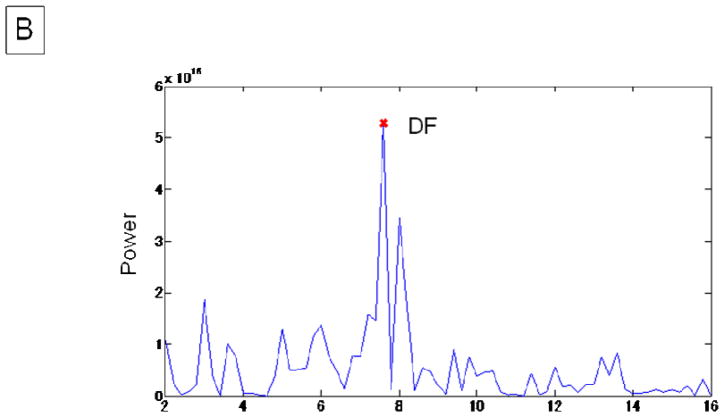
Figure 1. Measurement of atrial voltage and DF.
A. Electrogram voltage was defined as the mean of 10 the largest electrograms in a sampling window of 5000 ms and measured by custom software in the MatLab environment. The open green circles indicate the detection of peak electrogram amplitude. The red lines indicate the amplitude in this recoding period (0.28 mV).
B. The periodogram of the same recording period shown in panel A. The DF was 7.60 Hz.
DF: dominant frequency
Statistical Analysis
Continuous variables are expressed as mean ± 1 standard deviation and were compared by Student’s t-test or paired t-test, as appropriate. Categorical variables were compared by Chi-square analysis or with Fisher’s exact test, as appropriate. One-way analysis of variance (ANOVA) was used to compare continuous variables among multiple groups and post hoc analysis was performed using the Scheffe test. To determine the relationship between LA pressure and DF, an independent Pearson’s correlation coefficient was calculated for each of the 11 LA sites sampled. To account for these multiple comparisons, p-values were corrected using the Bonferroni correction. Thus, the relationship was deemed significant at the 0.05 level if the p-value was <0.0045 (i.e., 0.05/11). Multivariate regression analysis was performed to identify the predictors of the region with the highest DF. A p<0.05 indicated statistical significance in this case.
Results
Baseline characteristics
Age (58±8 vs.59±10 years, p=0.71) and time from the first diagnosis of AF (54±38 vs. 53±47 months, p=0.96) were similar between patients with paroxysmal and persistent AF (Table 1). The LA diameter was significantly larger in patients with persistent than in paroxysmal AF (48±6 vs. 38±4 mm, p<0.0001). Left ventricular systolic function was preserved in all patients (ejection fraction >50%). Hypertension (63% vs. 32%, p=0.03) and obesity indicated by body mass index (32±5 vs. 27±3 kg/m2, p=0.0001) were more prevalent in the patients with persistent AF.
LA pressure in paroxysmal and persistent AF
LA pressure was significantly higher in patients with persistent AF as compared to those with paroxysmal AF (18±5 vs.10±4 mm Hg, p<0.0001). The distribution of LA pressure in the paroxysmal AF group was too narrow to evaluate a relationship between atrial pressure and DF. Therefore, such a relationship was investigated only in the persistent AF group.
Regional DF in Persistent AF
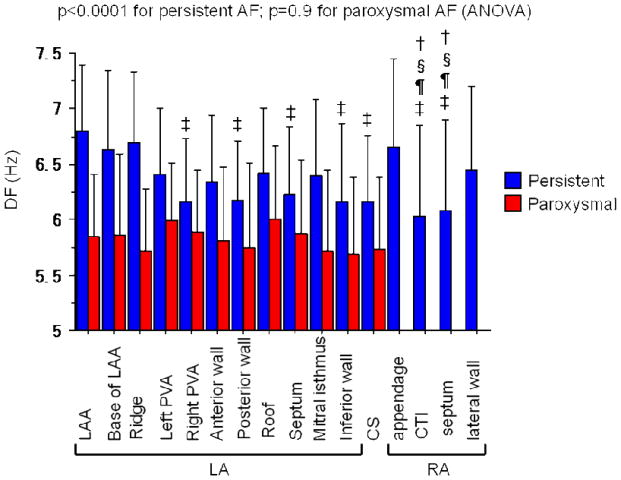
The mean DF of the LA and the RA was 6.4±0.7 and 6.3±0.8 Hz, respectively (p=0.17). The DF at the LA appendage (6.8±0.6 Hz), base of LA appendage (6.6±0.7Hz), ridge between the appendage and the left PVs (6.7±0.6 Hz) and RA appendage (6.7±0.8 Hz) were significantly higher than the other regions, for example, the posterior wall and inferior walls of the LA, and the CS (p<0.0001) (Figure 2). Among the LA sites, the LA appendage region (including the base and the ridge) had the highest DF (DFmax) in 24 of the 40 patients (60%). In the remainder, the DFmax was found at the left PV antrum in 5 (13%), the mitral isthmus in 3 (8%), the anterior wall in 2 (5%), the inferior wall in 2 (5%), posterior wall in 2 (5%), roof in 1 (2%), right PV antrum in 1 (2%) and septum in none of the patients (p=0.0006). The coefficient of variation (COV=standard deviation/mean × 100%) for DF in the LA in patients with persistent AF was 6.7±1.6%. There was no significant difference in regional DF in the LA in patients with paroxysmal AF (p=0.9; Figure 2).
Figure 2.
Mean DF at each region in persistent (blue) and paroxysmal (red) AF.
In patients with persistent AF, the mean DFs at 11 regions in the LA, at 4 regions in the RA, and in the CS are shown. There was a significant difference in the mean DF across the regions (p<0.0001, ANOVA).
‡: p<0.05 vs. DF at the LAA, §: <0.05 vs. DF at ridge, †: <0.05 vs. DF at base of LAA, ¶: <0.05 vs. DF at RAA.
In patients with paroxysmal AF, there was no difference in regional DF (p=0.9).
LA: left atrium, LAA: left atrial appendage, PVA: pulmonary vein antrum, CS: coronary sinus, RA: right atrium, CTI: cavotricuspid isthmus, RAA: right atrial appendage.
LA pressure
Patients with persistent AF were divided into 2 groups: a low pressure group with LA pressure of ≤18 mmHg, which was the mean and median LA pressure in the persistent AF group, and a high pressure group with LA pressure >18 mm Hg (Table 2). There was a borderline significant difference in the prevalence of the sleep apnea syndrome, which was higher in the high pressure group versus the low pressure group, respectively (29% versus 5%, p=0.05).
Table 2.
Characteristics of patients with normal and high LA pressure
| Persistent AF group (N=40) | P | ||
|---|---|---|---|
| Low LA pressure group (≤18mmHg, N=19) | High LA pressure group (>18mmHg, N=21) | ||
| Age | 60±9 | 57±11 | 0.44 |
| Gender (M/F) | 17/2 | 16/5 | 0.27 |
| Body mass index (kg/m2) | 33±5 | 31±4 | 0.19 |
| Sleep apnea syndrome | 1 | 6 | 0.05 |
| Hypertension | 10 | 15 | 0.22 |
| Duration of continuous AF (month) | 22±19 | 28±18 | 0.32 |
| LA diameter (mm) | 47±5 | 48±7 | 0.53 |
Data are shown as mean±SD. AF: atrial fibrillation, M: male, F: female, LA: left atrium, AADs: antiarrhythmic drugs, ACE: angiotensin converting enzyme, ARB: angiotensin II receptor blocker.
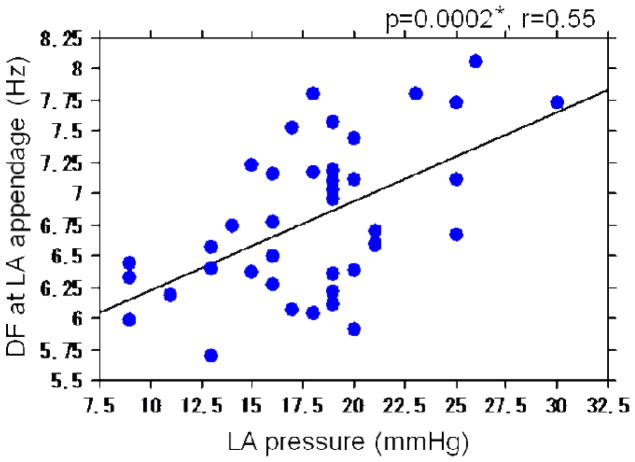
LA pressure and DF
There was a direct relationship between LA pressure and the DF of AF at the LAA (p=0.0002; r=0.55) (Figure 3) and the LA septum (p=0.003; r=0.46).
Figure 3.
Relationship between LA pressure and DF at the LAA.
*Significant at the 0.05 level if p <0.0045 by the Bonferroni correction.
Continuous AF and DF
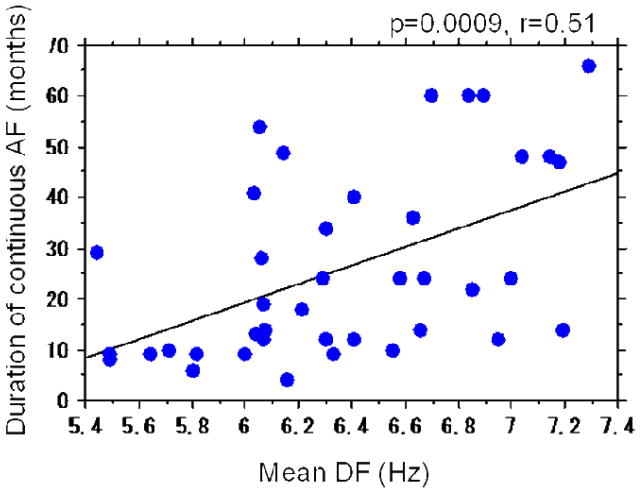
The mean DF in patients with paroxysmal and persistent AF was 5.8±0.5 Hz and 6.4±0.5, respectively (p=0.0006). There was no correlation between the time from first diagnosis of AF and mean DF in the paroxysmal AF group (p=0.5, r=0.18) and in persistent AF group (p=0.14, r=0.24). However, the duration of continuous (uninterrupted)AF in the persistent AF group was directly related to the mean DF of both left and right atria (p=0.0009, r=0.51) (Figure 4). The correlation between LA pressure and duration of continuous AF was weaker than the correlation between LA pressure and mean DF (p=0.04, r=0.33).
Figure 4.
Relationship between duration of continuous AF and mean DF.
Atrial Voltage
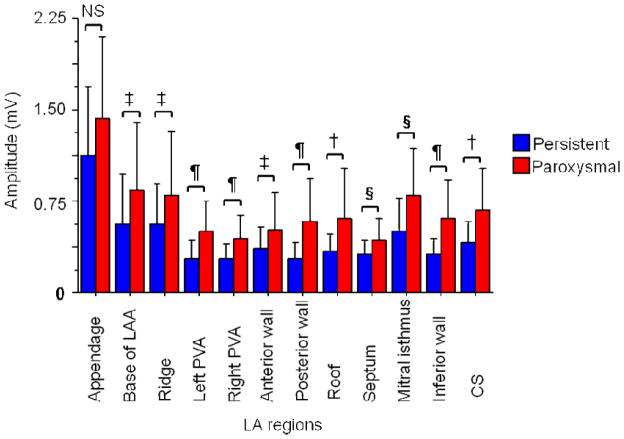
The mean LA voltage was significantly lower than the mean RA voltage (0.45±0.36 vs. 0.92±0.53 mV, p<0.0001) in patients with persistent AF. Mean LA voltage was significantly lower in patients with persistent AF than in patients with paroxysmal AF (0.45±0.36 vs. 0.64±0.39 mV, <0.0001). Regional atrial voltage was significantly lower at every LA site except the appendage, in patients with persistent AF as compared to those with paroxysmal AF (Figure 5). Although voltage at the LA appendage was also lower in patients with persistent versus paroxysmal AF (1.20±0.56 vs. 1.43±0.94 mV), this difference did not reach statistical significance (p=0.10). There were also significant regional differences in LA voltage in patients with both paroxysmal and persistent AF (Figure 5). Among patients with persistent AF, the mean voltage at the LA appendage and LA posterior wall was 1.20±0.56 and 0.28±0.12 mV, respectively (p<0.0001). In patients with paroxysmal AF, the mean voltage at the LAA and the posterior wall was 1.43±0.94 and 0.58±0.36 mV, respectively (p=0.0009). The COV for LA voltage in patients with persistent and paroxysmal AF was 65±19% and 53±17%, respectively (p=0.02).
Figure 5.
Regional LA voltage in patients with persistent (blue) and paroxysmal (red) AF. There were significant differences in the mean voltage across the regions (P<0.0001, ANOVA).
‡ : P<0.05, §: P<0.01, †: P<0.001, ¶: P<0.0001, NS: non-significant.
Determinants of DF in the LA Appendage
By univariate analysis, patients with DFmax in the LA appendage, as compared to those with DFmax elsewhere, had a significantly higher LA pressure (21±3 versus 17±5 mm Hg, p=0.009), lower age (52±9 versus 61±9, p=0.009) and higher voltage in the LA appendage (1.61±0.45 versus 0.95±0.50 mV, p=0.0006). There was no significant difference in gender (8/2 versus 25/5, p=0.81), duration of continuous AF (22±14 versus 27±20 months, p=0.41) and LA diameter (45±7 versus 49±5 mm, p=0.14). In a multivariate analysis using age, gender, LA pressure, duration of continuous AF, voltage in the LA appendage and LA diameter, the only independent predictor for DFmax in LA appendage was LA pressure (OR, 1.41, 95% CI 1.01–1.96; p=0.04). There were no predictors of DFmax at other regions.
Discussion
Main Findings
This study demonstrates that (1) The DF of AF is directly related to LA pressure in patients with persistent AF; (2) the DF of AF is directly related to the duration of continuous AF in patients with persistent AF; (3) LA pressure is higher in patients with persistent AF and is an independent predictor of DFmax in the LA appendage; and (4) regional LA voltage is lower in patients with persistent AF than in patients with paroxysmal AF.
LA pressure and DF
A stretch-related mechanism of AF has been proposed in a number of clinical conditions such as mitral valve disease and heart failure. It is thought that stretch may contribute to AF by evoking triggers9, 10 and/or promoting AF maintenance related to shortening of the atrial effective refractory period (ERP).11 Abbreviation of the atrial ERP would allow for a higher activation frequency. A prior animal study showed that an acute increase in LA pressure resulted in an increase in the DF, which is closely related to the atrial ERP.12, 13 To the best of our knowledge, this is the first human study to suggest that stretch imparted by elevated LA pressure may contribute to the maintenance of AF by stabilizing high frequency sources.
There are several therapeutic options that may be helpful in minimizing the detrimental consequences of stretch. For example, angiotensin receptor blockers may have a favorable effect by preventing stretch-induced electrical remodeling.14 Atrial stretch has also been implicated in the pathogenesis of AF related to sleep apnea,15 underscoring the importance of treating this condition. An animal study demonstrated that as LA pressure was lowered, AF sources became slow and unstable.2 Therefore, the role of diuretic therapy, even in the absence of heart failure, in reducing LA pressure and stretch should be explored. Also, specific inhibitors of stretch-activated channels would be a welcome addition to the armamentarium of antiarrhythmic medications. Although catheter ablation can eliminate persistent AF, it does not directly address elevated LA pressure and stretch. Interventions that have the potential to attenuate atrial stretch and/or its consequences may serve as adjuvant therapy after successful ablation to minimize the risk of late recurrence of AF.
Duration of Persistent AF and DF
Patients with persistent AF of long duration do not respond as well to medical therapy, or surgical16, or catheter ablation.17 The results of the current study suggest that patients with a longer duration of continuous AF tend to have a higher AF frequency, consistent with more extensive electrical remodeling. Experimental studies have demonstrated that longer lasting AF is associated with shortening of the atrial ERP, which then further perpetuates AF.13 It seems that AF also begets AF in humans by a similar mechanism.
Paroxysmal vs. Persistent AF
The factors that result in self-limited episodes in patients with paroxysmal AF as opposed to continuous, uninterrupted episodes in patients with persistent AF are not well understood. It is possible that the higher LA pressure in patients with persistent AF results in a greater degree of stretch-related electrical remodeling, resulting in a higher AF frequency. Indeed, prior studies,18 as well as the current study, have found that the DF in patients with persistent AF is higher than in patients with paroxysmal AF. Thus, a higher atrial activation rate in patients with persistent AF, related to a greater degree of stretch, may stabilize the arrhythmia, making it less likely to spontaneously terminate.2
DFmax in the LAA
The DFmax site in the majority of patients with persistent AF in this study was found at the region of the LA appendage. This observation may provide the rationale for ablation of complex electrograms around the LA appendage, which has been shown to be critical during the stepwise approach in acutely terminating AF.19 Also, ligation or excision of the LA appendage during a surgical ablation procedure for AF may not only help reduce the risk of thromboembolism, but may also directly eliminate a high frequency source responsible for maintaining AF.
LA pressure was found to be an independent predictor of DFmax in the LA appendage. This implies that the LA appendage may be more vulnerable to stretch-related electrical remodeling than other parts of the LA. It has been shown in an animal model that the LA appendage is more compliant than the body of the LA.20 In other words, for a given pressure, the LA appendage undergoes more distension than the LA body, which may render the former more susceptible to stretch-induced changes. In pathologic states in which the LA pressure is elevated, the relative blood flow to the LA appendage increases, consistent with its “reservoir function.”21 However, the results of the current study suggest that such compensation may actually be maladaptive since it may contribute to stretch-related remodeling of the LA appendage.
Regional Differences in LA Voltage
This study found that there were significant differences in regional LA voltage in paroxysmal and persistent AF. For example, in patients with persistent AF, the mean voltage at the LA appendage was more than four times larger than at the posterior wall of the LA. These results are consistent with those from an anatomic study, which reported that in patients with AF, there was a significantly higher prevalence of interstitial fibrosis in the posterior wall than in the LA appendage.22 There may be several explanations to help explain why the LA appendage seems to be relatively spared from structural remodeling in patients with AF. First, owing to their disparate embryological origins,23 the response of the LA posterior wall and appendage to various stimuli, for example, LA pressure, may be different. Another possibility may be related to wall tension.22 According to Laplace’s law, wall tension is directly related to pressure and radius, and indirectly related to wall thickness. Although the posterior LA and LAA are subject to the same pressure, the former would be expected to endure higher wall stress due to its larger radius and thinner myocardium. These mechanisms may help explain the substantial heterogeneity in regional LA voltage in both paroxysmal and persistent AF.
Regional Differences in DF
In an animal model, dispersion of atrial refractoriness was shown to be critical in the initiation and maintenance of AF.24 However, in the present study, the COV of DF was only about 6–7% as compared with animal studies in which the COV of ERP was reported to be about 21%. There may be several explanations for this discordance. First, the animals underwent the electrophysiology study after only 24 hours of rapid atrial pacing, as compared to patients in this study who were studied after more than a year of uninterrupted AF. It is possible that at early in the course of AF, there may is a large degree of ERP heterogeneity. However, as AF persists, the atria are likely to undergo global ERP remodeling, making it less likely to find marked differences in regional refractoriness. It is also possible that in the relatively short time frame in which the animal studies are conducted, structural changes, which have been shown to interfere with electrical remodeling,25 may not have yet occurred. Lastly, since AF frequencies are considerably higher in animal models than in patients with AF,26 the degree of fibrillatory conduction and the magnitude of frequency gradients are likely to be lower among the latter.27
Prior studies
Prior studies examining the impact of stretch on atrial refractoriness in humans have reached disparate conclusions. Some suggested that refractoriness was prolonged with increasing pressure,28, 29 while others found the opposite, or no difference.30, 31 These studies were limited by a lack of LA mapping and the fact that atrial pressure was assessed during non-physiologic conditions such as simultaneous atrio-ventricular pacing. To our knowledge, the current study is the first to analyze the relationship between atrial pressure and AF frequency at multiple biatrial sites in humans without any intervention to increase atrial pressure. More recent clinical studies have examined the impact of stretch in patients with mitral stenosis undergoing balloon commissurotomy. One study reported an increase in right atrial ERP at 3 months,32 whereas another found a decrease at 6 months after commissurotomy.33 These differences may be due to the fact that the former included patients with AF, whereas the latter examined the atrial substrate only in patients with sinus rhythm.
Limitations
The results of the current study suggest that there is an association between the LA pressure and AF frequency. A prospective clinical study utilizing saline infusion, as in animal studies, may be necessary to definitively demonstrate causation. However, acute instillation of saline, apart from being non-physiologic, may not be able to replicate the impact of chronic elevation in LA pressure. Another limitation of the study is that DF was determined by sequential as opposed to simultaneous atrial mapping. Also, atrial voltage was measured during AF as opposed to sinus rhythm. Lastly, the impact of the intrinsic cardiac autonomic system, one of the determinants of DF, was not analyzed.
Conclusions
LA pressure was significantly higher in patients with persistent AF, which implies that these patients may be more susceptible to stretch-related remodeling than patients with paroxysmal AF. The DF of AF was directly correlated with the LA pressure in patients with persistent AF, suggesting that atrial stretch may contribute to the maintenance of AF in humans.
Acknowledgments
Supported in part by a grant from the Leducq Transatlantic Network
Footnotes
Disclosures: Drs. Oral and Morady are co-founders of Ablation Frontiers
References
- 1.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–95. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 2.Kalifa J, Jalife J, Zaitsev AV, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–71. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. Jama. 1994;271:840–4. [PubMed] [Google Scholar]
- 4.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Davidson CJ, Bonow RO. Cardiac catheterization. In: Libby P, Bonow RO, Mann DL, Zipes DP, editors. Braunwald’s Heart Disease. 8. Philadelphia: Elsevier; 2008. pp. 439–63. [Google Scholar]
- 6.Yoshida K, Chugh A, Ulfarsson M, et al. Relationship between the spectral characteristics of atrial fibrillation and atrial tachycardias that occur after catheter ablation of atrial fibrillation. Heart Rhythm. 2009;6:11–7. doi: 10.1016/j.hrthm.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Ng J, Kadish AH, Goldberger JJ. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm. 2006;3:1295–305. doi: 10.1016/j.hrthm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Everett THt, Akar JG, Kok LC, Moorman JR, Haines DE. Use of global atrial fibrillation organization to optimize the success of burst pace termination. J Am Coll Cardiol. 2002;40:1831–40. doi: 10.1016/s0735-1097(02)02476-2. [DOI] [PubMed] [Google Scholar]
- 9.Lin WS, Prakash VS, Tai CT, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000;101:1274–81. doi: 10.1161/01.cir.101.11.1274. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 11.Qi J, Xiao J, Zhang Y, et al. Effects of potassium channel blockers on changes in refractoriness of atrial cardiomyocytes induced by stretch. Exp Biol Med (Maywood) 2009;234:779–84. doi: 10.3181/0902-RM-45. [DOI] [PubMed] [Google Scholar]
- 12.Misier AR, Opthof T, van Hemel NM, et al. Increased dispersion of “refractoriness” in patients with idiopathic paroxysmal atrial fibrillation. J Am Coll Cardiol. 1992;19:1531–5. doi: 10.1016/0735-1097(92)90614-s. [DOI] [PubMed] [Google Scholar]
- 13.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 14.Saygili E, Rana OR, Reuter H, et al. Losartan prevents stretch-induced electrical remodeling in cultured atrial neonatal myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H2898–905. doi: 10.1152/ajpheart.00546.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 16.Grubitzsch H, Grabow C, Orawa H, Konertz W. Factors predicting the time until atrial fibrillation recurrence after concomitant left atrial ablation. Eur J Cardiothorac Surg. 2008;34:67–72. doi: 10.1016/j.ejcts.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo S, Lellouche N, Wright M, et al. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J Am Coll Cardiol. 2009;54:788–95. doi: 10.1016/j.jacc.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 19.Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoit BD, Walsh RA. Regional atrial distensibility. Am J Physiol. 1992;262:H1356–60. doi: 10.1152/ajpheart.1992.262.5.H1356. [DOI] [PubMed] [Google Scholar]
- 21.Tabata T, Oki T, Yamada H, et al. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am J Cardiol. 1998;81:327–32. doi: 10.1016/s0002-9149(97)00903-x. [DOI] [PubMed] [Google Scholar]
- 22.Corradi D, Callegari S, Benussi S, et al. Myocyte changes and their left atrial distribution in patients with chronic atrial fibrillation related to mitral valve disease. Hum Pathol. 2005;36:1080–9. doi: 10.1016/j.humpath.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Douglas YL, Jongbloed MR, Gittenberger-de Groot AC, et al. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006;97:662–70. doi: 10.1016/j.amjcard.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation. 1998;98:2202–9. doi: 10.1161/01.cir.98.20.2202. [DOI] [PubMed] [Google Scholar]
- 25.Swartz MF, Fink GW, Lutz CJ, et al. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009;6:1415–22. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–6. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 27.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–42. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 28.Chen YJ, Chen SA, Tai CT, et al. Role of atrial electrophysiology and autonomic nervous system in patients with supraventricular tachycardia and paroxysmal atrial fibrillation. J Am Coll Cardiol. 1998;32:732–8. doi: 10.1016/s0735-1097(98)00305-2. [DOI] [PubMed] [Google Scholar]
- 29.Klein LS, Miles WM, Zipes DP. Effect of atrioventricular interval during pacing or reciprocating tachycardia on atrial size, pressure, and refractory period. Contraction-excitation feedback in human atrium. Circulation. 1990;82:60–8. doi: 10.1161/01.cir.82.1.60. [DOI] [PubMed] [Google Scholar]
- 30.Calkins H, el-Atassi R, Kalbfleisch S, Langberg J, Morady F. Effects of an acute increase in atrial pressure on atrial refractoriness in humans. Pacing Clin Electrophysiol. 1992;15:1674–80. doi: 10.1111/j.1540-8159.1992.tb02954.x. [DOI] [PubMed] [Google Scholar]
- 31.Tse HF, Pelosi F, Oral H, Knight BP, Strickberger SA, Morady F. Effects of simultaneous atrioventricular pacing on atrial refractoriness and atrial fibrillation inducibility: role of atrial mechanoelectrical feedback. J Cardiovasc Electrophysiol. 2001;12:43–50. doi: 10.1046/j.1540-8167.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 32.Fan K, Lee KL, Chow WH, Chau E, Lau CP. Internal cardioversion of chronic atrial fibrillation during percutaneous mitral commissurotomy: insight into reversal of chronic stretch-induced atrial remodeling. Circulation. 2002;105:2746–52. doi: 10.1161/01.cir.0000018441.64861.de. [DOI] [PubMed] [Google Scholar]
- 33.John B, Stiles MK, Kuklik P, et al. Reverse remodeling of the atria after treatment of chronic stretch in humans: implications for the atrial fibrillation substrate. J Am Coll Cardiol. 2010;55:1217–26. doi: 10.1016/j.jacc.2009.10.046. [DOI] [PubMed] [Google Scholar]