Abstract
Objective
Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS) are man-made compounds with widespread presence in human sera. In previous occupational and adult studies, PFOA and PFOS have been positively associated with serum lipids. Our objective is to interrogate the association between PFOA and PFOS and serum lipids in children.
Design
Cross-sectional community-based study.
Setting
Mid-Ohio river valley.
Participants
12,476 children included in the C8 Health Project, resultant from the pre-trial settlement of a class action lawsuit pursuant to PFOA contamination of the drinking water supply.
Main Outcome Measures
Serum lipids (total, HDL, and LDL cholesterol, and fasting triglycerides).
Results
Mean serum PFOA and PFOS concentrations were 69.2±111.9 ng/mL and 22.7±12.6 ng/mL. In linear regression after adjustment for covariables, PFOA was significantly associated with increased total and LDL cholesterol, and PFOS was significantly associated with increased total, HDL, and LDL cholesterol. In GLM ANCOVA analysis, between the 1st—5th quintiles of PFOA there was a 4.6 mg/dL and 3.8 mg/dL increase in the adjusted mean of total and LDL cholesterol, and an 8.5 mg/dL and 5.8 mg/dL increase in the adjusted mean for total and LDL cholesterol between the 1st—5th quintiles of PFOS. Increases were 10 mg/dL for some age- and gender-group strata. Observed effects were non-linear, with larger increases in total and LDL cholesterol occurring the lowest range of particularly PFOA.
Conclusions
While the epidemiologic and cross-sectional nature of the current study limit causal inferences, the consistent observed associations between increasing PFOA and PFOS and elevated total and LDL cholesterol warrant further study.
Keywords: Perfluorocarbons, Perfluoroalkyl acids, PFOA, C8, Toxic tort settlement, Environmental contamination, serum lipids, cholesterol, children
INTRODUCTION
Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS) are perfluoroalkyl acids, man-made compounds used as emulsifiers during the manufacture of fluoropolymers, chemicals that give non-stick heat resistance to cookware, or breathable yet waterproof properties to fabrics and upholstery. Perfluoroalkyl acids may also result from the metabolism or breakdown of fluorinated telomers, compounds used as coatings for commercial food packaging, factory treatments for fabrics and carpets, and manufacturer pre-treatment for “stain-resistant” clothing.
Recent reviews of the PFOA and PFOS scientific literature emphasize their environmental persistence and presence in a variety of marine and freshwater species.1–3 Human sera across myriad age groups and geographies are known to contain perfluoroalkyl acids generally, and PFOA and PFOS specifically. Recent results from the NHANES study reported detection of perfluoroalkyl acids in almost all samples, with a US-population median for PFOA of 5.2 ng/mL and 3.9 ng/mL (1999–2000 and 2003–2004 respectively).4,5 Identified sources of human exposure to PFOA and PFOS include drinking water, dust, food packaging, breast milk, cord blood, microwave popcorn, ambient air, and occupational exposure,1,6–8 although the relative contribution of each is unknown.
Animal studies have identified the liver as a primary target organ for perfluoroalkyl acid physiologic activity. Reported toxicological effects of exposure to PFOA and/or PFOS include hepatomegaly, reduction in serum triglycerides and cholesterol in some animal species, and alterations in coenzyme-A activity. These alterations in hepatic metabolism and function have been attributed to perfluoroalkyl acid action as a PPAR-α agonist with subsequent peroxisome proliferation.1–3
Compared to effects seen in animals, human studies have reported different associations between PFOA and lipids. In occupationally exposed employees, PFOA associations have included increased PFOA and: increased total cholesterol but not triglycerides or other lipids;9 increased total and LDL cholesterol but not HDL;10 increased total cholesterol but not triglycerides or HDL;11 and increased triglycerides but no association with total or LDL cholesterol.12 Study of a community cohort with known environmental PFOA contamination reported no association between PFOA and total cholesterol.13 A recent, much larger study of adults from the same and adjacent environmentally exposed communities reported a robust, positive association between PFOA and total and LDL cholesterol, and a less clear association of PFOA with triglycerides.14
No study has investigated potential associations between perfluoroalkyl acids and lipids in children. The importance of such studies is three-fold. First, if such associations are etiologic, exposure prevention would become important to reduce the long-term health consequences of elevations in known cardiovascular disease risk factors. Second, studying potential health consequences of an environmental exposure in children may provide greater insight as children likely have fewer factors confounding underlying associations (e.g., prevalent chronic or acute disease, medication use) compared to adults. Third, given possible differences in physiologic processes due to developmental changes in children and adolescents, toxicologic impact may be different compared to that observed in adults. Thus, the purpose of the current study is to interrogate associations between serum PFOA and PFOS and lipids in a large, community-based sample of children.
METHODS
Study Methods and Participants
Participants in the current study were 12,476 children, 1–17.9 years at their enrollment in the C8 Health Project (the Project). The Project has been more completely described elsewhere.15 Briefly, the Project resulted from a pre-trial settlement of the class action lawsuit, Leach v. E.I. du Pont de Nemours & Co., filed in 2002 after PFOA from the DuPont Washington Works facility near Parkersburg, WV was found to have infiltrated several local drinking water supplies along the mid-Ohio River valley. Project eligibility criteria included the ability to document consumption of contaminated drinking water (from one of 2 public water districts in West Virginia or 4 in Ohio, or from private water sources within the public water districts which contained ≥0.05 ppb PFOA) for at least one year between 1950 and December 3, 2004, at primary residence, place of employment, or school. Participants enrolled over a 13-month period in 2005–2006. Participation and enrollment included: documentation of identity and eligibility; a self-reported survey of demographics, personal health history and lifestyle habits; self-reported height and weight; and voluntary submission of a blood sample. As we have previously reported, approximately 60% of participants were resident in an eligible water district at the time of their enrollment in the Project, and an estimated 80% of people resident in the eligible water districts at the time of the Project enrolled as participants.15 With 69,030 total participants (12,476 children), the Project represents the largest community-based study investigating potential associations between PFOA exposure and human health effects undertaken to date.
Blood Sample Processing and Laboratory Methods
Children voluntarily submitted blood samples, a maximum 26 cc. Tubes were spun, aliquotted, and refrigerated at community-based data collection sites until shipping to laboratories for analysis.
Clinical laboratory tests were performed at an accredited clinical diagnostic laboratory (LabCorp, Inc, Burlington, NC, USA). The lipid panel included total, HDL, and LDL cholesterol (total-C, LDL-C, HDL-C), and triglycerides. LDL-C was calculated using the Friedewald formula for those with triglycerides <400mg/dL. While fasting was not a requirement for phlebotomy, the time of last meal was reported.
The primary laboratory performing perfluorocarbon analysis (Exygen Research Inc., State College, PA, USA) was also used for an independent study of a smaller group of residents in one water district included in the Project.16 Full perfluoroalkyl acid analytic techniques and quality assurance protocols for the Project have been published.15 Briefly, the analytic protocol used was a modification of a previously described protocol that used a protein precipitation extraction together with reverse-phase high-performance liquid chromatography/tandem mass spectrometry.17 Spectrometric detection was performed using a triple quadrupole mass spectrometer in selected reaction monitoring mode, monitoring for the individual m/z transitions for the perfluoroalkyl acid and 13C-PFOA surrogate.
Consenting Procedures
Project data collection administered by Brookmar, Inc. was conducted under the authority and supervision of the Court. Brookmar Inc. employed a consenting procedure, approved by parties to the Settlement, which included language specific to the Project’s purpose and data collection procedures, and the mandatory documentation requirements to demonstrate Class eligibility. All participants submitting a voluntary blood sample completed the standard consent and release forms of the clinical laboratory contracted for phlebotomy. The Project group at West Virginia University and the C8 Science Panel obtained IRB approval, from their own, academic institutions permitting access to anonymous Project data.
Statistical Analysis
The outcome variables were total-C, LDL-C, HDL-C, and triglycerides. For simplicity, the same covariables were considered for all analytic models: age, gender (except for models stratified by gender), BMI z-score, time of fasting (minutes), and whether the participant had a regular exercise program. Age was included as a continuous variable and as 2 strata (1–11.9 and 12–17.9 years of age) to assess possible age and developmental confounding. For all analyses using quantiles, grouping was established within age group and gender, and so quantiles are age-group and gender specific. Presence of a regular exercise program was self-reported as part of the survey (“Do you engage in an exercise program?” with a “Yes/No” response option), as was fasting, height, and weight. Analyses including triglycerides were conducted for only those reporting a fast ≥6 hours. BMI z-score was calculated using EpiInfo™ (CDC, 2000 Reference dataset).
Multiple, linear regression was performed to assess for the presence of an overall, linear association between PFOA or PFOS and lipids. Both PFOA or PFOS and the dependent variable were natural-log transformed.
General linear model (GLM) analysis ANCOVA was performed to estimate predicted lipids (estimated marginal mean; EMM) after adjustment for covariables based on increasing PFOA or PFOS quintiles. The serum lipid was defined as the dependent variable, PFOA or PFOS quintile as a fixed factor, and the other independent variables (age, gender, BMI z-score, exercise, fasting) as covariates. The covariable-adjusted EMM for each quintile is presented graphically for the overall population (all age groups and genders combined). The difference in the covariable-adjusted EMM between the 5th and 1st quintile was established for each age group and gender strata. To interpret the statistical significance of the trend in the quintile-based change in the covariable-adjusted EMM, linear regression analysis was used to estimate the β-coefficient (and corresponding p value) for the PFOA or PFOS quintile and is reported as a β-for-trend.
To assess the linearity or non-linearity of any association between PFOA or PFOS and serum lipids, 20-group quantiles were determined and the population median for each group calculated. GLM ANCOVA analysis was again used to determine the covariable-adjusted EMM for each quantile, which was plotted against its PFOA or PFOS median.
Binary logistic regression analysis was performed to assess the odds of abnormal lipids with increasing PFOA or PFOS. For total-C, LDL-C and triglycerides, categorization as abnormal was based on American Heart Association-endorsed cut-off values for “borderline” or “high” in children (total-C ≥170 mg/dL, LDL-C ≥110 mg/dL, triglycerides ≥150 mg/dL).18 For HDL cholesterol, children with values <40 mg/dL were classified as abnormal. Logistic regression analysis was performed using PFOA or PFOS quintile dummy variables, where the 1st quintile group was considered the referent group.
Interaction between PFOA and PFOS was dually assessed. Using logistic regression analysis, with models otherwise constructed as described above, was performed with PFOA quintile, PFOS quintile, and the product of quintiles (interaction term) included in the analytic model. The statistical significance of the interaction term (p-interaction) is reported. Logistic regression analysis was also used to assess for the presence of multiplicative interaction using models also as otherwise described above. Four groups were created based on both PFOA and PFOS quintile: Group 1 (PFOA 1st–4th quintile & PFOS 1st–4th quintile); Group 2 (PFOA 5th quintile & PFOS 1st–4th quintile); Group 3 (PFOA 1st–4th quintile & PFOS 5th quintile); Group 4 (PFOA 5th quintile & PFOS 5th quintile). Logistic regression was then completed using 3 dummy variables, where Group 1 was considered the referent group.
Sensitivity analyses were also conducted for the impacts of fasting and socioeconomic status. Using models otherwise constructed as described above, multiple, linear regression was performed for models with and without household income (dichotomized at ≤$30,000/year or >$30,000/year), and models adjusted for fasting status or with only participants having completed ≥6 hour fast. The magnitude and statistical significance of β-coefficients for the different models were then compared. Both PFOA or PFOS and the dependent variable were natural-log transformed.
All analyses were performed using SPSS© (Chicago, IL).
RESULTS
General characteristics of participants are reported in Table 1. The average age was 11.1±4.5 years (mean± standard deviation) and participation was similar across genders (49% girls and 51% boys). Of the 12,476 children included in the current study, >10,000 (>80%) had perfluoroalkyl acid quantification and serum lipids available for analysis. Consistent with Census Bureau estimates for the area, more than 95% reported their ethnicity as “White”, almost 40% (39.7%) were classified as overweight or obese (≥85th BMI percentile), and approximately one-third (36.7%) reported having a regular exercise program. Based on participant-reported residence at the time of their enrollment, a slightly higher proportion of participants were from Ohio compared to West Virginia (54% vs. 44%, respectively).
Table 1.
Participant Characteristics
| Variable | Parameter / Stratification | Boys | Girls | Total |
|---|---|---|---|---|
| Age (Years) | Median / Mean (± Standard Deviation) | 11.65 / 11.2±4.41 | 11.52 / 11.08±4.52 | 11.6 / 11.14±4.46 |
| Age Group | 1 – 11.9 Years | 3287 (51.7%) | 3249 (53.2%) | 6536 (52.4%) |
| 12 – 17.9 Years | 3072 (48.3%) | 2862 (46.8%) | 5934 (47.6%) | |
| Regular Exercise | Yes | 2491 (39.1%) | 2085 (34.1%) | 4576 (36.7%) |
| No | 3872 (60.9%) | 4028 (65.9%) | 7900 (63.3%) | |
| BMI Percentile | Underweight (<5th) | 272 (4.8%) | 291 (5.4%) | 563 (5.1%) |
| Normal(5th–84.9th) | 2947 (51.7%) | 3168 (58.9) | 6115 (55.2%) | |
| Overweight (85th–95th) | 934 (16.4%) | 911 (16.9%) | 1845 (16.6%) | |
| Obese (>95th) | 1550 (27.2%) | 1011 (18.8%) | 2561 (23.1%) | |
| Hours Fasting | <6 Hrs | 4591 (72.2%) | 4400 (72.0%) | 8991 (72.1% |
| ≥6 Hrs | 1394 (21.9%) | 1346 (22.0%) | 2740(22.0%) | |
| Not Reported | 378 (5.9%) | 367 (6.0%) | 745 (6.0%) | |
| Household Income | ≤$30,000/year | 2471 (49.3%) | 2303 (47.6%) | 4774 (48.4%) |
| >$30,000/year | 2540 (50.7%) | 2540 (52.4%) | 5080 (51.6%) | |
| Ethnicity | White | 6061 (96.3%) | 5853 (96.0%) | 11894 (96.1%) |
| Black | 106 (1.7%) | 96 (1.6%) | 202 (1.6%) | |
| All Others | 145 (2.0%) | 130 (2.4%) | 275 (2.3%) | |
| Residence (State) at Time of Enrollment | Ohio | 3476 (54.6%) | 3320 (54.3%) | 6796 (54.5%) |
| West Virginia | 2817 (44.3%) | 2703 (44.2%) | 5520 (44.2%) | |
| Other | 70 (1.1%) | 90 (1.5%) | 160 (1.3%) | |
| Total Cholesterol | <170 mg/dL | 3729 (68.6%) | 3207 (62.8%) | 6936 (65.8%) |
| ≥170 mg/dL | 1706 (31.4%) | 1901 (37.2%) | 3607 (34.2%) | |
| LDL Cholesterol | <110 mg/dL | 4581 (85.3%) | 4158 (82.0%) | 8739 (83.7%) |
| ≥110 mg/dL | 788 (14.7%) | 915 (18.0%) | 1703 (16.3%) | |
| HDL Cholesterol | <40 mg/dL | 1313 (24.3%) | 760 (14.9%) | 2073 (19.7%) |
| ≥40 mg/dL | 4348 (75.8%) | 4122 (85.1%) | 8470 (80.3%) | |
| Fasting Triglycerides | <150 mg/dL | 1244 (84.6%) | 1201 (86.7%) | 2445 (85.6%) |
| ≥150 mg/dL | 227 (15.4%) | 185 (13.3%) | 412 (14.4%) | |
| PFOA (ng/mL) | 1 – 11.9Years | 35.1 / 82.1±129.2 | 30.7 / 73.1±120.1 | 32.6 / 77.7±124.9 |
| 12 – 17.9 Years | 30.1 / 69.3±107.1 | 22.9 / 53.7±88.1 | 26.3 / 61.8±98.8 | |
| PFOS (ng/mL) | 1 – 11.9Years | 21.7 / 24.6±13.4 | 19.9 / 22.6±12.6 | 20.7 / 23.6±13.1 |
| 12 – 17.9 Years | 20.3 / 23.2±12.9 | 18.2 / 20.5±11.3 | 19.3 / 21.9±12.2 | |
Mean total-C was 160.7±29.3 mg/dL, with 65.8% classified as acceptable (<170 mg/dL). Mean calculated LDL-C was 87.3±25.2 mg/dL, with 83.7% classified as acceptable (<110 mg/dL). Mean HDL-C was 49.3±11.3 mg/dL, with 92.2% classified as ideal (≥35 mg/dL) and 19.7% <40 mg/dL. Finally, mean fasting triglycerides was 99.1±56.0 mg/dL, with 85.7% classified as acceptable (≤150 mg/dL).
Mean serum PFOA and PFOS concentration was 69.2±111.9 ng/mL and 22.7±12.6 ng/mL respectively, with serum concentrations statistically significantly higher in males and younger children for both PFOA and PFOS, particularly PFOA. Consistent with the environmental (drinking water) contamination and as previously reported,15 serum PFOA concentration for 12–19 year olds in the Project population was substantially above the reported concentration for 12–19 year olds in the 2003–2004 NHANES study,5 while PFOS concentrations were similar (29.3 ng/mL vs. 3.9 ng/mL and 19.1 ng/mL vs 19.3 ng/mL respectively).
Results from regression analysis (not shown) demonstrated that, after adjustment for covariables, total-C and LDL-C were linearly and positively associated with both PFOA and PFOS (p<0.0001 for all models). Triglycerides were also linearly and positively associated with PFOA (p=0.019) but not PFOS. HDL-C was not linearly associated with PFOA but was positively associated with PFOS. For linear regression models and other analyses reported here, it was almost always the case that all covariables were also statistically significantly associated with the dependent variable (not shown).
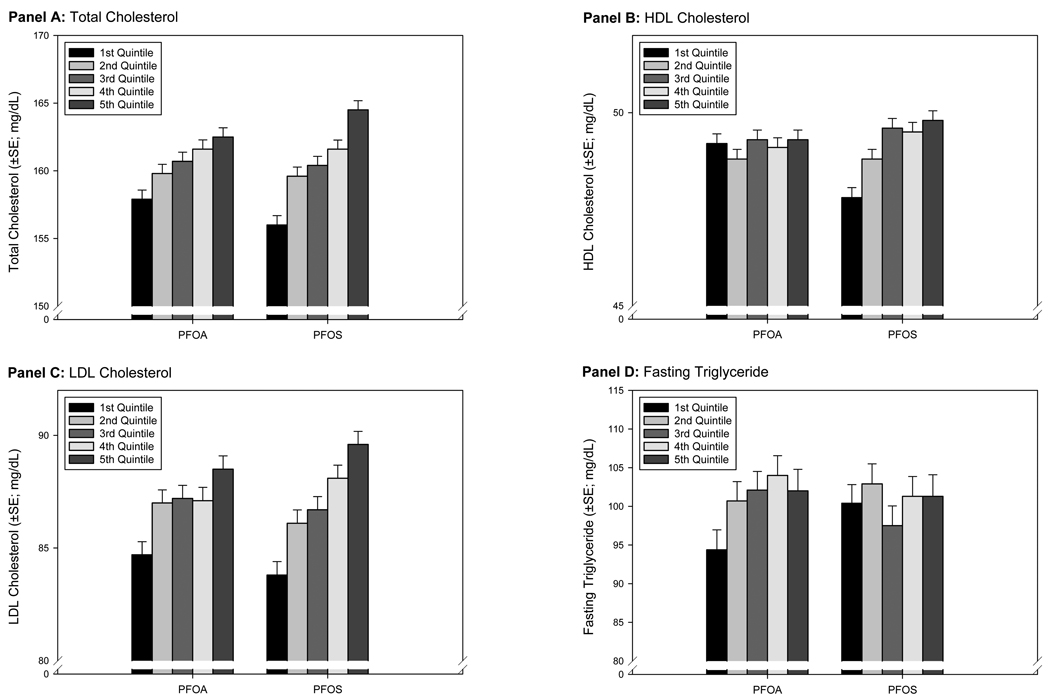
Associations between increasing PFOA and PFOS quintiles and the covariable-adjusted EMM of serum lipids are depicted in Figure 1. Both total-C and LDL-C demonstrated a consistent increase for each increase in PFOA or PFOS quintile: a 4.6 mg/dL and 3.8 mg/dL increase in the covariable-adjusted EMM of total-C and LDL-C between the 1st and 5th quintile of PFOA; and a 8.5 mg/dL and 5.8 mg/dL increase in the covariable-adjusted EMM of total-C and LDL-C between the 1st and 5th quintile of PFOS. Overall associations between PFOA and PFOS and HDL-C and triglycerides were less clear with no apparent association between PFOA quintile and covariable-adjusted EMM for HDL-C, or PFOS quintile and covariable-adjusted EMM for triglycerides. There was a small increase in the covariable-adjusted EMM of HDL-C and the 1st and 3rd quintile of PFOS, but not the 3rd to 5th quintile of PFOS. There was no difference between the covariable-adjusted EMM for triglycerides and the 1st, 2nd, or 3rd PFOA quintile, but an increase between the 3rd and 4th or 5th quintile of PFOA.
Figure 1.
Changes in Covariate-Adjusted Estimated Marginal Means (GLM Analysis) Across PFOA and PFOS Quintiles
These associations are more fully characterized in Table 2, which reports differences between the 5th–1st quintile and β-for-trend for age group and gender strata. For PFOA and total-C and LDL-C, there was a trend toward a larger increase in the covariable-adjusted EMM in younger compared to older age groups (5.8 mg/dL vs. 4.2 mg/dL for total-C and 4.92 mg/dL vs. 3.24 mg/dL for LDL-C), and in boys compared to girls (within each age group). The β-for-trend for each of these age group and gender strata were all statistically significant (p<0.05). For PFOS, there was a trend toward a larger increase in the covariable-adjusted EMM in older compared to younger age groups (9.5 mg/dL vs. 5.5 mg/dL for total-C and 7.5 mg/dL vs. 3.4 mg/dL for LDL-C), and a trend toward larger increases for boys compared to girls (within each age group). The β-for-trend for each of these age group and gender strata were also all statistically significant (p<0.05).
Table 2.
Differences in PFOA between 1st–5th Quintile Estimated Marginal Means (GLM Analysis) and Assessment of Quintile Trend (Regression Analysis)
| Age Group |
Gender | n (Fasting¥) |
Total Cholesterolφ | HDL Cholesterolφ | LDL Cholesterolφ,ς | Fasting Triglycerides* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference in EMM¥ |
β for Trend (β±SE / pβ) |
Difference in EMM¥ |
β for Trend (β±SE / pβ) |
Difference in EMM¥ |
β for Trend (β±SE / pβ) |
Difference in EMM¥ |
β for Trend (n / β±SE / pβ) |
||||
| PFOA | 1 – 11.9 Years | Both | 3857 (803) | 5.8 mg/dL | 1.3±0.3/<0.0001 | <1 mg/dL | −0.02±0.1/0.877 | 4.9 mg/dL | 1.0±0.3/0.001 | 2.0 mg/dL | 2.0±1.3/0.1 |
| Girls | 1886 (397) | 5.8 mg/dL | 1.1±0.4/<0.0001 | <1 mg/dL | 0.02±0.2/0.9 | 5.4 mg/dL | 0.8±0.4/0.04 | 16.2 mg/dL | 4.0±1.9/0.04 | ||
| Boys | 1971 (406) | 6.3 mg/dL | 1.6±0.4/<0.0001 | <1 mg/dL | −0.06±0.2/0.7 | 4.8 mg/dL | 1.1±0.4/0.004 | 5.3 mg/dL | 0.4±1.9/0.8 | ||
| 12 – 17.9 Years | Both | 5293 (1428) | 4.2 mg/dL | 1.1±0.3/<0.0001 | <1 mg/dL | 0.1±0.1/0.2 | 3.2 mg/dL | 0.7±0.2/0.004 | 3.8 mg/dL | 1.5±1.1/0.1 | |
| Girls | 2520 (687) | 3.9 mg/dL | 1.0±0.4/0.02 | <1mg/dL | 0.3±0.2/0.09 | 3.2 mg/dL | 0.7±0.4/0.05 | 1.8 mg/dL | 0.8±1.4/0.6 | ||
| Boys | 2773 (741) | 4.8 mg/dL | 1.1±0.4/0.005 | <1 mg/dL | 0.03±0.1/0.8 | 3.5 mg/dL | 0.7±0.3/0.03 | 5.9 mg/dL | 2.4±1.6/0.1 | ||
| PFOS | 1 – 11.9 Years | Both | 3857 (803) | 5.5 mg/dL | 1.3±0.3/<0.0001 | 1.6 mg/dL | 0.3±0.1/0.007 | 3.4 mg/dL | 0.9±0.3/0.002 | 2.8 mg/dL | 0.1±1.4/0.99 |
| Girls | 1886 (397) | 4.6 mg/dL | 1.3±0.5/0.004 | <1 mg/dL | 0.1±0.2/0.5 | 2.6 mg/dL | 0.8±0.4/0.04 | 7.6 mg/dL | 0.6±1.9/0.7 | ||
| Boys | 1971 (406) | 6.2 mg/dL | 1.2±0.5/0.01 | 2.6 mg/dL | 0.5±0.2/0.003 | 4.1 mg/dL | 0.9±0.4/0.03 | −1.4 mg/dL | −0.3±2.0/0.9 | ||
| 12 – 17.9 Years | Both | 5293 (1428) | 9.5 mg/dL | 2.1±0.3/<0.0001 | 1.5 mg/dL | 0.4±0.1/0.001 | 7.5 mg/dL | 1.7±0.2/<0.0001 | <1 mg/dL | −0.1±1.0/0.9 | |
| Girls | 2520 (687) | 9.4 mg/dL | 1.9±0.4/<0.0001 | 1.8 mg/dL | 0.3±0.2/0.06 | 6.9 mg/dL | 1.5±0.4/<0.0001 | −13.4 mg/dL | −3.0±1.3/0.02 | ||
| Boys | 2773 (741) | 9.3 mg/dL | 2.1±0.4/<0.0001 | 1.1 mg/dL | 0.4±0.1/0.003 | 7.9 mg/dL | 1.8±0.3/<0.0001 | 11.1 mg/dL | 2.2±1.6/0.2 | ||
Sample size for fasting triglyceride models only
Models adjusted for age, estimated time of fasting, BMI Z-score, gender, and regular exercise; gender stratified models not adjusted for gender
Calculated for those with triglyceride <400 mg/dL regardless of fasting status
Defined as self-reported fasting ≥6 hours prior to phlebotomy
In contrast, the β-for-trend for age group and gender strata for the associations between PFOA and HDL-C and triglycerides were not statistically significant (except for one stratum), making differences in the covariable-adjusted EMM difficult to interpret. For associations between PFOS and HDL-C, the β-for-trend was statistically significant for boy and both genders combined (but not girls) for both age groups, though marginal increases in covariable-adjusted EMM were small (1.1 mg/dL to 2.6 mg/dL). Similarly, the β-for-trend for age group and gender strata for the associations between PFOS and triglycerides were not statistically significant (except for one stratum), making differences in the covariable-adjusted EMM difficult to interpret.
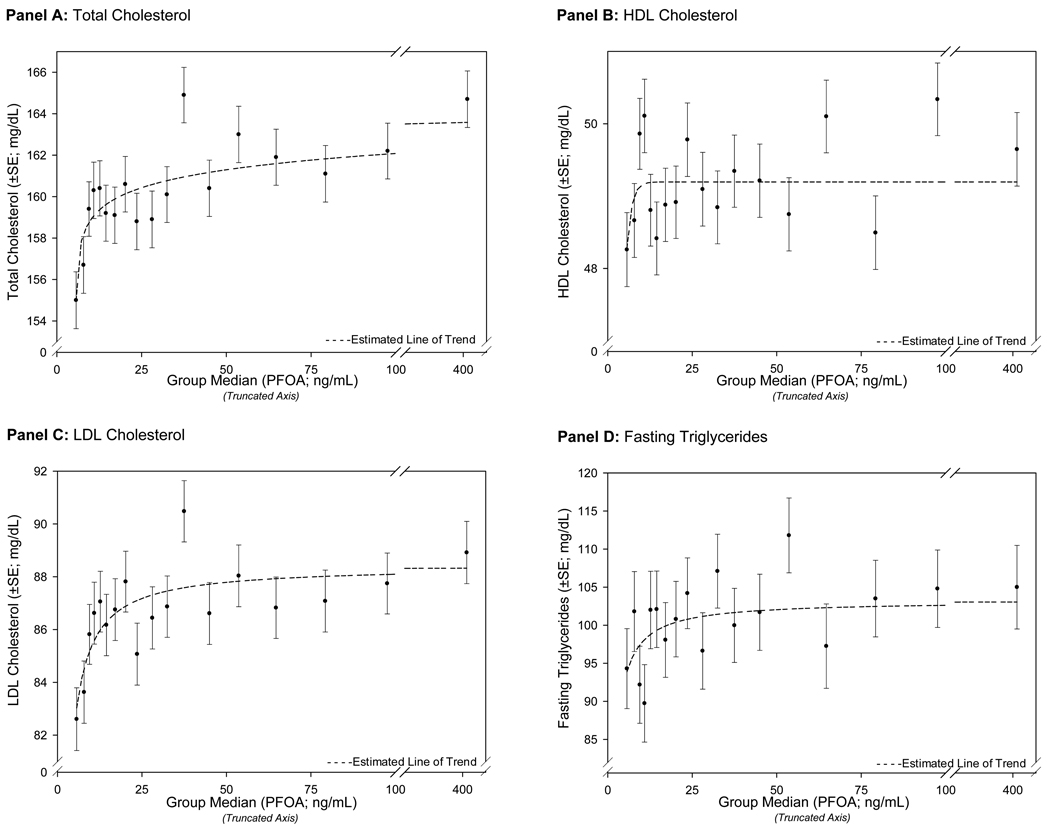
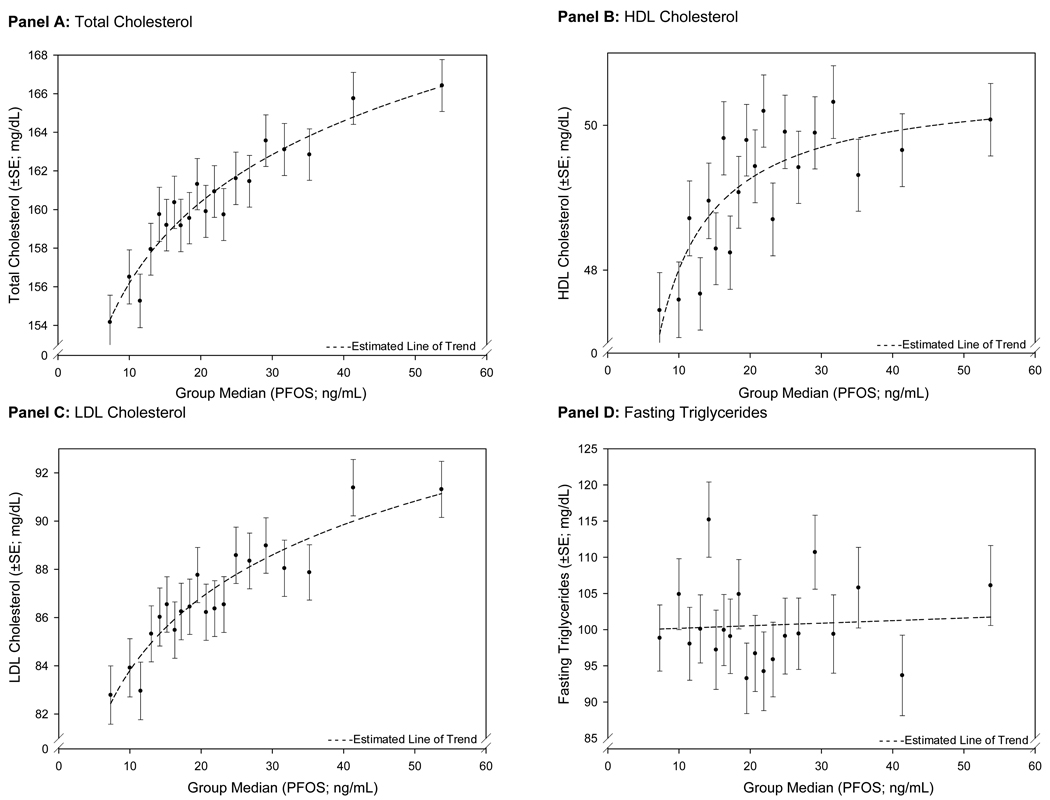
In Figures 2 and 3, the covariable-adjusted EMM for serum lipids for 20-group quantiles of PFOA or PFOS are plotted against the median of PFOA or PFOS for the quantile. For both PFOA and PFOS, the results demonstrated a non-linear association between increasing PFOA or PFOS and total-C and LDL-C. For PFOA, the largest increases in the covariable-adjusted EMM of total-C and LDL-C were seen at the lowest range of PFOA concentrations; the slope attenuated at higher serum concentrations. Although population exposure and corresponding serum concentrations of PFOS were lower, the relationship between increasing PFOS quantiles and the covariable-adjusted EMM of total-C and LDL-C was similar across the spectrum of serum PFOS concentrations. HDL-C also demonstrated a small, non-linear association with increasing PFOS.
Figure 2.
Non-Linear Changes in Covariate-Adjusted Estimated Marginal Means (GLM Analysis) Across PFOA 20-Group Quantiles
Figure 3.
Non-Linear Changes in Covariate-Adjusted Estimated Marginal Means (GLM Analysis) Across PFOS 20-Group Quantiles
Logistic regression results are shown in Table 3. Both increasing PFOA and PFOS quintiles were positively associated with an increased risk of abnormal total-C (adjusted odds ratio 1.2 (95% CI 1.1–1.4) and 1.6 (1.4–1.9) respectively) and LDL-C (1.4 (1.2–1.7) and 1.6 (1.3–1.9) respectively); increasing PFOS quintiles were also associated with decreased risk of low HDL-C (0.7 (0.6–0.9)). Neither PFOA nor PFOS were associated with increased risk for abnormal triglycerides.
Table 3.
Risk of Abnormal Blood Lipids (Logistic Regression Analysis) Based on Increasing PFOA & PFOS Quintiles
| Quintile | Total Cholesterolφ | HDL Cholesterolφ | LDL Cholesterolφ,ς | Fasting Triglycerides* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval |
Odds Ratio | 95% Confidence Interval |
Odds Ratio | 95% Confidence Interval |
Odds Ratio | 95% Confidence Interval |
||
| PFOA | 1st Quintile | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) | ||||
| 2nd Quintile | 1.1 | (1.0–1.3) | 1.0 | (0.8–1.2) | 1.2 | (1.0–1.5) | 1.0 | (0.7–1.5) | |
| 3rd Quintile | 1.2 | (1.0–1.4) | 1.0 | (0.8–1.2) | 1.2 | (1.0–1.4) | 1.3 | (0.9–1.9) | |
| 4th Quintile | 1.2 | (1.1–1.4) | 1.0 | (0.9–1.2) | 1.2 | (1.0–1.4) | 1.6 | (1.1–2.3) | |
| 5th Quintile | 1.2 | (1.1–1.4) | 0.9 | (0.8–1.1) | 1.4 | (1.2–1.7) | 1.0 | (0.7–1.6) | |
| PFOS | 1st Quintile | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) | ||||
| 2nd Quintile | 1.3 | (1.1–1.4) | 0.9 | (0.8–1.1) | 1.2 | (1.0–1.5) | 1.3 | (0.9–1.8) | |
| 3rd Quintile | 1.3 | (1.2–1.5) | 0.8 | (0.7–1.0) | 1.2 | (1.0–1.5) | 1.0 | (0.7–1.4) | |
| 4th Quintile | 1.3 | (1.2–1.6) | 0.8 | (0.7–0.9) | 1.3 | (1.1–1.6) | 1.1 | (0.7–1.6) | |
| 5th Quintile | 1.6 | (1.4–1.9) | 0.7 | (0.6–0.9) | 1.6 | (1.3–1.9) | 1.2 | (0.8–1.5) | |
Models adjusted for age, estimated time of fasting, BMI Z-score, gender, and regular exercise; gender stratified models not adjusted for gender
Calculated for those with triglyceride <400 mg/dL regardless of fasting status
Defined as self-reported fasting ≥6 hours prior to phlebotomy
Results for analyses assessing interaction are shown in Table 4. For total-C, LDL-C and triglycerides, results do not support interaction between PFOA and PFOS in the prediction of these lipids. There is some evidence of multiplicative interaction between PFOA and PFOS in the reduction of the risk of low HDL-C (the odds ratio for Group 4 exceeds the product of the odds ratios for Group 2 and Group 3).
Table 4.
Assessment of Interaction (Logistic Regression Analysis) Coincident PFOA & PFOS Quintile Groups
| Quintile | Total Cholesterolφ | HDL Cholesterolφ | LDL Cholesterolφ,ς | Fasting Triglycerides* | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval |
Odds Ratio | 95% Confidence Interval |
Odds Ratio | 95% Confidence Interval |
Odds Ratio | 95% Confidence Interval |
|
| Group 1‡ | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) | 1.0 (Referent) | ||||
| Group 2† | 1.1 | (0.9–1.2) | 1.0 | (0.8–1.1) | 1.2 | (1.0–1.4) | 0.9 | (1.6–1.3) |
| Group 3¥ | 1.3 | (1.2–1.5) | 0.9 | (0.8–1.0) | 1.3 | (1.1–1.6) | 1.2 | (0.8–1.8) |
| Group 4€ | 1.4 | (1.2–1.7) | 0.7 | (0.5–0.9) | 1.2 | (0.8–1.8) | 0.8 | (0.4–1.7) |
| p-interaction | >0.2 | >0.2 | >0.2 | >0.2 | ||||
Models adjusted for age, estimated time of fasting, BMI Z-score, gender, and regular exercise; gender stratified models not adjusted for gender
Calculated for those with triglyceride <400 mg/dL regardless of fasting status
Defined as self-reported fasting ≥6 hours prior to phlebotomy
Group 1: PFOA 1st–4th quintile & PFOS 1st–4th quintile
Group 2: PROA 5th quintile & PFOS 1st–4th quintile
Group 3: PFOA 1st–4th quintile & PFOS 5th quintile
Group 4: PFOA 5th quintile & PFOS 5th quintile
Sensitivity analyses were conducted to assess the stability of the association between PFOA or PFOS and lipids after the inclusion of household income and for fasting-only participants. Results (not shown) suggested that, after adjustment for the same covariables, models were stable and associations unaltered. The positive and statistically significant association between both PFOA and PFOS and total-C and LDL-C was not altered after the inclusion of household income, a proxy for socioeconomic status, or when analysis was performed only on the subset of participants who had completed a ≥6 hr fast. Likewise, the positive, statistically significant association between PFOA and fasting triglycerides was not altered after the inclusion of household income. PFOS was not associated with fasting triglycerides with or without the inclusion of household income. The association between PFOA and HDL-C was not statistically significant with or without the inclusion of household income, or for the fasting or non-fasting participants. The positive, linear association between PFOS and HDL-C was statistically significant with and without the inclusion of household income, and for both the fasting and non-fasting participants.
DISCUSSION
This study reports the first known assessment of associations between PFOA and PFOS and serum lipids in children from the largest community-based study of the effects of PFOA exposure to date. Across several types of analyses, results consistently provided evidence for a positive association between PFOA and PFOS and serum lipids, specifically an increase in total-C and LDL-C with increasing PFOA and PFOS. Dose-response relationships were non-linear, with larger increases in lipids at the lower range of PFOA concentration in particular. Additionally, results suggested that the magnitude of association between PFOS and total-C and LDL-C was higher than that between PFOA and total-C and LDL-C. Finally, there was a statistically significantly increased risk for abnormal total-C and LDL-C with increasing PFOA and PFOS.
Results reported here are consistent with previous studies in adults which have generally shown a trend toward a positive association between PFOA and cholesterol. Observations reported here are also consistent with a study of adults (≥18 years) from the same Project population, where authors reported an 11–12 mg/dL increase in total-C from the lowest to highest decile of serum PFOA (vs. a 4.6 mg/dL from the lowest to highest quintile reported here) and a corresponding 8–9 mg/dL increase in LDL-C (vs. 3.8 mg/dL reported here), and a 10–12 mg/dL increase in total-C from the lowest to highest decile of serum PFOS (vs. 8.5 mg/dL from the lowest to highest quintile reported here) and a corresponding 11–12 mg/dL increase in LDL-C (vs. 5.8 mg/dL reported here).14
Evidence from animal studies has suggested that activation of PPAR-α is an important mechanism through which PFOA and PFOS exert biologic effects, though it is unclear that this animal-based evidence can be extrapolated to humans. PFOA and PFOS elimination time in humans is substantially longer compared to rodents resulting in a longer duration to reach a steady-state dose,19 PPAR-α is expressed in human liver tissue at approximately 10% of rodent levels, and humans and other primates are refractory or less responsive to PPAR-α agonists compared to rodents.20 Thus, associations reported here are etiologically plausible if a non-PPAR-α mechanistic pathway is operant in humans in addition to or instead of a PPAR-α pathway; some studies humans and other primates have reported lipidemic effects through non- PPAR-α-dependent mechanisms.21
The non-linear nature of the observed associations, particularly for PFOA, suggests a possible saturation point in an underlying physiologic mechanism. Further, while serum PFOA levels in this study population exceeded levels from a nationally representative sample, PFOS levels were similar (as above). Regardless, the magnitude of the observed associations between PFOS and total-C and LDL-C were at least similar and in some instances larger to those observed for PFOA. Thus, PFOA and PFOS specifically, and possibly perfluoroalkyl acids as a general class, appear to be associated with serum lipids, and the association appears to exist at levels of PFOA and PFOS exposure which are in the range characterized by nationally representative studies.
The strengths of this study include the large sample size and participation rate (and consequent ability to examine associations in different age groups and genders), and childhood replication of observations made in adult populations. The replication of observations in a sample free from some factors that can confound associations in adult samples contributes additional confidence to observations reported here. While confounding from gender or developmental effects was controlled through stratification, the prevalent design of the Project did not permit assessment of effects of cumulative exposure, nor residual or persistent effects of prenatal exposure. The long-term health consequences of elevated serum lipids in the ranges observed in this study (3–10 mg/dL) are unclear.
The cross-sectional nature of this study is an acknowledged weakness, and thus causal inference is limited. Additional potential limitations include self-reported survey data and limited availability of covariables known to be associated with lipids, and uncertainty of fasting status for analysis of triglycerides.
Studying the susceptibility of children to these chemicals has been identified as a particular data need and research priority.22 We have previously reported a U-shaped pattern in serum concentrations of PFOA in boys and girls and PFOS in girls in this population (i.e., serum concentrations were higher in younger age groups, decreased into early- to middle-adulthood, and then increased again through adulthood).15 Further, the effects of either perfluoroalkyl acids on developing physiologic systems, or the effects of sustained, long-term exposure are unknown. The large sample size of the current study, and findings consistent with results from adult occupational and larger community studies, support a strong need to prioritize the study of the effects of perfluoroalkyl acids in children to assess whether the clear associations reported here are etiologic.
CONCLUSION
In conclusion, while the epidemiologic and cross-sectional nature of the current study inherently limit causal inference, the consistent observed associations between increasing PFOA and PFOS and elevated total-C and LDL-C warrant further study. Should the association prove to be etiologic, the cumulative effects of such an elevation in cholesterol on long-term cardiovascular health are unclear given the early age in which these associations were observed.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions of Cathy Lally and Jessica MacNeil from the Emory University Rollins School of Public Health for their expertise and assistance in data cleaning, and personnel at Exygen Research Corporation and Axys Analytics for ensuring accurate descriptions of laboratory methods. Finally, the scale C8 Health Project necessitated that many individuals provided valuable assistance during its development and implementation. The authors gratefully acknowledge these contributions and thank all those individuals who supported the execution of the Project.
FUNDING SOURCES:
The C8 Health Project was funded by the Settlement Agreement in the case of Leach v. E.I. DuPont de Nemours & Co., Civil Action No. 01-C-608 (W. Va. Circuit Court of Wood County, 2004), which resulted from drinking water contamination by the chemical perfluorooctanoic acid (PFOA or C8). The Court authorized the creation of Brookmar, Inc., an independent company created solely to design, publicize, and implement the C8 Health Project, including all enrollment and data collection. Brookmar, Inc. received funding exclusively from the Settlement, administered through a Court-approved financial professional (the Health Project Administrator). Brookmar, Inc. made data from the C8 Health Project available to the C8 Science Panel, also created pursuant to the Settlement Agreement, and to a group at West Virginia University, pursuant to a contractual relationship with Brookmar, Inc. The design of the Project was developed in consultation with, though not subject to, the wishes of the settling parties. All authors declare that their ability to design, conduct, interpret, and publish this research study was unimpeded and fully independent of the Court and settling parties, who had no role in preparing, reviewing, or approving the manuscript.
Footnotes
COMPETING INTERESTS DECLARATION:
For SJF, AS, SSK, AMD:
These authors were engaged in the Project pursuant to a contractual relationship between Brookmar, Inc. and West Virginia University. This study was funded in part by that contract. These authors have no current or prior competing financial or non-financial interests to disclose.
For KS, DAS, TF:
These authors are members of the separate C8 Science Panel, whose Court-appointed obligation is to determine “probable links” between PFOA exposure and health outcomes. Panel membership was jointly agreed to by both the Plaintiffs and DuPont, and then approved by the Court. As such, these authors are in receipt of funding from the C8 Class Action Settlement Agreement. These authors have no other financial disclosures to make, and no competing non-financial interests to disclose.
REFERENCES
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutches A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicolog Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy GL, Jr, Butenoff JL, Olsen GW, O’Connoar JC, Seacat AM, Perkins RG, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34(4):351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- 3.Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animal. J Toxicolog Sci. 2003;28(2):49–57. doi: 10.2131/jts.28.49. [DOI] [PubMed] [Google Scholar]
- 4.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Sci Technol. 2007;41(7):2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115(11):1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, et al. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect. 2007;115(2):226–230. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112(11):1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen GW, Logan PW, Hansen KJ, Simpson CA, Burris JM, Burlew MM, et al. An occupational exposure assessment of a perfluorooctanesulfonyl fluoride production site: biomonitoring. AIHA J (Fairfax, Va) 2003;64(5):651–659. doi: 10.1202/375.1. [DOI] [PubMed] [Google Scholar]
- 9.Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med. 2007;49(8):872–879. doi: 10.1097/JOM.0b013e318124a93f. [DOI] [PubMed] [Google Scholar]
- 10.Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med. 2007 Oct;49(10):1086–1096. doi: 10.1097/JOM.0b013e318156eca3. [DOI] [PubMed] [Google Scholar]
- 11.Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009 Mar;51(3):364–372. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- 12.Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (FPOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health. 2007;81(2):231–246. doi: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- 13.Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, et al. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J Occup Environ Med. 2006;48(8):771–779. doi: 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenland K, Tinder S, Frisbee SJ, Ducatman AM, Vaccarino V. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009;170(10):1268–1278. doi: 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- 15.Frisbee SJ, Brooks AP, Jr, Maher A, Flensborg P, Arnold S, Fletcher T, et al. The C8 Health Project : Design, methods, and participants. Environ Health Perspect. 2009;117(12):1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med. 2006;48(8):759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty JM, Connolly PD, Decker ER, Kennedy SM, Ellefson ME, Reagen WK, et al. Quantitative determination of perfluorooctanoic acid in serum and plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 May 25;819(2):329–338. doi: 10.1016/j.jchromb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.American Heart Association. AHA Scientific Position. Cholesterol and Atherosclerosis in Children. [Accessed 10.19.2009]; Available at: http ://www.americanheart.org/presenter.jhtml?identifier=4499.
- 19.Hundley SG, Sarrif AM, Kennedy GL., Jr Absorption, distribution, and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem Toxicol. 2006;29(2):137–145. doi: 10.1080/01480540600561361. [DOI] [PubMed] [Google Scholar]
- 20.Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, et al. PPARα agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33(6):655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- 21.Okochi E, Nishimaki-Mogami T, Suzuki K, Takahashi A. Perfluorooctanoic acid, a peroxisome-proliferating hypolipidemic agent, dissociates apolipoprotein B48 from lipoprotein particles and decreases secretion of very low density lipoproteins by cultured rat hepatocytes. Biochimica et Biophysica Acta. 1999 Mar 25;1437(3):393–401. doi: 10.1016/s1388-1981(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 22.Draft Toxicological Profile for Perfluoroalkyls. Atlanta, GA: Department of Health and Human Services; 2009. Public Health Service, Agency for Toxic Substances and Disease Registry. [Google Scholar]