Abstract
Lymphodeleption prior to adoptive transfer of tumor-specific T cells greatly improves the clinical efficacy of adoptive T-cell therapy for patients with advanced melanoma, and increases the therapeutic efficacy of cancer vaccines in animal models. Lymphodepletion reduces competition between lymphocytes, and thus creates “space” for enhanced expansion and survival of tumor-specific T cells. Within the lymphodepleted host, Ag-specific T cells still need to compete with other lymphocytes that undergo lymphopenia-driven proliferation. Herein, we describe the relative capacity of naïve T cells, Treg, and NK cells to undergo lymphopenia-driven proliferation. We found that the major population that underwent lymphopenia-driven proliferation was the CD122+ memory-like T-cell population (CD122+CD8+ Treg), and these cells competed with Ag-driven proliferation of melanoma-specific T cells. Removal of CD122+CD8+ Treg resulted in a greater expansion of tumor-specific T cells and tumor infiltration of functional effector/memory T cells. Our results demonstrate the lymphopenia-driven proliferation of CD122+CD8+ Treg in reconstituted lymphodepleted mice limited the antitumor efficacy of DC vaccination in conjunction with adoptive transfer of tumor-specific T cells.
Keywords: Cancer vaccine, CD8+ Treg, Lymphopenia-driven proliferation
Introduction
Due in large part to the limited expansion and survival of vaccine-induced tumor Ag-specific T cells, active specific immunotherapy of tumor-bearing hosts with tumor vaccines has generally been ineffective [1]. Therefore, a major goal of current T-cell based immunotherapy protocols is to induce a large number of tumor-specific T cells capable of mediating regression of established tumors and maintaining long-term memory to prevent tumor recurrence. Lymphodepletion has been recently demonstrated to facilitate the expansion and survival of therapeutic, adoptively transferred in vitro-expanded T cells, which induced tumor regression in patients with melanoma (see review in [2]). Concurrently, we and others have demonstrated that vaccination induced a dramatic expansion of tumor-specific T cells, and improved the efficacy of active immunotherapy in reconstituted lymphodepleted mice [3–7]. While lymphopenic conditioning has been shown to benefit antitumor immunity, and aids in the establishment of the T-cell repertoire in neonatal mice [8], it was detrimental for transplant tolerance [9], and precipitated the development of autoimmune disease [10].
Homeostatic proliferation, or more precisely, lymphopenia-driven proliferation of lymphocytes in irradiated or lymphocyte-deficient mice, is a well-studied phenomenon (see review [11]). Both cytokines, IL-7 and IL-15, and self-MHC/peptide ligands are required for the maintenance of the relatively stable size of the naïve and memory-like-cell pools in normal lymphoreplete mice, and for the spontaneous T-cell proliferation observed in lymphodepleted mice in the absence of exposure to exogenous Ag. However, the proliferation of naïve and memory T cells in lymphodepleted mice is regulated differently; homeostasis of naïve CD8+ T cells is regulated by IL-7 and self-MHC/peptide ligands, whereas homeostasis of memory-like CD8+ T cells is MHC-independent, and controlled by both IL-7 and IL-15. In addition to lymphopenia-driven proliferation, the co-transfer of a small number of Ag-specific TCR transgenic T cells into irradiated mice following Ag exposure resulted in a dramatic expansion of Ag-specific T cells [12]. Our recent published data also demonstrated Ag-induced proliferation of melanoma-specific T cells in lymphodepleted hosts, and showed that both Ag-induced expansion and lymphopenia-driven proliferation of non-Ag specific T cells were IL-7 dependent [6]. The more rapid expansion of Ag-activated T cells enabled them to outpace the lymphopenia-driven proliferation of non-Ag specific T cells during the first 2 wk of immune reconstitution, but contraction followed. The contraction was presumably due to the suppression mediated by Treg [13–15], or competition with other lymphocyte subsets that undergo delayed proliferation driven by the lymphopenic condition [16].
The disruption of T-cell homeostasis leads to profound changes in programs of T-cell activation, differentiation, and survival. Different programming might promote or dampen T-cell reactivity to Ag [17, 18]. Thus, it is critically important to determine how to set the T-cell regulating programs and determine what underlying mechanisms promote the development of effective antitumor immunity during immune reconstitution in lymphodepleted hosts. Various investigators have provided data to suggest that improved activation of T cells may be the result of elimination of Treg, creation of space, or removal of cytokine sinks [7, 19]. However, the relative contribution of these mechanisms needs to be further characterized. In this report, we carefully assessed the effect of lymphopenia-driven proliferation of different subsets of lymphocytes on the concomitant Ag-driven proliferation of melanomas-specific T cells, and the antitumor efficacy of adoptive T-cell therapy in melanoma-bearing mice.
Results
Combined CD25/CD122 depletion improved antitumor immune response
We have previously documented that vaccination with peptide-pulsed DC induced a rapid and large expansion of melanoma-specific T cells in lymphodepleted mice that was followed by a delayed lymphopenia-driven proliferation of co-transferred polyclonal naïve spleen cells [6]. We hypothesized that the delayed proliferation of co-transferred spleen cells could reduce the maximum expansion of tumor-specific T cells, and thus limit the therapeutic activity of adoptively transferred T cells. First, we mixed titrated numbers of pmel-1 spleen cells together with 10 million WT spleen cells before adoptive transfer to identify the minimal number of pmel-1 needed to be able to measure quantitatively adequate numbers in blood after vaccination with peptide-pulsed DC (Supporting Information Fig. 1). We found that 104 was the optimal number of pmel-1 spleen cells that could be mixed with 107 WT spleen cells. Compared with WT spleen cells, donor spleen cells from IL-15 KO mice has a significantly less suppressive effect on the primary response of pmel-1 T cells to peptide-pulsed DC than spleen cells from WT mice (Supporting Information Fig. 2). The suppression mediated by co-transfer of WT spleen cells was even more dramatic when the secondary response of pmel-1 T cells to DC vaccination was measured. Surprisingly, the co-transfer of spleen cells from IL-15 KO mice did not suppress but increased the secondary response of pmel-1 T cells. IL-15 KO mice are known to have deficient numbers of CD122+CD8+ memory-like (sometimes referred to as “memory-phenotype” or “innate”) T cells, NK, and NKT cells, but have sufficient numbers of CD25+CD4+ Treg (see review [11], and Supporting Information Fig. 2), suggesting that lymphocytes other than CD25+CD4+ Treg played the key suppressive role in our model. Consistent with this notion, CD122+CD8+ memory-like cells constituted the major population of lymphocytes that underwent lymphopenia-driven proliferation when adoptively transferred into sub-lethally irradiated mice (Supporting Information Fig. 3).
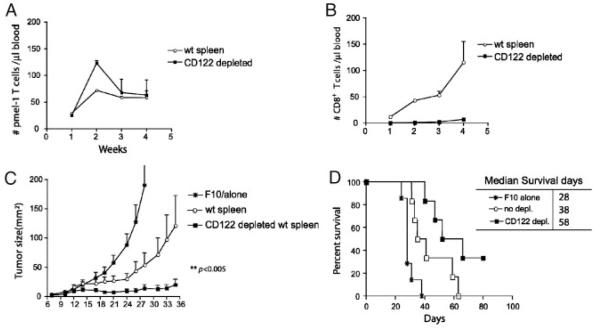
To substantiate our initial observations and determine the effect of CD122 depletion on the therapeutic efficacy of adoptive T-cell therapy in lymphopenic mice, we treated mice bearing 6-day subcutaneous F10 tumors with irradiation, followed by adoptive transfer of 104 pmel-1 spleen cells and 107 congenic spleen cells with or without prior depletion of CD122+ cells, and vaccination with peptide-pulsed DC. The absolute numbers of pmel-1, congenic, and host T cells in the blood were enumerated at different intervals after vaccination. We found that depletion of CD122+ cells doubled the number of pmel-1 T cells found in the blood of vaccinated mice 2 wk after vaccination (Fig. 1A), and there was no recovery of congenic T cells when CD122+ cells were depleted (Fig. 1B). CD122+ lymphocytes rather than CD122− cells were the primary lymphocyte subpopulation that underwent lymphopenia-driven proliferation. In contrast, host T-cell recovery, which is reflected by the thymic output of naïve T cells, did not differ in recipients of CD122-depleted and non-depleted T cells. Most importantly, depletion of CD122+ lymphocytes resulted in a greater antitumor efficacy (Fig. 1C and D). Depletion of CD122+ cells from congenic donor spleen cells led to a significantly longer delay of tumor growth and an increase in median survival of tumor-bearing mice (from 38 days to 58 days). Moreover, 35% of mice receiving CD122-depleted cells were rendered tumor-free and survived more than 80 days; whereas, none of tumor-bearing mice that underwent adoptive transfer of undepleted congenic spleen cells and pmel-1 T cells survived. In addition, co-transfer of CD122-depleted spleen cells exhibited no effect on the tumor-growth and survival of melanoma-bearing mice after treatment with transfer of pmel-1 T cells and DC vaccination (Supporting Information Fig. 4), further supporting the notion that CD122+ cells were the major suppressor cells in naïve spleens. Since CD122+CD8+ T cells that functioned as Treg have been described in autoimmune disease models (see review [20]), we will hereafter refer to these cells as the CD122+CD8+ Treg.
Figure 1.
Removal of CD122+ cells from adoptively co-transferred cells enhanced antitumor activity and expansion of pmel-1 T cells in vaccinated lymphodepleted mice. Naïve C57BL/6 mice (n=10 per group) were injected with 2×105 B16-F10 tumor cells s.c. at day 0. Five days later, the tumor-bearing mice were sublethally irradiated. At day 6 after F10 inoculation, irradiated mice were adoptively reconstituted with 104 pmel-1 T cells together with 107 of either congenic splenocytes or CD122-depleted congenic spleen cells, followed by s.c. vaccination of 2×106 peptide-pulsed DC loaded with hgp-9 peptide at days 6 and 12 post tumor injection. Tumor size was measured three times each week. Mice were sacrificed when the tumor size exceeded 200 mm2. (A) The number of pmel-1 T cells, data show mean±SEM (n=3). (B) The number of congenic CD8+ T cells in blood drawn weekly from vaccinated mice, data show mean±SEM. (C) Tumor growth and (D) survival rate of mice. Time-to-death (or tumor-free survival, etc.) between groups was compared using the nonparametric log-rank test, and median survival time was estimated from the Kaplan–Meier survival curves. All results shown are representative of three independent experiments.
Combined CD25/CD122 depletion enhanced expansion and survival of memory pmel-1 T cells
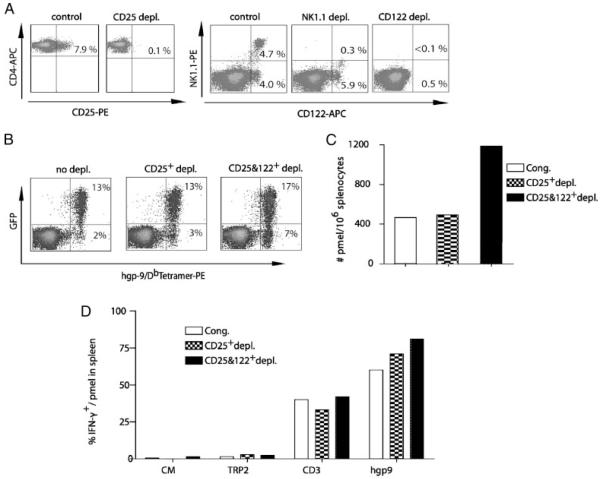
The beneficial antitumor effects that follow depletion of CD4+CD25+ natural Treg have been well described [21]. We sought to determine whether depletion of CD122+CD8+ Treg in addition to CD4+CD25+ natural Treg would further enhance the expansion and survival of pmel-1 T cells. Since NK cells and NK T cells were the other major CD122+ populations, their contribution to immune regulation was also investigated. Spleen cells from WT mice were subjected to depletion of CD25+ cells alone, CD25+ and NK1.1+ cells, and CD25+ and CD122+ T cells using magnetic beads. As expected, depletion with anti-CD25 or NK1.1 antibodies resulted in near-complete disappearance of cells expressing CD25 or NK1.1, respectively. NK depletion resulted in elimination of both NK and NKT cells, while the CD122+ non-NK1.1 expressing cells remained. CD122− depletion resulted in near complete elimination of both NK1.1+ cells and CD8+CD122+ T cells (Fig. 2A). At wk 4 after vaccination, depletion of CD25+ cells from naïve spleen before adoptive transfer had no effect on the number of pmel-1 T cells in blood (13% of CD8+ T cells) or spleen (400/106 spleen cells) (Fig. 2B and C). However, CD25- and CD122-depleted mice also exhibited a pronounced increase in the number of endogenous peptide-specific T cells, identified by hgp9-Db tetramer staining (GFP-tetramer+) (Fig. 2B). In addition, 7% of total CD8+ T cells in the blood of mice with CD25 and CD122 depletion were positive for hgp9-tetramer+ GFP−, compared with 2 or 3% of CD8+ T cells in the control or CD25 only depletion group. Thus, the removal of CD122+ cells in addition to CD25+ cells led to expansion of both transgenic pmel-1 T cells and non-transgenic peptide-specific T cells. Four weeks after adoptive transfer the number of pmel-1 T cells in the spleen of mice from the CD25 and CD122 depletion group was threefold greater than in the control or CD25 depletion group (Fig. 2C). The function of pmel-1 T cells found in spleens among all three groups of mice was comparable as demonstrated by a similar production of IFN-γ upon ex vivo stimulation with peptide (Fig. 2D). Taken together, these experiments showed that lymphopenia-driven proliferation of CD4+CD25+ and CD122+CD8+ T cells negatively regulated proliferation of Ag-specific pmel-1 T cells and non-transgenic T cells in lymphodepleted mice.
Figure 2.
The effects of CD25, NK, CD25, and CD122 depletion on the pmel-1 T-cell response. (A) Depleting splenocytes in vitro with anti-CD122 antibody conjugated to MACS MicroBeads removed NK and CD122+CD8+ T cells, while antibodies against NK1.1 or CD25 only removed the corresponding cell population. After MACS separation, different depleted cell populations were stained with anti-CD4, anti-CD25, anti-CD122, and anti-NK1.1 antibody for flow cytometric analysis. (B) Both Tg pmel-1 and non-Tg gp100-specific T cells were increased after CD25 and CD122 double depletion. Blood samples from each group as described in (A) were collected at wk 4 and pooled to stain with anti-CD8 Ab and hgp-9/Db MHC tetramers for flow cytomteric analysis. The numbers indicate the percentage of tetramer-positive pmel-1 transgenic T cells or endogenous non-transgenic T cells in total gated CD8+ cells. (C) Spleens from each group as described in (B) were harvested at wk 4, the absolute number of pmel-1 T cells in 106 splenocytes was determined by flow cytometric analysis. (D) 2×106 splenocytes pooled from five mice of each group as described in (B) were stimulated for 6 h in medium containing 1g/mL hgp9 peptide derived from melanoma Ag hgp100, 5 g/mL anti-CD3 Ab, 1 g/mL TRP2 or CM alone, respectively. Results shown are the percentages of IFN-γ+gated pmel-1+ T cells determined by intracellular staining and flow cytometric analysis. Representative results from three independent experiments are shown.
Combined CD25/CD122 depletion improved survival and enhanced tumor infiltration by pmel-1 T cells
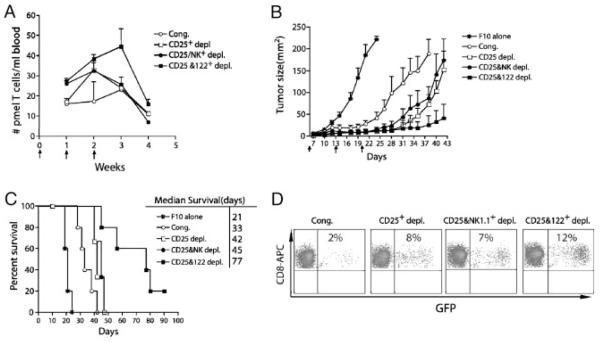
The effect of the removal of different subsets of lymphocytes on the antitumor efficacy of vaccination in lymphodepleted tumor-bearing mice was assessed in mice bearing 6-day established subcutaneous B16F10 tumors. Ten million WT congenic spleen cells depleted or non-depleted were adoptively transferred into irradiated mice with 104 naïve pmel-1 spleen cells, and were subsequently followed by three weekly vaccinations with peptide-pulsed DC. The absolute numbers of pmel-1 T cells from wk 1–4 after adoptive transfer was determined (Fig. 3A). Compared with the non-depleted control group, CD25 depletion increased the number of pmel-1 T cells only at wk 2, whereas depletion of both CD25 and NK cells increased the number of pmel-1 T cells on both wk 1 and 2. As shown for pmel-1 T cell numbers in mice with single depletion of CD122 (Fig. 1A), the number of pmel-1 T cells at wk 3 or 4 in mice subjected to CD25 alone or CD25 and NK double depletion did not differ from mice that received undepleted naïve spleen cells (Fig. 3A). However, the number of pmel-1 T cells in tumor-bearing mice gradually increased until wk 3, whereas mice that received CD25 alone, or CD25 and NK double depletion contrasted similarly to control mice at wk 3 despite being vaccinated at wk 2. Thus, our data indicated that CD25 or NK depletion acted on the early expansion phase of pmel-1 T-cell proliferation, while CD122 depletion acted on late phases of T-cell survival to enable persistent expansion of pmel-1 T cells. Depletion of CD25 and CD122 expressing cells acted synergistically in this model to augment the expansion and survival of tumor-reactive T cells after vaccination.
Figure 3.
Removal of both CD25+ and CD122+ cells significantly reduced the suppression and enhanced the antitumor efficacy of adoptive immunotherapy and vaccination in lymphodepleted mice. (A) CD25 and CD122 double depletion greatly promoted the expansion and survival of pmel-1 T cells. Irradiated mice (n=5–6 per group) were adoptively co-transferred with 104 pmel-1/GFP double Tg spleen cells together with 107 congenic spleen cells, CD25-depleted cells, CD25- and NK-depleted cells, CD25- and CD122-depleted congenic spleen cells, respectively; and followed immediately by three, weekly vaccinations with peptide-pulsed DC. Absolute number of pmel-1 T cells (gated on CD8+GFP+CD45.2+) in blood samples at the indicated times. Data show mean±SEM. (B) Naïve C57BL/6 mice (n=10 per group) were injected with 2×105 B16-F10 tumor cells s.c. at day 0. Five days later, the tumor-bearing mice were sublethally irradiated. At day 6 after F10 inoculation, irradiated mice were adoptively transferred with 104 pmel-1 T cells together with 107 of either congenic splenocytes or with CD25−, NK−, CD25+NK, or CD25+CD122-depleted spleen cells, in conjunction with peptide-pulsed DC vaccination at days 6, 13, and 20 after F10 inoculation. (B) Tumor growth and (C) survival of mice. Results shown are representative of three independent experiments. Time-to-death (or tumor-free survival, etc.) between groups was compared using the nonparametric log-rank test and median survival time was estimated from the Kaplan–Meier survival curves. Results shown are representative of one of three independent experiments. (D) At wk 5 after F10 inoculation, tumor tissues from the vaccinated mice were harvested. Percentage of pmel-1 T cells in total gated CD8+ T cells in digested tumors was determined by flow cytometry analysis of GFP and CD8 expression of transferred pmel-1 T cells.
When 104 pmel-1 spleen cells (approximately 2000 pmel-1 hgp100-specific CD8+ T cells) were adoptively transferred together with untreated congenic spleen cells, a small but significant delay of tumor growth occurred (Fig. 3B). CD25 depletion further retarded tumor growth and also prolonged median survival. Depletion of CD122+ cells, but not NK cells, combined with CD25- depletion to result in a much greater delay of tumor growth and prolonged survival of mice. Only mice reconstituted with CD25- and CD122-depleted congenic spleen cells exhibited tumor-free survival more than 90 days after tumor inoculation (Fig. 3C). These results further demonstrated that CD122+CD8+ T cells were the other major population of Treg that inhibited vaccine-induced proliferation of pmel-1 T cells and antitumor efficacy in lymphodepleted tumor-bearing mice. Next, we examined the effect of depletion on the relative infiltration of tumors with GFP+ pmel-1 T cells (Fig. 3D). The highest percentage of pmel-1 T cells (12%) was observed when the co-transferred cells were depleted of both CD25+ and CD122+ cells. In the absence of direct imaging, it is difficult to know whether increased infiltration of pmel-1 T cells resulted from increased trafficking or increased expansion of pmel-1 T cells in situ after removal of CD25+ and CD122+ cells.
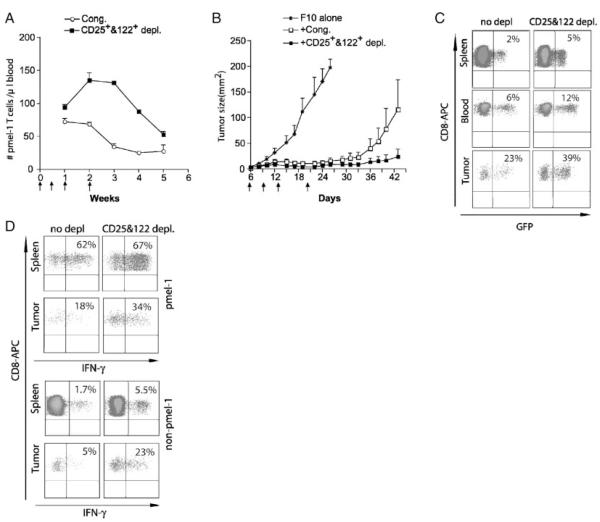
We sought to test whether multiple DC vaccinations at short intervals could lead to sustained Ag-induced proliferation in lymphodepleted mice. Mice were vaccinated with peptide-pulsed DC on days 6, 9, 12, and 19 post tumor injection. Tumor-bearing mice were irradiated 6 days after tumor injection and reconstituted with 104 naïve pmel-1 spleen cells together with 107 congenic spleen cells, with or without CD25 and CD122 depletion (Fig. 4A). After multiple DC vaccinations, Pmel-1 T cells still contracted immediately when co-transferred with undepleted spleen cells after the last vaccination. However, CD25 and CD122 depletion led to a prolonged expansion and delayed contraction of pmel-1 T cells. These results suggested that the suppression of tumor-reactive T cells mediated by CD25+ and CD122+ T cells could not be overcome by multiple vaccinations alone. Tumor-growth in melanoma-bearing mice subjected to reconstitution with pmel-1 T cells together with CD25- and CD122-double depleted spleen cells was significantly delayed compared with mice that received pmel-1 T cells together with undepleted spleens (Fig. 4B).
Figure 4.
Removal of CD25+ and CD122+ cells from co-transferred naïve spleen cells enhanced the tumor infiltration of IFN-γ-producing pmel-1 and non-pmel-1 effector/memory T cells. Naïve C57BL/6 mice (n=10 per group) were injected with 2×105 B16-F10 tumor cells s.c. at day 0. Five days later, the tumor-bearing mice were sublethally irradiated. At day 6 after F10 inoculation, irradiated mice were adoptively transferred with 104 pmel-1 T cells together with 107 of either congenic splenocytes or with CD25- and CD122-depleted spleen cells, in conjunction with peptide-pulsed DC vaccination at days 6, 9, 13, and 20 after F10 inoculation. (A) The absolute number of pmel-1 T cells was determined in blood of mice at indicated time points after tumor injection (n=5 per group). Data show mean±SEM. (B) Growth of B16F10 tumors were monitored. Results shown are representative of two independent experiments. (C) At wk 5 after F10 inoculation, blood, spleen, and tumor tissues from the vaccinated mice were harvested. Percentage of pmel-1 T cells as a fraction of total CD8+ T cells in tumors was determined by flow cytometric analysis of GFP and CD8 expression of pmel-1 T cells. (D) Spleen cells were stimulated in vitro with hgp-9 peptide, and IFN-γ production by pmel-1 T cells (GFP+, top panel) and non-pmel-1 T cells (GFP−, bottom panel) in spleens, blood, and tumors was determined by flow cytometry analysis after intracellular staining of stimulated T cells with PE-conjugated anti-IFN-γ antibody. Results shown are representative of two independent experiments.
To further characterize pmel-1 T cells in different organs, and in tumors, treated mice were sacrificed on day 44. Spleen, blood, and tumors were collected for the analysis of the abundance of pmel-1 T cells (Fig. 4C). The percentage of CD8+ T cells that were GFP+(pmel-1 T cells) found in the spleen and blood or in the tumors of mice reconstituted with depleted spleen cells was double that of mice reconstituted with undepleted spleen cells. The majority (around 67%) of pmel-1 T cells, and a significant fraction of non-pmel-1 T cells found in the spleen produced IFN-γ (Fig. 4D), with or without depletion. Thus, pmel-1 T cells in peripheral tissues of tumor-bearing mice were functional effector/memory T cells. However, depletion did increase the percentage of IFN-γ producing non-pmel-1 T cells, primarily due to an increased frequency of peptide-specific T cells. A much lower percentage of pmel-1 T cells (18%) found in tumors were able to produce IFN-γ as compared with pmel-1 T cells found in spleens (62%). These results strongly suggested that functional inactivation of pmel-1 T cells occurred locally in tumor sites. Interestingly, this inactivation could be ameliorated by CD25 and CD122 depletion, which almost doubled the percentage of IFN-γ-producing pmel-1 T cells from 18 to 34%. A much more dramatic increase of IFN-γ-producing, peptide-specific non-pmel-1 T cells was found in tumors from mice reconstituted with CD25- and CD122-depleted cells (5–23%). This could result from both an increased frequency and functionality of these tumor-specific T cells in tumor sites after depletion of Treg.
Exogenous IL-7 reduced suppression and prolonged mice survival after reconstitution and vaccination
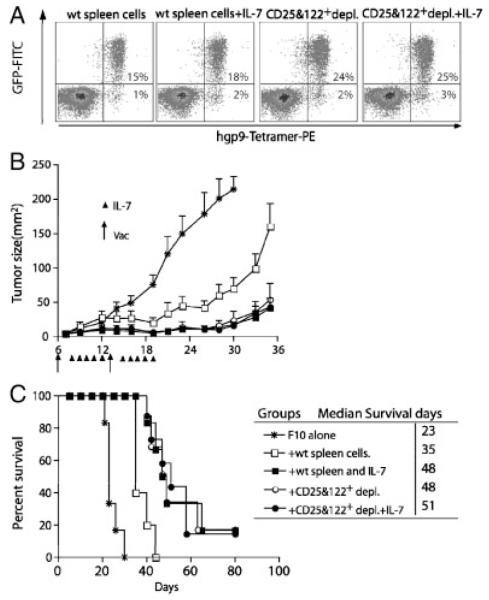
Because both the expansion and survival of vaccine-induced pmel-1 T cells and lymphopenia-driven proliferation of CD122+CD8+ T cells are IL-7 dependent [6], we sought to determine whether administration of excess IL-7 would minimize the competition and improve the proliferation and expansion of pmel-1 T cells. Tumor-bearing mice were irradiated at day 6 and reconstituted with pmel-1 T cells together with congenic spleen cells with or without CD25 and CD122 depletion. Vaccines were given at days 6 and 13 and recombinant human IL-7 was administrated i.p. every day for 5 days. At 3 wk after adoptive transfer, IL-7 administration resulted in marginal, but statistically insignificant, increase in the percentage of pmel-1 T cells in the blood (from 15 to 18%). This number was higher in the blood of mice that received co-transfer of CD25- and CD122-depleted naïve spleen cells (24%). However, IL-7 did not further increase the number of pmel-1 T cells (from 24% to 25%) in mice that received CD25- and CD122-depleted spleen cells (Fig. 5A). Similarly, non-transgenic hgp9-specific T cells were only slightly increased by IL-7 administration. Despite the marginal increase of peptide-specific T cells, IL-7 administration did result in a significant delay of tumor growth (Fig. 5B) and prolonged survival of tumor-bearing mice to the same degree as that produced by depletion of CD25+ and CD122+cells (Fig. 5C). The median survival for the IL-7 group and for the CD25 and CD122 double depletion group was the same (48 days compared with 35 days in the control group). The addition of IL-7 to CD25 and CD122 depletion did not further improve antitumor efficacy. These results strongly suggested that consumption of IL-7 by CD122+ T cells may be one potential limiting factor that restricts Ag-induced proliferation and expansion, and the functional differentiation of pmel-1 T cells. The profound effect on the tumor growth by IL-7 administration is not simply caused by its effect on pmel-1 expansion or survival.
Figure 5.
Administration of exogenous recombinant IL-7 mimics the effect of CD25 and CD122 depletion. Naïve C57BL/6 mice (n=10 per group) were injected with 2×105 B16-F10 tumor cells s.c. at day 0. Five days later, the tumor-bearing mice were sublethally irradiated. At day 6 after F10 inoculation, irradiated mice were adoptively transferred with 104 pmel-1 T cells together with 107 of either congenic splenocytes or with depletion of both CD25+ and CD122+ cells, in conjunction with peptide-pulsed DC vaccination at days 6 and 13 after F10 inoculation. Two days after each vaccination, exogenous protein of IL-7 (1 μg) was administrated IP twice daily five times. (A) Blood samples were collected 3 wk after vaccination, and stained with hgp-9/Db MHC tetramers. The numbers indicate the percentage of tetramer-positive pmel-1+ T cells, or pmel-1− CD8+ T cells from pooled blood of three to five mice. (B) Tumor growth and (C) survival rate of mice. Mice were sacrificed when the tumor size exceeded 200 mm2. Time-to-death (or tumor-free survival, etc.) between groups was compared using the nonparametric log-rank test and median survival time was estimated from the Kaplan–Meier survival curves. Results shown are representative of two independent experiments.
Discussion
A dramatic expansion of Ag-specific CD8+ T cells is usually observed during primary and secondary infections [22, 23]; however, the same type of expansion is rarely seen during tumor progression or after vaccination with tumor-associated Ag. There are too many examples of early and late development of therapeutic cancer vaccines that end up in failure [24]. One might argue that the meager, usually barely detectable, CD8+ T-cell response to tumor Ag is the culprit, and active immunotherapy will be effective only when the antitumor immune response achieves a level comparable to that seen following infection. In contrast to the dismal success of active immunotherapy, adoptive immunotherapy with tumor-reactive T cells after lymphodepletion has yielded exceptionally high rates of tumor regression in patients with advanced melanoma [2]. Therefore, it is reasonable to think that therapeutic cancer vaccines could be effective if the resulting expansion and persistence of tumor-reactive T cells reach the levels of adoptive-transferred T cells in lymphodepleted hosts. Previously, we and others demonstrated that vaccination during reconstitution of lymphodepleted hosts enabled selective expansion from the polyclonal naïve T cell repertoire and long-term survival of tumor-reactive T cells [3–7]. This novel active immunotherapy strategy rekindled the interest in cancer vaccines and provides a strong basis to combine active immunization with adoptive T-cell transfer.
Depletion of Treg and removal of cytokine sinks have been proposed as mechanisms to explain the phenomena that results in the preferential expansion of Ag-specific T cells in the lymphodepleted host [13–15]. Using the same tumor model and pmel-1 TCR transgenic T cells, Restifo’s group showed that the preferential expansion of Ag-induced T-cell responses was primarily due to the removal of γc responsive lymphocytes, including T cells and NK cells, by lymphodepletion, which would effectively reduce their consumption of IL-7 and IL-15 [7]. However, γc deficiency resulted in the complete absence of multiple lymphocyte subsets, and thus the relative contribution of different individual subsets was not addressed. In this report, we used antibody depletion and reconstitution to show that CD4+CD25+ and CD8+CD122+ T cells underwent lymphopenia-driven proliferation, and both populations negatively regulated vaccine-induced expansion and survival of tumor-specific T cells. Although NK cells, NKT cells, and γδ T cells also undergo lymphopenia-driven proliferation, their effect on Ag-induced antitumor CTL responses is less pronounced than that of CD4+CD25+ Treg and CD8+CD122+ Treg. We found that removal of CD4+CD25+ and CD122+CD8+ Treg led to a marked increase in the number and function of tumor-infiltrating T cells, suggesting that Treg may also affect trafficking, secondary expansion of tumor-specific T cells, and their functional differentiation in tumor sites. In an autoimmune diabetes model, CD4+CD25+ T cells also appeared to diminish autoreactive T cells primarily in the target organ [25].
The major finding of the current study was the identification of CD8+CD122+ Treg as another, yet more potent, negative regulator of vaccine-induced expansion and survival of tumor-specific T cells. During acute viral infection, both attrition of memory CD8+ T cells and lymphopenia can be observed and may account for the dramatic expansion of virus-specific CD8+ T cells [26, 27]. The rapid attrition of pre-existent memory-like CD8+ T cells during viral or bacterial infection was thought to be due to the strong type I or II IFN response invoked by viral or bacterial replication [28, 29]. The early attrition of memory-like CD8+ T cells allows more room for the vigorous T-cell expansion and a more diverse T-cell response. It is interesting that our rather serendipitous finding that lymophodepletion enhanced antitumor immune responses [4] was an active strategy utilized by the immune system to combat natural infection. This could also explain why the strong inflammatory response to viral infection, which is missing during tumor progression, is critically important for the rapid expansion of viral Ag-specific effector/memory T cells. Understanding the mechanism by which viruses induce attrition of memory T cells could help us to induce a higher level of antitumor CD8+ T cells without the need for the toxic methods currently used solely for the purpose of lymphodepletion.
Earlier published work and our current study established that CD8+CD122+ Treg are the major population that undergoes lymphopenia-driven proliferation. They may also serve a regulatory function and prevent the development of dangerous self-reactive T cells in the lymphodepleted mice and in the mouse models of EAE and Graves’ hyperthyroidism [20, 30–32]. Recent studies demonstrated the key role of IL-10 produced by CD8+CD122+ Treg in their suppressive function [32–34]. The role of IL-10 in our model needs to be determined. In lymphoreplete mice, CD8+CD122+ Treg and CD4+CD25+ Treg are maintained primarily by IL-15 produced by DC [35] and IL-2 produced by naïve CD4+ T cells, respectively [36]. Our data indicate that both IL-7 and IL-15 are required for the maximum proliferation of CD8+CD122+ Treg in lymphodepleted mice (Supporting Information Fig. 3). Only overexpression of IL-7 but not the normal levels of IL-7 found in IL-15-deficient mice could rescue CD8+CD122+ Treg, strongly suggesting these Treg could act as a cytokine sink in lymphodepleted mice [37, 38]. Recently, it was found that CD8+CD122+ T cells with innate function are enriched in mice lacking the IL-2-inducible T-cell kinase and primarily selected by on hemato-poietic cells in thymus [39–44]. The innate T cells shared same memory T-cell markers with CD8+CD122+ Treg; however, it remains to be determined whether they are functionally similar to NKT cells, i.e. they could play a dual role in both innate immunity and as Treg.
Our study did not differentiate these cells from among all CD122+ T cells. A caveat of our study pertains to the face we relied on the co-transfer of competing cell populations rather than the depletion of endogenous CD122+ cells in a replete host – it was proved to be impossible to deplete endogenous CD122+ cells without affecting expanded pmel-1 T cells that acquired CD122 after activation. Nevertheless, our results do suggest that regulatory CD8+ cells impede the response of tumor reactive cells by competition for limiting cytokines (especially IL-7).
Another interesting observation is that depletion of CD122+ cells from spleen cells co-transferred with pmel-1 cells showed a dramatic effect on tumor growth (Fig. 3C). However, depletion of CD122+ cells increased the number of pmel-1 cells only at the peak of expansion (2 wk after tumor inoculation); no significant difference of pmel-1 cell number was observed at 3–4 wk after tumor inoculation (Fig. 1A), when tumor growth was most critically affected (Fig. 1C). This result indicates that there was not only a quantitative change but also some qualitative change that occurred in pmel-1 cells, which was caused by the depletion of CD122+ cells. Consistent with this notion, significantly more IFN-γ-producing pmel-1 T cells and endogenous peptide-specific T cells were found in tumor sites from mice that received CD25 and CD122 double depletion (Fig. 4D). This qualitative change might be due to better differentiation of effector/memory T cells in tumor sites after depletion of Treg. This result might not be readily explained by the disappearance of simple competition for IL-7 between pmel-1 cells and CD122+ cells. However, our data did suggest that a large amount of exogenous IL-7 (1 μg×10 times, Fig. 5) could mimic certain aspects of CD25 and CD122 depletion. The administration of a super-physiological amount of IL-7 could have also resulted in other qualitative changes in pmel-1 cells.
Together with recent findings that CD122+CD8+ Treg can suppress autoimmunity in the murine Graves’ hyperthyroidism and EAE model independent of lymphopenia-driven proliferation [31, 32], our results indicate that, like CD25+CD4+ Treg, CD122+CD8+ Treg are in fact another group of bona fide natural Treg, whose immune regulatory functions and suppressive mechanisms are waiting to be exploited in the near future.
Materials and methods
Mice
Mice were purchased from the Jackson Laboratory (Bar Harbor, Main) and from Charles River Laboratories (Wilmington, MA). Pmel-1 transgenic mice, Pmel-1 and GFP double transgenic mice, and IL-15 knockout mice (IL-15−/− ) were described before [6]. All animal protocols were approved by the Earle A. Chiles Research Institute Animal Care and Use Committee.
DC preparation and loading of peptide
DC were generated and isolated as described previously [6]. Briefly, bone marrow cells were isolated and cultured in complete media supplemented with murine GM-CSF (50 ng/mL) for 8–10 days. Expanded cells were harvested and frozen in mulitple aliquots in LN2. Frozen DC were rapidly thawed at 37°C and pulsed for 2–4 h at 37°C with 10 μg/mL of the appropriate peptide in complete medium. In all experiments, the H-2Db-restricted human gp100 (KVPRNQDWL; hgp-9) was used. Loaded DC were washed with PBS before injection.
Immunotherapy of melanoma-bearing mice with irradiation, adoptive T-cell transfer, and vaccination
The detailed immunotherapy protocol has been described elsewhere [6]. Briefly, C57BL/6 mice subcutaneously injected with B16F10 melanoma were subjected to whole body irradiation (500 Gy) on day 5, and adoptively transferred with naïve spleen cells from mice as indicated. In some experiments, CD25+ cells alone or together with NK cells or CD122+ cells (including T cells and NK cells) were depleted using biotin-conjugated anti-CD25, anti-CD122, or anti-NK1.1 antibodies and strepavidin-conjugated MACS MicroBeads (Milenyi Biotec) before adoptive transfer into tumor-bearing mice (n=5–8 per group). Adoptive transfer was followed immediately by s.c. vaccination with 1~2×106 DC pulsed with hgp-9 peptide. In some experiments, additional DC vaccinations were administrated at indicated intervals. Tumor size was measured three times a week, and mice were sacrificed when one diameter exceeded 150 mm. All experiments were carried out in a blinded and randomized fashion. In some experiments, IL-7 was blocked by injection of mice with 1 mg purified monoclonal anti-IL-7 antibody (clone M25).
Flow cytometry
Single-cell suspensions prepared from blood, spleen, or F10 tumor tissues were stained with APC-labeled anti-CD8 and PE–labeled anti-CD45.1 antibodies (eBiosciences, San Diego, CA) for FACS® analysis. Pmel-1 transgenic T cells were gated on GFP and CD8 double-positive populations (GFP+CD8+CD45.1−). GFP-CD8+CD45.1+ cells were the adoptively transferred congenic T cells, whereas GFP-CD8+ CD45.1− cells are repopulated host T cells after irradiation. At least 20 000 live cell events, gated using scatter plots, were analyzed for each sample. In some experiments, APC-labeled hgp-9/H-2Db MHC tetramer was used to stain peptide-specific cells (obtained from the NIH tetramer core facility).
For cell division analysis, spleen cells were labeled with CFSE (5 μmol/L) according to the suggested protocol from Molecular Probes (Eugene, OR). For pmel-1 T cells functional analysis, single-cell suspensions prepared from blood, spleen, or F10 tumor tissues were stimulated for 6 h in medium containing 1 μg/mL hgp-9, 5 μg/mL anti-CD3 Ab, 1 μg/mL TRP2, or CM alone respectively, and then cells were harvested to stain for intracellular IFN-γ. Flow cytometric analysis was done with the FACSCalibur and Cellquest software (Becton Dickinson, Mountain View, CA).
Statistical analysis
Log-rank nonparametric analysis was used to analyze the tumor-free survival data. Each group consisted of at least six mice, and no animal was excluded from the statistical evaluation. Student’s t-test was used to analyze the number of T cells and percentage of T cells producing IFN-γ. A two-sided p<0.05 was considered significant.
Supplementary Material
Acknowledgements
This work was supported by the Providence Portland Medical Foundation, the M. J. Murdock Charitable Trust, the American Cancer Society research scholar grant LIB-106810 (HMH), Human Services Public Health Service grant R01 CA107243 (H.M.H.), and National Natural Science Foundation of China (L.W. and H.M.H.) (grant number 30771999).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Supporting Information available online
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang J, Morgan RA, Dudley M. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 5.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur. J. Immunol. 2003;33:2123–2132. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 6.Wang LX, Li R, Yang G, Lim M, O’Hara A, Chu Y, Fox BA, et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 11.Surh C, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J. Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 13.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+ CD25+ regulatory T cells. Nat. Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 16.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J. Exp. Med. 2003;197:451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin. Immunol. 2005;17:231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Fry TJ, Mackall CL. Immune reconstitution following hemato-poietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005;35:S53–S57. doi: 10.1038/sj.bmt.1704848. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, Shi Z, Okuno Y, Isobe K. Are CD8+ CD122+cells regulatory T cells or memory T cells? Human Immunology. 2008;69:751–754. doi: 10.1016/j.humimm.2008.08.285. [DOI] [PubMed] [Google Scholar]
- 21.Curiel TJ. Regulatory T cells and treatment of cancer. Curr. Opin. Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doherty PC, Christensen JP. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 23.Pamer EG. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 24.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat. Biotechnol. 2009;27:129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+T reg cells impinge on autoimmune diabetes. J. Exp. Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- 29.Dudani R, Murali-Krishna K, Krishnan L, Sad S. IFN-gamma induces the erosion of preexisting CD8 T cell memory during infection with a heterologous intracellular bacterium. J. Immunol. 2008;181:1700–1709. doi: 10.4049/jimmunol.181.3.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+regulatory T cells in the maintenance of T cell homeostasis. J. Exp. Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology. 2007;148:6040–6046. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 32.Lee YH, Ishida Y, Rifa’i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 33.Endharti AT, Rifa’I M, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, et al. Cutting edge: CD8+CD122+regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+T cells. J. Immunol. 2005;175:7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 34.Rifa’i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int. Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 35.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naïve and memory CD8+T cells. J. Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+T cells. J. Exp. Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+cells but are not required for memory phenotype CD4+cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubois S, Waldmann TA, Müller JR. ITK and IL-15 support two distinct subsets of CD8+T cells. Proc. Natl. Acad. Sci. USA. 2006;103:12075–12080. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atherly LO, Brehm MA, Welsh RM, Berg LJ. Tec kinases Itk and Rlk are required for CD8+T cell responses to virus infection independent of their role in CD4+T cell help. J. Immunol. 2006;176:1571–1581. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- 41.Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8+T cells with innate function selectively develop in the absence of active Itk. Eur. J. Immunol. 2007;37:2892–2899. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, August A. Naïve and innate memory phenotype CD4+T cells have different requirements for active Itk for their development. J. Immunol. 2008;180:6544–6552. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prince AL, Yin CC, Enos ME, Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate conventional versus innate T-cell development. Immunol. Rev. 2009;228:115–131. doi: 10.1111/j.1600-065X.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Readinger JA, Mueller KL, Venegas AM, Horai R, Schwartzberg PL. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 2009;228:93–114. doi: 10.1111/j.1600-065X.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.