Abstract
Background: High rates of fracture following liver transplantation were reported in earlier years, but the impact of subsequent changes in immune suppression and the introduction of bone-protective therapy on fracture rate have not been reported.
Aim: The aim of this study was to document clinical fracture incidence during the period 1998–2008 in a single transplant centre, following the introduction of a bone management protocol.
Design: It was designed as a retrospective cohort.
Methods: Records were retrieved from 531 of 592 eligible patients in an audit of all patients undergoing a first liver transplant during the 10-year period. All fractures were verified radiologically.
Results: The mean follow-up period was 61.4 months. Prior to transplantation 5.6% of patients had a history of fracture. Incident clinical fractures following transplantation were recorded in just 15 (3.5%) patients. The most common fracture site was the spine and the median time from transplant to fracture was 26 months (range 2–83 months).
Conclusion: There was a low fracture rate in patients undergoing liver transplantation in this centre over the past 10 years. This rate is lower than that in previous reports, which is likely to reflect the use of lower doses of prednisolone for immune suppression and the administration of bone-protective therapy to high-risk patients.
Introduction
Following the introduction of liver transplantation as a treatment for end-stage liver disease, a high incidence of osteoporosis was reported post-operatively. High rates of bone loss and fracture were documented in the first 6–12 months post-transplant, resulting in significant morbidity.1–17 Many risk factors for bone disease were recognized, including glucocorticoid therapy and other immunosuppressive agents, vitamin D insufficiency, poor nutrition, hypogonadism and pre-existing bone disease.
Since these early studies were reported, there have been a number of changes in the management of patients undergoing liver transplantation. In particular, the dose and duration of glucocorticoid therapy for immunosuppression have been reduced substantially and many centres now have management protocols to identify and treat individuals at risk of fracture, both before and after transplantation. However, the impact of such measures on fracture incidence has not been reported formally. The aim of this study was to examine fracture incidence in our Liver Transplant Unit over a 10-year period, following the introduction of a protocol for bone health assessment and management in patients undergoing liver transplantation.
Materials and methods
An audit was performed at the Liver Transplant Unit at Addenbrooke’s Hospital, Cambridge, UK. This was approved by the Addenbrooke’s Hospital Clinical Governance and Audit Department. All patients who underwent their first deceased donor orthotopic liver transplantation (OLT) between 2 January 1998 and 31 December 2007 were included. This period was chosen to match the introduction of bone health assessment and treatment of osteoporosis. Patients who had undergone liver transplantation prior to that date were excluded even if a subsequent transplant was undertaken between 1998 and 2007. The follow-up time was set from 2 January 1998 to 31 December 2008 and the audit was performed between 2 January 2009 and 4 June 2010.
Data were retrieved from the patients’ hospital notes, the liver transplant unit database and Addenbrooke’s Hospital electronic records (eMR and iSOFT). All notes were reviewed carefully for data on age at time of transplant, height, weight, gender, alcohol use, tobacco use, underlying liver disease, use of bone-protective medication, calcium and vitamin D supplements, glucocorticoid and other immunosuppressive drugs and a history of fractures. Bone mineral density (BMD) in the lumbar spine (L1–L4) and proximal hip was measured by dual energy X-ray absorptiometry (DXA) using a Hologic bone densitometer (Hologic QDR 4500A, Hologic Inc., Bedford, MA, USA). The short-term in vivo precision of measurement at these two sites is 1 and 1–2%, respectively. Total hip BMD T-scores were calculated using data from the National Health and Nutrition Examination Survey (NHANES) reference female population. For fractures reported in the patient records, confirmation was obtained from an X-ray report. In addition, all available lateral spine X-ray reports were reviewed.
The Liver Transplant Bone Protocol, introduced in 1997, consists of a bone health assessment pre-transplant including BMD measurement by DXA and a lateral spine X-ray. For any patient with a BMD T-score <−1.5 and/or a previous fracture bisphosphonate therapy is recommended with repeat BMD measurement in 1 year. Subsequently, bisphosphonate use is reviewed and maintained if BMD remains low and/or glucocorticoid therapy is continued beyond 1 year. Calcium and vitamin D supplements are recommended for vitamin D-deficient subjects.
Statistical analysis
The descriptive statistic is presented as mean [standard deviation (SD)], median [inter-quartile range (IQR)] or n/n total (percent). Kaplan–Meier statistic was used to estimate the incidence of fractures. The changes in BMD measurements before transplant and between 24 and 48 months after the transplant were evaluated by paired Student’s t-test. Additionally, Fisher exact test, χ2-test, Student’s t-test and Mann–Whitney test were used to compare the differences between groups. Differences were considered statistically significant when the two-tailed P-value was <0.05. The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS®) for Windows® version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Among the patients undergoing liver transplantation between 2 January 1998 and 31 December 2007 at Addenbrooke’s Hospital, 592 subjects were eligible to be included in this audit. We were able to retrieve the notes from 446 (96.7%) patients who are still alive and 85 (64.9%) of those who died during the follow-up period. Of these patients, 49 included in the audit underwent a second transplant during the study period. There were no significant differences in age, gender or BMD between the deceased and non-deceased subjects.
Characteristics of the patients at the time of transplantation are shown in Table 1. BMD was measured in 67.4% of subjects before the transplant and a spine X-ray was performed in 86.1%. Osteoporosis (BMD T-score ≤−2.5 in the hip and/or spine) was diagnosed in 18% of the patients in whom BMD was measured. Prior to liver transplantation, 6.1% of patients were on bone-protective medication and 15.8% were on calcium and vitamin D supplements. A history of previous fracture was reported in 5.6% of the patients. These data are shown in Table 2.
Table 1.
Characteristics of audited patients at time of transplantation
| Characteristic | n/n total (%)* |
|---|---|
| Age, mean (SD) (years) | 51.7 (12.2) |
| Height, mean (SD) (m) | 1.71 (0.10) |
| Weight, mean (SD) (kg) | 77.12 (17.03) |
| BMI, mean (SD) (kg/m2) | 26.39 (4.84) |
| Gender | |
| Males | 327/531 (61.6) |
| Females | 204/531 (38.4) |
| Current use of alcohol | 103/483 (21.3) |
| Current smoking | 90/459 (19.6) |
| Aetiology of the transplant | |
| Alcohol-related cirrhosis | 134/531(25.2) |
| Primary sclerosing cholangitis | 51/531 (9.6) |
| Chronic hepatitis C virus infection | 49/531 (9.2) |
| Primary biliary cirrhosis | 46/531 (8.7) |
| Primary hepatocellular carcinoma with cirrhosis | 44/531(8.3) |
| Acute/subacute fulminant hepatic failure | 41/531 (7.7) |
| Cryptogenic cirrhosis | 30/531 (5.6) |
| Other | 136/531 (25.8) |
*Values are represented as n/n total (%) unless otherwise noted.
Table 2.
Bone assessment prior to transplant
| Characteristic | n/n total (%) |
|---|---|
| Bone densitometry (DXA) | 356/528 (67.4) |
| T-score ≤ −1.5 | 167/350 (47.7) |
| Osteoporosis (T-score ≤ −2.5) | 63/350 (18) |
| Use of bone medication | 31/507 (6.1) |
| Type of bone medication | |
| Bisphosphonates | 28/31 (90.3) |
| HRT | 2/31 (6.5) |
| Raloxifene | 1/31 (3.2) |
| Use of calcium plus vitamin D | 80/507 (15.8) |
| Use of glucocorticoids | 29/498 (5.8) |
| Lateral spine X-ray | 432/502 (86.1) |
| Fracture pre-transplant | 28/498 (5.6) |
| Pre transplant fracture site | |
| Spine | 19/28 (67.9) |
| Wrist | 2/28 (7.1) |
| Lower leg | 2/28 (7.1) |
| Clavicle | 1/28 (3.6) |
| Ribs | 1/28 (3.6) |
| Unknown | 3/28 (10.7) |
During the follow-up period, 22.1% of the patients died (mean 61.4 months, range 0–131 months). Prednisolone was prescribed in 97.8% of patients and the median prednisolone regimen duration was 3 months (range 0–120 months). The other most commonly prescribed immunosuppressive drugs were tacrolimus and azathioprine. These data are shown in Table 3.
Table 3.
Characteristics of audited patients after post-transplant
| Characteristic | n/n total (%)* |
|---|---|
| Follow-up time, mean (SD) (months) | 61.4 (34.9) |
| Deceased | 131/592 (22.1) |
| Pulsed methylprednisolone use | 98/482 (20.3) |
| Prednisolone use | 494/505 (97.8) |
| Prednisolone regimen duration, median (IQR) (months) | 3 (3–5) |
| Tacrolimus use | 479/528 (90.7) |
| Azathioprine use | 488/526 (92.8) |
| Sirolimus use | 136/526 (25.9) |
| Mycophenolate use | 60/526 (11.4) |
| Cyclosporine use | 58/527 (11.0) |
| Use of other immune suppressive agents | 7/526 (1.3) |
| Liver re-graft (re-transplant) | 49/531 (9.2) |
*Values are represented as n/n total (%) unless otherwise noted.
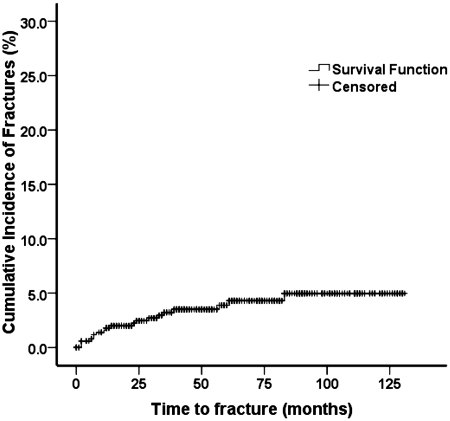
The cumulative incidence of fracture in the follow-up period is shown in Figure 1. During this period 20 clinical fractures were recorded (Tables 4 and 5) in 19 patients. One subject presented with a spine fracture and a rib fracture at two different time points. The median time from transplant to fracture was 12 months (range 2–83 months). Fifty-five percent of incident fractures were vertebral fractures (Table 5).
Figure 1.
Incidence of fracture after the orthotopic liver transplant.
Table 4.
Bone assessment post-transplant
| Characteristic | n/n total (%)* |
|---|---|
| Incident fracture post-transplant | 19/520 (3.7) |
| Median (IQR) months to fracture | 12 (6.5–34) |
| Mean difference (95% CI) in BMD at 24–48 months (T-score; n = 52) | |
| Spine | −0.64 (−0.89, −0.40) |
| Total hip | 0.06 (−0.3, 0.25) |
| Use of bone medication after transplant | 155/526 (29.5) |
| Bisphosphonates | 144/154 (93.5) |
| HRT | 6/154 (3.9) |
| Raloxifene | 2/154 (1.3) |
| Strontium | 2/154 (1.3) |
| Use of calcium plus vitamin D post-transplant | 198/524 (37.8) |
*Values are represented as n/n total (%) unless otherwise noted.
Table 5.
Post-transplant fracture site (n = 20)
| Fracture site | n (%) |
|---|---|
| Spine | 11 (55) |
| Wrist | 2 (10) |
| Humerus | 1 (5) |
| Hip | 1 (5) |
| Lower leg | 1 (5) |
| Hand | 1 (5) |
| Ribs | 1 (5) |
| Sacrum | 1 (5) |
| Ischial tuberosity | 1 (5) |
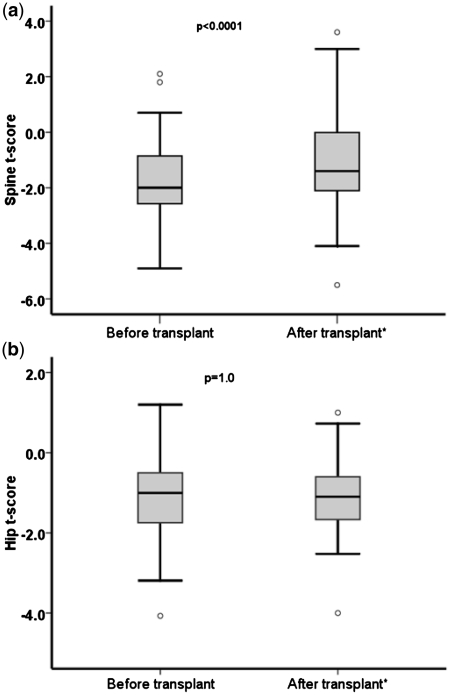
At least one BMD measurement was performed post-transplant in 48.3% of the subjects. A new diagnosis of osteoporosis was made in 1.7% of these individuals and the overall prevalence of osteoporosis after transplant was 17.7%. BMD measurements before and between 24 and 48 months after transplant were available in 52 patients (Figure 2). There was a significant increase in the spine T-score after transplant [–1.73 (SD 1.39) vs. –1.09 (SD 1.62), pre-transplant vs. post-transplant, P < 0.0001], but no significant change in total hip T-score [−1.05 (SD 1.02) vs. −1.11 (SD 0.96), P = 1.0] (Table 4).
Figure 2.
Lumbar spine (a) and total hip (b) BMD T-score before and after liver transplant (n = 52).
Bone-protective medication was taken by 29.5% of patients after transplantation (Table 4). These patients were more likely to be females (49 vs. 33.4%, P < 0.0001) and to have a T-score lower than −1.5 at the hip and/or spine at any time (73.9 vs. 32.3%, P < 0.0001) (data not shown). Similarly, calcium and vitamin D supplementation, taken by 37.8% of subjects post-transplant, was more common in women (48.5 vs. 32.5%, P < 0.0001) and in patients with a T-score lower than −1.5 at the hip and/or spine at any time (68.7 vs. 32.5%, P < 0.0001).
Subjects with incident fracture were older (P < 0.01), were more commonly women (P < 0.01) and had taken prednisolone for longer post-transplant (P < 0.01). Additionally, bone-protective medication (P < 0.05) and calcium plus vitamin D (P < 0.01) were more likely to be prescribed after an incident fracture (Table 6). There were no significant differences between the subjects with and without incident fracture after Bonferroni correction for multiple comparisons.
Table 6.
Comparison between patients with and without incident fractures
| Characteristic | With incident fracture | Without incident fracture | P-value |
|---|---|---|---|
| Age, mean (SD) (years) | 56.7 (8.5) | 51.5 (12.3) | <0.01 |
| BMI, mean (SD) (kg/m2) | 26.6 (4.7) | 26.3 (4.9) | NS |
| Gender: females | 13/19 (68.4) | 312/499 (62.3) | <0.01 |
| Current use of alcohol | 2/18 (11.1) | 97/454 (21.4) | NS |
| Current smoking | 5/16 (31.2) | 84/432 (19.4) | NS |
| Use of glucocorticoids pre-transplant | 0/18 (0.0) | 29/470 (6.2) | NS |
| Use of bone medication pre-transplant | 1/19 (5.3) | 30/477 (6.3) | NS |
| Use of calcium plus vitamin D pre-transplant | 4/19 (21.1) | 76/477 (15.9) | NS |
| BMD T-score ≤−1.5 pre-transplant | 7/13 (53.8) | 157/333 (47.1) | NS |
| BMD T-score ≤−2.5 pre-transplant | 4/13 (30.8) | 59/333 (17.7) | NS |
| Fracture pre-transplant | 3/19 (15.8) | 24/476 (5.1) | NS |
| Liver re-graft | 2/19 (10.5) | 44/499 (8.8) | NS |
| Deceased | 4/19 (21.1) | 78/499 (15.6) | NS |
| Use of pulse methylprednisolone post-transplant | 2/17 (11.8) | 95/454 (20.9) | NS |
| Prednisolone regimen duration (months) post-transplant | 4 (Quartiles 3, 10.5) | 3 (Quartiles 3, 5) | <0.05 |
| Tacrolimus use post-transplant | 17/19 (89.5) | 454/498 (91.2) | NS |
| Azathioprine use post-transplant | 18/19 (94.7) | 460/496 (92.7) | NS |
| Sirolimus use post-transplant | 7/19 (36.8) | 128/496 (25.8) | NS |
| Mycophenolate use post-transplant | 2/19 (10.5) | 57/496 (11.5) | NS |
| Cyclosporine use post-transplant | 3/19 (15.8) | 52/497 (10.5) | NS |
| Use of calcium plus vitamin D post-transplant | 13/19 (68.4) | 182/496 (36.7) | <0.01 |
| Use of bone medication post-transplant | 10/19 (52.6) | 141/498 (28.3) | <0.05 |
Values are represented as n/n total (%) unless otherwise noted. NS = non significant.
Discussion
The results demonstrate a low incidence of fracture following liver transplantation in this centre over the past 10 years. In common with other groups, we had reported a high incidence of fractures in the early post-operative period; 27% of 37 adults undergoing liver transplantation between 1993 and 1995 developed new vertebral fractures within the first three post-operative months.14 In contrast, this audit of the years 1998–2008 demonstrated a fracture incidence post-transplant of only 3.7%.
The reasons for the lower fracture incidence in recent years cannot be defined precisely on the basis of this audit, but are likely to include reduction in the dose and duration of glucocorticoid therapy and the use of bone-protective therapy in individuals at high risk of fracture. In the early days of liver transplantation, very large doses of prednisolone were used for prolonged periods, whereas much lower doses are used now and for shorter periods. For example, in the early 1990s the starting dose of prednisolone used in most patients in the 1980s was 60 mg/day falling to 30 mg/day in the early 1990s and this was continued for 6 months with gradual reductions thereafter. In contrast, in current practice the initial dose is 20 mg/day with the aim of stopping glucocorticoids between 6 and 12 weeks for patients transplanted for conditions other than autoimmune liver disease. Further, some patient groups such as those transplanted with hepatitis C virus infection are not treated with glucocorticoids at all. Secondly, an established standard protocol for bone health assessment prior to transplantation identifies individuals at high risk of fracture for bone-protective therapy before and after transplant. Both these changes in clinical practice are likely to have contributed to the low-fracture incidence post-transplant.
In addition, it is possible that improved nutritional support prior to and after transplantation may have played a role. Evidence for better bone health prior to transplant can be obtained from a comparison of the prevalence of osteoporosis (BMD T-score ≤ −2.5) in earlier and later studies from this centre. Thus, we reported osteoporosis in 36.6% of 243 consecutive patients undergoing liver transplantation in the early to mid-1990s,18 whereas osteoporosis was present in only 18% of patients pre-transplant in the present study. Furthermore, the prevalence of fracture prior to transplantation was low (5.6%) in this study.
In the general population, the use of clinical risk factors in addition to BMD is widely used to improve prediction of fracture risk, using tools such as FRAX®, the WHO-supported risk algorithm.19–22 While this approach is reasonably well validated at a population level, risk factors for fracture post-transplantation are less clearly documented. Older age,8,15,23 the presence of a prevalent vertebral fracture prior to transplantation8,15 and in some studies, chronic cholestatic liver disease,3,13 have been associated with increased fracture risk in patients undergoing liver transplantation, but a clear relationship with BMD has not emerged from most studies. The contribution of other clinical risk factors such as smoking, alcohol abuse and a parental history of a fractured hip to fracture risk in the transplant population has not been assessed and requires further study. However, at present it seems reasonable to recommend bone-protective therapy in all patients with a previous history of fragility fracture and/or those with low BMD, although the threshold for the latter is arbitrary and to some extent age dependent. The T-score of ≤−1.5 chosen in this centre was based on the UK guidelines for glucocorticoid therapy;24 however, because of the strong independent effect of age on fracture risk, treatment might be advised at a higher BMD in elderly subjects and a lower BMD in younger subjects.
Although a standard management protocol was in place during the period of the audit, not all individuals who fulfilled the criteria for bone-protective therapy received treatment. Thus, ∼30% of patients received treatment post-transplant, while 47.7% had a BMD T-score ≤−1.5 before the transplant. We were not able to identify the reasons for this apparent under-treatment in the audit; only oral bisphosphonates were approved for osteoporosis during almost the whole period of the audit and contraindication to or intolerance of oral bisphosphonate therapy is likely to have prevented treatment in some cases, particularly those with a history of haemorrhage from oesophageal varices. In the relatively small number of cases in whom BMD measurements were available before and 24–48 months after transplantation, there was a significant increase in BMD in the spine, but not the hip. Other studies have mostly demonstrated an increase in BMD after the first year or so after transplantation, although recovery in the hip may be slower than in the spine.16,25,26
A number of studies have investigated the effect of bone-protective therapy in patients undergoing liver transplantation.17,27–36 However, these have often been relatively small and not all have been randomized or controlled; while beneficial effects on BMD have been demonstrated in some, none has been adequately powered to demonstrate reduction in fracture. Most data exist for the bisphosphonates pamidronate, alendronate and zoledronate, all of which have been shown to reduce or prevent bone loss in the spine and in most cases, also the proximal femur. Of these, zoledronate may be the most appropriate treatment option in many of the liver transplant population in view of its intravenous mode of administration and infrequent dosing regimen. However, although untested in the transplant population, once yearly infusion of 5 mg is likely to be adequate37 rather than the more frequent dosing regimens used in the studies reported to date.34,35
Our study has several limitations. It was not possible to retrieve all the medical records of patients undergoing liver transplant during the audit period, particularly of those who had died. For those in whom records were available, there were some missing data, for example, only about two-thirds and one-half, respectively, of patients had BMD measurement before and after transplantation. Similarly, only 59 patients had a spine X-ray both before and after transplantation and since the majority of vertebral fractures are asymptomatic,38 it is possible that the incidence of vertebral fractures was underestimated. Finally, we relied on medical records and X-ray reports for fracture diagnosis and this provides another possible reason for underestimating fracture incidence.
In summary, our audit has demonstrated a low incidence of clinical fracture in patients undergoing liver transplantation in our unit over the period 1998–2008. This is likely to reflect reduction in the total dose of glucocorticoids used for immune suppression and the use of bone-protective therapy in individuals at high risk of fracture. Bisphosphonates provide a rational approach to the prevention of fracture in the transplant population; because of its low cost, alendronate is the front-line option, but intravenous zoledronate should be considered in patients who cannot take or are intolerant to alendronate and in those unlikely to be compliant with long-term oral bisphosphonate therapy. The optimal duration of therapy has not been defined but in view of the tendency for BMD to improve 1–2 years post-transplant and the prolonged action of zoledronate, one dose may be sufficient for many patients.
Funding
National Institute of Health Research; Cambridge Biomedical Research Centre; CAPES foundation, Ministry of Education, Brazil (Grant number 1204-08-4 to M.O.P.).
Conflict of interest: None declared.
Acknowledgements
The authors acknowledge all people whose help made this audit possible.
References
- 1.Compston JE. Osteoporosis after liver transplantation. Liver Transplant. 2003;9:321–30. doi: 10.1053/jlts.2003.50044. [DOI] [PubMed] [Google Scholar]
- 2.Weaver GA, Franck WA, Streck WF, Starzl TE. Hepatic osteodystrophy after liver transplantation in a patient with primary biliary cirrhosis. Am J Gastroenterol. 1983;78:102–6. [PubMed] [Google Scholar]
- 3.Haagsma EB, Thijn CJP, Post JG, Slooff MJH, Gips CH. Bone disease after orthotopic liver transplantation. J Hepatol. 1988;6:94–100. doi: 10.1016/s0168-8278(88)80467-7. [DOI] [PubMed] [Google Scholar]
- 4.Porayko MK, Wiesner RH, Hay JE, Krom RAF, Dickson ER, Beaver S, et al. Bone disease in liver transplant recipients: incidence, timing, and risk factors. Transplant Proc. 1991;23:1462–5. [PubMed] [Google Scholar]
- 5.Eastell R, Dickson ER, Hodgson SF, Wiesner RH, Porayko MK, Wahner HW, et al. Rates of vertebral bone loss before and after liver transplantation in women with primary biliary cirrhosis. Hepatology. 1991;14:296–300. [PubMed] [Google Scholar]
- 6.McDonald JA, Dunstan CR, Dilworth P, Sherbon K, Sheil AGR, Evans RA, et al. Bone loss after liver transplantation. Hepatology. 1991;14:613–19. doi: 10.1016/0270-9139(91)90047-y. [DOI] [PubMed] [Google Scholar]
- 7.Arnold JC, Hauser D, Ziegler R, Kommerell B, Otto G, Theilmann L, et al. Bone disease after liver transplantation. Transplant Proc. 1992;24:2709–10. [PubMed] [Google Scholar]
- 8.Meys E, Fontanges E, Fourcade N, Thomasson A, Pouyet M, Delmas PD. Bone loss after orthotopic liver transplantation. Am J Med. 1994;97:445–50. doi: 10.1016/0002-9343(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 9.Hussaini SH, Oldroyd B, Stewart SP, Roman F, Smith MA, Pollard S, et al. Regional bone mineral density after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 1999;11:157–63. doi: 10.1097/00042737-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Leidig-Bruckner G, Hosch S, Dodidou P, Ritschel D, Conradt C, Klose C, et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357:342–7. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 11.Abdelhadi M, Eriksson SA, Eriksson SL, Ericzon BG, Nordenstrom J. Bone mineral status in end-stage liver disease and the effect of liver transplantation. Scand J Gastroenterol. 1995;30:1210–15. doi: 10.3109/00365529509101633. [DOI] [PubMed] [Google Scholar]
- 12.Floreani A, Fries W, Luisetto G, Burra P, Fagiuoli S, Boccagni P, et al. Bone metabolism in orthotopic liver transplantation: a prospective study. Liver Transplant Surg. 1998;4:311–19. doi: 10.1002/lt.500040413. [DOI] [PubMed] [Google Scholar]
- 13.Keogh JB, Tsalamandris C, Sewell RB, Jones RM, Angus PW, Nyulasi IB, et al. Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition. 1999;15:661–4. doi: 10.1016/s0899-9007(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 14.Navasa M, Monegal A, Guanabens N, Peris P, Rimola A, Munoz-Gomez J, et al. Bone fractures in liver transplant patients. Brit J Rheumatol. 1994;33:52–55. doi: 10.1093/rheumatology/33.1.52. [DOI] [PubMed] [Google Scholar]
- 15.Ninkovic M, Skingle SJ, Bearcroft PWP, Bishop NJ, Alexander GJM, Compston JE. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2000;12:931–5. doi: 10.1097/00042737-200012080-00013. [DOI] [PubMed] [Google Scholar]
- 16.Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba MJ, et al. Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int. 2001;12:484–92. doi: 10.1007/s001980170094. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins FG, Leon M, Lopez MB, Valero MA, Larrodera L, Garcia-Garcia I, et al. Bone loss and turnover in patients with liver transplantation. Hepato-Gastroenterol. 1994;41:158–61. [PubMed] [Google Scholar]
- 18.Ninkovic M, Love S, Tom BDM, Alexander GJM, Compston JE. High prevalence of osteoporosis in patients with chronic liver disease prior to transplantation. Calcif Tissue Int. 2001;69:321–6. doi: 10.1007/s00223-001-2028-4. [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA, Oden A, Johnell O, Johansson H, DeLaet C, Brown J, et al. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–36. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, Oden A, Johnell O, Johansson H, DeLaet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA on behalf of the World Health Organization Scientific Group. Technical Report. WHO Collaborating Centre, University of Sheffield, UK; 2008. Assessment of osteoporosis at the primary health-care level. [Google Scholar]
- 22.Compston J. Recent advances in the management of osteoporosis. Clin Med. 2009;9:565–9. doi: 10.7861/clinmedicine.9-6-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taillandier J, Alemanni M, Samuel D, Bismuth H. Rheumatic complications following liver transplantation. Transplant Proc. 1999;31:1717–18. doi: 10.1016/s0041-1345(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 24.Guidelines Working Group for the Bone and Tooth Society, National Osteoporosis Society and Royal College of Physicians. Glucocorticoid-induced Osteoporosis: Guidelines for Prevention and Treatment. London: Royal College of Physicians; 2002. [Google Scholar]
- 25.Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transplant. 2006;12:1390–402. doi: 10.1002/lt.20874. [DOI] [PubMed] [Google Scholar]
- 26.Hamberg SM, Piers DA, van den Berg AP, Slooff MJH, Haagsma EB. Bone mineral density in the long term after liver transplantation. Osteoporos Int. 2000;11:600–6. doi: 10.1007/s001980070081. [DOI] [PubMed] [Google Scholar]
- 27.Valero MA, Loinaz C, Larrodena L, Leon M, Moreno E, Hawkins F. Calcitonin and bisphosphonate treatment in bone loss after liver transplantation. Calcif Tissue Int. 1995;57:15–19. doi: 10.1007/BF00298990. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus R, Lohmann R, Platz KP, Guckelberger O, Schon M, Lang M, et al. Treatment of osteoporosis after liver transplantation. Transplant Proc. 1995;27:1226–7. [PubMed] [Google Scholar]
- 29.Hay JE, Malinchoc M, Dickson ER. A controlled trial of calcitonin therapy for the prevention of atraumatic fractures in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 2001;34:292–8. doi: 10.1016/s0168-8278(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 30.Reeves HL, Francis RM, Manas DM, Hudson M, Day CP. Intravenous bisphosphonate prevents symptomatic osteoporotic vertebral collapse in patients after liver transplantation. Liver Transplant Surg. 1998;4:404–9. doi: 10.1002/lt.500040508. [DOI] [PubMed] [Google Scholar]
- 31.Ninkovic M, Love S, Tom BDM, Bearcroft PWP, Alexander GJM, Compston JE. Lack of effect of intravenous pamidronate on fracture incidence and bone mineral density after orthotopic liver transplantation. J Hepatol. 2002;37:93–100. doi: 10.1016/s0168-8278(02)00100-9. [DOI] [PubMed] [Google Scholar]
- 32.Millonig G, Graziadei IW, Eichler D, Pfeiffer KP, Finkenstedt G, Muehllechner P, et al. Alendronate in combination with calcium and vitamin D prevents bone loss after orthotopic liver transplantation. Liver Transplant. 2005;11:960–6. doi: 10.1002/lt.20466. [DOI] [PubMed] [Google Scholar]
- 33.Atamaz F, Hepguler S, Akyildiz M, Karasu Z, Kilic M. Effects of alendronate on bone mineral density and bone metabolic markers in patients with liver transplantation. Osteoporos Int. 2006;17:942–9. doi: 10.1007/s00198-006-0082-5. [DOI] [PubMed] [Google Scholar]
- 34.Crawford BA, Kam C, Pavlovic J, Byth K, Handelsman DJ, Angus PW, et al. Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:239–48. doi: 10.7326/0003-4819-144-4-200602210-00005. [DOI] [PubMed] [Google Scholar]
- 35.Misof BM, Bodingbauer M, Roschger P, Wekerle T, Pakrah B, Haas M, et al. Short-term effects of high-dose zoledronic acid treatment on bone mineralisation density distribution after orthotopic liver transplantation. Calcif Tissue Int. 2008;83:167–75. doi: 10.1007/s00223-008-9161-2. [DOI] [PubMed] [Google Scholar]
- 36.Kaemmerer D, Lehmann G, Wolf G, Settmacher U, Hommann M. Treatment of osteoporosis after liver transplantation with ibandronate. Transplant Int. 2010;23:753–9. doi: 10.1111/j.1432-2277.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- 37.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 38.Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–7. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]