Abstract
The etiology of preeclampsia is complex, with susceptibility being attributable to multiple environmental factors and a large genetic component. Although many candidate genes for preeclampsia have been suggested and studied, the specific causative genes still remain to be identified. Catechol-O-methyltransferase (COMT) is an enzyme involved in catecholamine and estrogen degradation and has recently been ascribed a role in development of preeclampsia. In the present study, we have examined the COMT gene by genotyping the functional Val108/158Met polymorphism (rs4680) and an additional single-nucleotide polymorphism, rs6269, predicting COMT activity haplotypes in a large Norwegian case/control cohort (ncases= 1135, ncontrols= 2262). A low COMT activity haplotype is associated with recurrent preeclampsia in our cohort. This may support the role of redox-regulated signaling and oxidative stress in preeclampsia pathogenesis as suggested by recent studies in a genetic mouse model. The COMT gene might be a genetic risk factor shared between preeclampsia and cardiovascular diseases.
Keywords: preeclampsia, catechol-O-methyltransferase, COMT, Val108/158Met, haplotypes
Introduction
The pregnancy-associated complication preeclampsia is a leading cause of maternal and fetal morbidity and mortality. Approximately 3% of all pregnant women in populations of European descent are affected by preeclampsia (Saftlas et al., 1990). In severe cases of preeclampsia, the only effective treatment is delivery, irrespective of gestational age. The classical clinical manifestations of preeclampsia are elevated blood pressure and proteinuria. The etiology is complex and like in other common complex disorders both genetic and environmental factors influence the risk of developing the disease. Genetic factors are suggested to be responsible for >50% of the liability to preeclampsia (Salonen Ros et al., 2000; Moses et al., 2006), and several candidate genes have been studied. However, the results are inconsistent and specific causative genes involved in preeclampsia still remain to be identified (Broughton Pipkin, 1999; Roberts and Cooper, 2001; Consortium, 2005; Chappell and Morgan, 2006; Mutze et al., 2008; Nejatizadeh et al., 2008).
A recent animal study put forward the suggestion that deficiency in catechol-O-methyltransferase (COMT) is associated with preeclampsia (Kanasaki et al., 2008). COMT is a key enzyme in the degradation of both catecholamines and estrogens (Creveling, 2003). High- and low-activity variants of COMT, due to single base changes, have been discovered (Diatchenko et al., 2005). One polymorphism with functional implications is a non-synonymous G to A base change (rs4680; NM_000754.2), the COMT Val108/158Met polymorphism. This polymorphism results in a substitution of the amino acid valine for methionine at codon 108 and 158 in the soluble and membrane bound isoforms of COMT, respectively. The Met(A)-allele of this polymorphism is associated with a 3- to 4-fold decrease in COMT enzyme activity (Lotta et al., 1995), and several clinical conditions such as pain perception (Zubieta et al., 2003; Diatchenko et al., 2005), psychiatric disorders (Woo et al., 2002; Azzam and Mathews, 2003; Prasad et al., 2008), hypertension (Happonen et al., 2006; Hagen et al., 2007a, b; Annerbrink et al., 2008) and heart disease (Eriksson et al., 2004; Hagen et al., 2007a, b; Voutilainen et al., 2007) have been reported to be associated with this base change.
Inspired by Kanasaki et al.'s hypothesis that COMT deficiency is associated with preeclampsia, we examined the potential role of high and low activity haplotypes in the central region of COMT. The functional COMT Val108/158Met polymorphism and an additional single-nucleotide polymorphism (SNP; rs6269) were genotyped to account for the three major haplotypes observed in the central region of COMT in populations of European descent (Gabriel et al., 2002; Diatchenko et al., 2005).
Materials and Methods
The HUNT population
All women subjected to genotyping were retrospectively identified from the second Nord-Trøndelag Health Study (HUNT2) (Holmen et al., 2003). Preeclampsia was defined as the onset of persistent hypertension (exceeding 140/90 mmHg), in combination with proteinuria (exceeding 300 mg/l per day) after 20 weeks gestation. Women with preeclamptic (cases) and non-preeclamptic (controls) singleton pregnancies in the HUNT2 cohort were identified by linking the HUNT database to the Medical Birth Registry of Norway (MBRN) (Moses et al., 2008). The inhabitants of Nord-Trøndelag county are well suited for genetic studies due to ethnic homogeneity (<3% non-Caucasians) (Holmen et al., 2003, 2004). The HUNT2 preeclampsia cohort is described in detail elsewhere (Moses et al., 2008; Fenstad et al., 2010).
Clinical characterization of the HUNT2 preeclampsia cohort
Cases registered with one preeclamptic pregnancy were defined as non-recurrent, and cases with more than one preeclamptic pregnancy were defined as recurrent. The non-recurrent preeclampsia group also included women with only one registered pregnancy in the MBRN. Preterm delivery was defined as delivery before 37 weeks (Gifford et al., 2000). Small for gestational age (SGA) was defined as an infant with a birthweight ≤2 standard deviations (SDs) below the expected weight for gestational age and sex, corresponding to the 2.5 percentile (Marsal et al., 1996). For assessment of metabolic syndrome, an International Diabetes Federation (IDF) proxy definition [waist circumference ≥80 cm plus any two of high density lipid (HDL) cholesterol <1.29, treatment for hypertension or blood pressure ≥130/≥85 mm Hg, diabetes diagnosed after age of 30] (Hildrum et al., 2007) was used, as fasting blood glucose was not available for all the individuals in the study cohort. Using the IDF proxy definition in a cross-sectional analysis of 10 206 HUNT2 participants, Hildrum et al. showed that there was no differences in the prevalence of metabolic syndrome between fasting and non-fasting groups (Hildrum et al., 2007).
SNP genotyping
DNA for genotyping was extracted from blood samples stored in the HUNT biobank, as described elsewhere (Moses et al., 2008). Applied Biosystems’ TaqMan genotyping assays (Applied Biosystems, Foster City, CA, USA) were selected to genotype the rs4680 (Val108/158Met) and rs6269 SNPs using 5 ng of genomic DNA from each of the case and control samples. The assays were performed on an Applied Biosystems 7900HT Fast Real-Time PCR System at HUNT biobank and sample genotypes were interrogated using the integrated 7900HT system data analysis software. The genotyping procedure has been validated at HUNT biobank by LightCycler (Roche Diagnostics Scandinavia AB, Stockholm, Sweden) hybridization probe genotyping and DNA sequencing of rs4680.
Haplotype analysis
Haplotypes were predicted from genotype information from each individual using the computer program Phase (http://stephenslab.uchicago.edu/home.html) (Stephens et al., 2001; Stephens and Donnelly, 2003). Only individuals with both SNPs successfully genotyped were included in the haplotype analysis (n= 3036; ncontrols= 2029, ncases= 1007; nnon-recurrent= 888, nrecurrent= 119). The frequency of the haplotypes was also calculated based on this number of individuals.
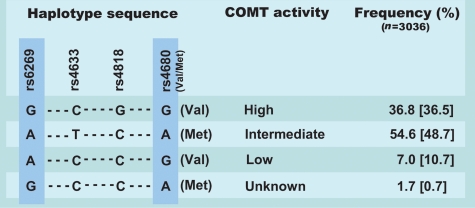
Only a few common COMT haplotypes are observed in populations of European descent (Gabriel et al., 2002; Diatchenko et al., 2005), and three major COMT haplotypes accounting for ∼96% of all detected haplotypes in the coding region determine the COMT activity in humans (Diatchenko et al., 2005). Figure 1 shows these three major haplotypes that are demonstrated to constitute four central SNPs (rs6269, rs4633, rs4818 and rs4680) (Diatchenko et al., 2005). These three haplotypes are associated with very different COMT enzyme activities (Nackley et al., 2006), and have also been demonstrated to be associated with variation in sensitivity to experimental pain. They were therefore designated as low pain sensitivity (LPS), average pain sensitivity (APS) and high pain sensitivity (HPS) (Diatchenko et al., 2005). There is an inverse correlation between pain sensitivity and COMT activity, meaning that the LPS haplotype represents the high COMT activity haplotype, whereas the HPS represents the low COMT activity haplotype and the APS represents the intermediate COMT activity haplotype.
Figure 1.
Haplotypes in the central region of the COMT gene. [(Figure modified from Andersen and Skorpen (2009)]. A total of four central SNPs in the COMT gene have been demonstrated to combine into three common haplotypes (Diatchenko et al., 2005) which have been associated with variation in COMT enzyme activity (Nackley et al., 2006). The two SNPs marked with a pale blue rectangle, rs6269 and rs4680, in combination differentiate between the three common activity haplotypes (Halleland et al., 2009) and were the ones genotyped in the present study. Frequencies for the haplotypes detected shown in this figure are consistent with previous findings and the frequencies observed by Diatchenko et al. are shown in brackets.
In this study, the genotyped SNPs (rs4680 and rs6269) were selected based on the observation that only two of the central four SNPs were needed to tag the variation in a Norwegian sample set (Halleland et al., 2009). It was observed that there is strong pair wise linkage disequilibrium (LD) with almost perfect correlation (r2> 0.97) between rs6269–rs4818 and rs4633–rs4680, and that the rs6269 SNP tags the high COMT activity haplotype (Halleland et al., 2009).
Statistical analysis
Clinical characterisation
The software package SPSS 16.0 for Windows was used to compute descriptive statistics such as mean and SD. P-values were computed based on t-test statistics for normally distributed variables. Non-parametrical methods (χ2) were used for categorical variables. The non-recurrent and recurrent preeclampsia groups were analyzed separately. Each preeclamptic group was compared with the non-preeclamptic group. Multivariate logistic regression was used to model preeclampsia as the (dichotomous) dependent variable against maternal age. A threshold of α = 0.05 was set for statistical significance of all computed analyses.
SNP and haplotype association analysis
Concordance with Hardy–Weinberg proportions was tested using a χ2 goodness-of-fit statistic. The SNP association analyses for the Val108/158Met (rs4680) and rs6269 SNPs and haplotype association analyses for the four possible haplotypes (Fig. 1) were carried out in PASW Statistics version 17 using a Pearson's χ2 statistic. The SNPs and haplotypes were analyzed separately for the subgroups of preeclamptic women (non-recurrent and recurrent) against non-preeclamptic control women. An additive (A allele frequency versus G allele frequency) genetic model was used for the SNP association analysis. For the haplotype association analyses, we tested whether carrying one of the four possible haplotypes was associated with disease state. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A threshold of α = 0.05 was set for statistical significance of all computed analyses.
Ethics
The study was approved by the Regional Committee for Medical Research Ethics, the National Data Inspectorate and The Directorate of Health and Social Welfare in Norway.
Results
Statistical power analysis
Using a relevant range of minor allele frequencies (30–50%) [National Center for Biotechnology Information (NCBI) SNP database], a priori power calculations ad modum Lalouel and Rhorwasser (Lalouel and Rohrwasser, 2002) for the genotyped SNPs demonstrated 80% power to detect an effect size (OR) difference of 1.25 for the non-recurrent group (n= 1003) and 1.65–1.75 for the subgroup of women with recurrent preeclampsia (n= 136).
Clinical characterization
The original HUNT2 preeclampsia cohort (1139 cases and 2269 controls) was used when performing the clinical characterisation (Moses et al., 2008; Fenstad et al., 2010). Mean follow-up time from index pregnancy recorded in MBRN to inclusion in the present study was 25 ± 10 years. Gestational age and birthweight differed between the neonates in preeclamptic and non-preeclamptic pregnancies, the preeclamptic women had a higher risk of preterm delivery, and of delivering a SGA neonate (Table I, P< 0.001). Metabolic syndrome was evaluated by data from the HUNT2 study and was higher in the case groups when compared with controls (Table I, P< 0.001). After adjusting for maternal age, the differences in clinical phenotype between case and control groups remained significant (Table I, P< 0.001).
Table I.
Clinical characteristics of the HUNT2 preeclampsia case/control cohort.
| Preeclampsia (non-recurrent, n= 1003) | Preeclampsia (recurrenta, n= 136) | Control (n= 2269) | |
|---|---|---|---|
| Maternal age at index pregnancy (years) | 27 ± 6* | 25 ± 5 | 25 ± 5 |
| Gestational age (days) | 275 ± 22* | 271 ± 20* | 282 ± 18 |
| Birthweight (g) | 3.238 ± 837* | 3.040 ± 846* | 3.483 ± 592 |
| SGAb | 147 (15)* | 26 (20)* | 87 (4) |
| Preterm birthc | 132 (14)* | 29 (22)* | 114 (5) |
| Maternal age at inclusion in HUNT2 | 40 ± 11 | 37 ± 9* | 40 ± 11 |
| Metabolic syndromed | 163 (16)* | 30 (22)* | 212 (9) |
Data presented as mean ± SD or number (percentage). P-values are computed by comparing each preeclamptic group to the non-preeclamptic control group.
IDF, the International Diabetes Federation; HDL, high-density lipoprotein; CI, confidence interval.
aMore than one preeclamptic pregnancy.
b≤2SD of expected weight.
cDelivery before Week 37.
dIDF-proxy definition; waist circumference ≥80 cm plus any two of (HDL cholesterol <1.29, treatment for hypertension or blood pressure ≥130/≥85 mmHg, diabetes diagnosed after age of 30 or fasting plasma glucose ≥5.6 mmol/l) (Hildrum et al., 2007).
*P< 0.001.
We also observed clinical differences between the group of women with recurrent and non-recurrent preeclampsia (Table I). The women with recurrent preeclampsia delivered earlier (P= 0.018) and had a higher prevalence of preterm birth (22%) compared with the women with non-recurrent preeclampsia (16%; P< 0.01). The neonates from the recurrent preeclamptic pregnancies had a lower birthweight (adjusted for gestational age, P= 0.055), but the seemingly different prevalence of SGA (20 versus 15% recurrent versus non-recurrent) was not statistically significant (P= 0.2). The P-values were adjusted for maternal age. The group of women with recurrent preeclampsia also had a higher prevalence of metabolic syndrome at inclusion in the HUNT2 study compared with the women with non-recurrent preeclampsia when adjusting for age at inclusion (P= 0.019).
COMT genotyping and association analysis
DNA samples were available for 1135 women registered with preeclamptic pregnancies and 2262 controls. We observed a high genotyping success rate for the rs4680 and rs6269 SNPs in both cases (94%) and controls (95%), and both SNPs conformed to Hardy–Weinberg proportions (P> 0.05). Approximately 10% of our samples were genotyped on both the TaqMan and the LightCycler genotyping system with a concordance of 99%. No association between the two studied COMT SNPs and non-recurrent preeclampsia was observed in our Norwegian cohort (Table II). However, a significant overrepresentation of the wild type allele (Val (G)), not the low activity allele (Met (A)), of the Val108/158Met polymorphism (rs4680) was observed in the group of women with recurrent preeclampsia (P= 0.047, OR = 0.77, CI 0.6–1.0) (Table II). No association was observed between rs6269 and recurrent preeclampsia (Table II).
Table II.
Distribution of COMT genotypes and alleles in the HUNT2 preeclampsia case/control cohort.
| SNP | Genotype (NN) |
Preeclampsia non-recurrent |
Preeclampsia recurrent |
Control |
OR |
CI |
|---|---|---|---|---|---|---|
| Allele (N) | n (proportion of total) | n (proportion of total) | n (proportion of total) | |||
| rs4680 (Val108/158Met) | GG | 174 (0.18) | 36 (0.28) | 412 (0.19) | ||
| AG | 461 (0.48) | 60 (0.46) | 1097 (0.50) | |||
| AA | 335 (0.35) | 35 (0.27) | 678 (0.31) | |||
| A (Met) | 1131 (0.58) | 130 (0.50) | 2453 (0.56) | 1.1a | 1.0–1.2a | |
| G (Val) | 809 (0.42) | 132 (0.50) | 1921 (0.44) | 0.8b,* | 0.6–1.0b | |
| rs6269 | AA | 361 (0.39) | 47 (0.39) | 771 (0.37) | ||
| GA | 412 (0.45) | 52 (0.43) | 1035 (0.49) | |||
| GG | 143 (0.16) | 23 (0.19) | 289 (0.14) | |||
| A | 1134 (0.62) | 146 (0.60) | 2577 (0.62) | 1.0a | 0.9–1.1a | |
| G | 698 (0.38) | 98 (0.40) | 1613 (0.39) | 0.9b | 0.7–1.2b |
OR, odds ratio; CI, 95% confidence interval.
aPreeclampsia non-recurrent versus control.
bPreeclampsia recurrent versus control.
*Significantly different from the value for the control group when compared with the frequency of the G allele using Pearson's χ2 analysis in a 2 × 2 contingency table (χ2= 4.185, P = 0.047).
The three common COMT haplotypes as well as the less frequent G-A (rs6269–rs4680) haplotype were detected in our Norwegian cohort (Table III and Fig. 1). The frequencies of the three common haplotypes in our cohort are shown in Fig. 1 and are consistent with frequencies observed in other studies (Fig. 1) (Diatchenko et al., 2005; Rakvag et al., 2008; Halleland et al., 2009). The less frequent G–A haplotype observed in our cohort is likely to be the G–C–C–A haplotype observed by Diatchenko et al. (Diatchenko et al., 2005), as this is the only haplotype with G–A at the two SNPs genotyped in the current study. We found that carrying the low COMT activity haplotype was significantly associated with recurrent preeclampsia (P= 0.018, OR = 1.8, CI 1.1–2.8) (Table III). The non-recurrent preeclampsia group did not show association with any of the haplotypes.
Table III.
COMT haplotypes in the HUNT2 preeclampsia case/control cohort.
| Haplotype | rs6269 –rs4680 (N-N) | Preeclampsia non-recurrent (proportion) | Preeclampsia recurrent (proportion) | Control (proportion) | OR | CI |
|---|---|---|---|---|---|---|
| 1 (high activity) | G–G | 516 (0.58) | 72 (0.61) | 1237 (0.61) | 0.9a | 0.8–1.0a |
| 1.0b | 0.7–1.4b | |||||
| 2 (intermediate activity) | A–A | 710 (0.80) | 87 (0.73) | 1627 (0.80) | 1.0a | 0.8–1.2a |
| 0.7b | 0.4–1.0b | |||||
| 3 (low activity) | A–G | 110 (0.12) | 25 (0.21) | 263 (0.13) | 1.0a | 0.8–1.2a |
| 1.8b* | 1.1–2.8b | |||||
| 4 (unknown activity) | G–A | 30 (0.03) | 2 (0.02) | 58 (0.03) | 1.2a | 0.8–1.9a |
| 0.6b | 0.1–2.4b |
Proportions represent the proportion of individuals being a carrier of the haplotype tested (number of individuals carrying haplotype X divided on total number of individuals in the studied subgroup).
OR, odds ratio; CI, 95% confidence interval.
aPreeclampsia non-recurrent versus control.
bPreeclampsia recurrent versus control.
*Significantly different from the value for the control group when compared with the frequency of the other haplotypes combined using Pearson's χ2 analysis in a 2 × 2 contingency table (χ2= 0.57, P= 0.018).
Discussion
Growing evidence supports the role of COMT in human pregnancy. The COMT enzyme is reported to be active in both placenta (Barnea et al., 1988) and decidua (Casey and MacDonald, 1983), and expression in human fetal membranes has recently been reported (Harirah et al., 2009). Decreased placental COMT activity was first reported to be associated with hypertension in pregnancy (Barnea et al., 1988). More recently, reduced placental COMT protein expression has been observed in women with severe preeclampsia (Kanasaki et al., 2008). On the basis of the latter observation, together with observations from studying COMT knockout mice, COMT was introduced as a preeclampsia susceptibility gene (Kanasaki et al., 2008). The Comt−/− mice developed a preeclampsia-like syndrome, with elevated blood pressure, albuminiuria, glomerular changes, placental thrombosis, and hypoxia and preterm birth. However, administration of 2-methoxyestradiol (2-ME), a natural estrogen metabolite produced by COMT, to pregnant Comt−/− mice ameliorated the preeclampsia-like symptoms (Kanasaki et al., 2008). It was suggested that genetic variation within the COMT gene could be an explanation for disruption of COMT and 2-ME in preeclamptic women (Kanasaki et al., 2008).
SNPs in the COMT gene have been shown to significantly affect enzyme activity (Lotta et al., 1995; Diatchenko et al., 2005; Nackley et al., 2009). It was therefore reasonable to hypothesize that SNPs in this gene are associated with preeclampsia pathogenesis. Recently, the Val108/158Met polymorphism was shown to be associated with preeclampsia in a small Korean population cohort of 164 preeclamptic and 182 normotensive patients (Lim et al., 2010). However, it has become clear that the Val108/158Met polymorphism alone is not likely to account for the variation of COMT enzyme activity. Four central SNPs (rs6269, rs4633, rs4818 and rs4680) in the COMT gene combine to form three common haplotypes (Diatchenko et al., 2005), and these are associated with varying levels of COMT enzyme activity (Nackley et al., 2006) (Fig. 1). The fact that the wild type Val108/158 (G) allele is present in both the high and low COMT activity haplotypes (Diatchenko et al., 2005) demonstrates the importance of studying haplotypes rather than single SNPs. We therefore performed haplotype analysis to see whether any of the three common haplotypes (Fig. 1) were associated with preeclampsia. We found that the low COMT activity haplotype, with a 58-fold reduced enzyme activity (Nackley et al., 2009), was significantly associated with recurrent preeclampsia (P= 0.018), with an OR of 1.8 (CI 1.1–2.8) for carrying this haplotype. Consistent with other studies, our group of women with recurrent preeclampsia showed the highest risk of preterm labor, low fetal birthweight and the highest risk of later life cardiovascular disease (assessed as metabolic syndrome) (Sibai et al., 1991; Odegard et al., 2000; Magnussen et al., 2009). Therefore, our findings support the hypothesis that lower maternal COMT enzyme activity predisposes to severe preeclampsia.
Angiogenesis, the formation of new blood vessels, is a central process in development of both preeclampsia and cardiovascular diseases. Alterations in angiogenesis during early pregnancy contribute to incomplete remodeling of uterine spiral arteries and abnormal placental vascular development (Roberts and Cooper, 2001). Decreased COMT activity and subsequent reduced levels of estrogen metabolites, such as 2-ME, may impair vascular health in several ways. (LaVallee et al., 2003; Barchiesi et al., 2006; Dubey et al., 2007; Dubey and Jackson, 2009). It has been demonstrated that 2-ME has antiangiogenic effects (Fotsis et al., 1994), suppressing hypoxia-inducible factor-1α (HIF-1α), which plays an essential role in angiogenesis. This transcription factor is responsible for the induction of genes that facilitate the adaption and survival of cells during low-oxygen levels (Wang et al., 1995; Semenza, 1998), including soluble fms-like tyrosine kinase 1. 2-ME has recently been suggested to be an important co-stimulator together with low-oxygen levels for induction of the invasiveness of trophoblasts (Lee et al., 2010). Thus, it has been suggested that 2-ME plays a role in maintaining placental homeostasis (Kanasaki and Kalluri, 2009). Disturbances in COMT and 2-ME homeostasis and regulation may cause placental pathology in different ways at different stages of the pregnancy. A premature increase in 2-ME has been hypothesized to disturb hypoxia-driven trophoblast invasion and vascular remodeling and therefore contribute to preeclampsia pathogenesis (Lee et al., 2010). In late pregnancy, decreased COMT activity, thus lower levels of 2-ME and decreased inhibition of HIF-1α could potentially cause vascular pathology and inflammatory activation (Banerjee et al., 2009). Studies examining the expression and activity of the COMT enzyme throughout pregnancy are warranted to clarify the role of COMT/2-ME at different stages of the pregnancy.
Acting as a pro-oxidant, 2-ME has direct involvement in redox-regulated signaling (Banerjee et al., 2009), a possible shared disease mechanism between preeclampsia and cardiovascular diseases. Furthermore, the COMT enzyme is also important for homocysteine metabolism, a known cardiovascular risk factor (Shenoy et al., 2010). A combination of high serum homocysteine levels and the low activity Val108/158 allele conferred increased risk (hazard risk ratio of 2.94) of acute coronary events in middle-aged men from eastern Finland (Voutilainen et al., 2007). A similar mechanism has been suggested for preeclampsia (Shenoy et al., 2010). Such epigenetic effects might explain the diverging results found in both genetic studies concerning COMT and epidemiologic studies concerning vitamin B and homocysteine levels (Ray and Laskin, 1999; Sanchez et al., 2001; Eriksson et al., 2004; Mignini et al., 2005; Happonen et al., 2006; Hagen et al., 2007a, b; Voutilainen et al., 2007; Annerbrink et al., 2008; Hintsanen et al., 2008; Guven et al., 2009; Nackley et al., 2009; Ntaios et al., 2009; Ciaccio and Bellia, 2010; Lim et al., 2010). In summary, the COMT gene may be a candidate gene for the genetic liability possibly shared between preeclampsia and cardiovascular disease. Altered COMT enzyme activity and 2-ME production is likely to be of great importance in the development of both preeclampsia and cardiovascular diseases (Dubey and Jackson, 2009).
Based on research showing that due to strong pair wise LD between rs6269–rs4818 and rs4633–rs4680, two of these central four SNPs were considered to be sufficient to tag the variation in a Norwegian population (Halleland et al., 2009). In the present study, the two SNPs (rs6269 and rs4680) were selected in order to represent three major haplotypes and a fourth less frequent haplotype (Fig. 1) in the central region of COMT. However, we do acknowledge that the multiple SNPs within the COMT gene give rise to a multitude of possible haplotype combinations, where minor differences between haplotypes may have profound effects on the COMT activity. To further investigate the role of genetic variation affecting COMT activity, one should extend the haplotype analysis to include the entire COMT gene. Only then can the ‘true’ effect of the high, intermediate and low activity haplotypes examined in the present study be controlled for. This can be done by looking at how these haplotypes are combined with the different haplotypes of flanking haploblocks. However, this will require very large study samples in order to account for the multitude of possible diplotype combinations (Andersen and Skorpen, 2009).
The major COMT haplotypes differ with respect to mRNA secondary structure (Diatchenko et al., 2005; Nackley et al., 2006). The low COMT activity haplotype has been suggested to have notable functional consequences due to an RNA folding structure substantially deviating from the structure of the most frequent and older high COMT activity haplotype (Nackley et al., 2006). Reduced enzymatic activity corresponding to the low COMT activity haplotype has been shown to be paralleled by reduced protein levels (Nackley et al., 2006). Differences in the local secondary structure of mRNA are likely to result in differences in protein translation efficiency (Nackley et al., 2006). The observation that the minor allele of an SNP (rs2097603) located in the promoter region of the membrane bound form of COMT producing a 1.5-fold reduction in lymphocyte COMT activity independent of the Val158Met allele supports this hypothesis (Chen et al., 2004). Another study found that a haplotype consisting of two non-coding SNPs (rs737865 in intron 1 and rs165599 in the 3′ untranslated region) reduced expression of COMT mRNA (Bray et al., 2003). The effect of the minor allele of three minor SNPs (rs6267, rs74062 and rs8192488) linked to the low COMT activity haplotype was recently studied. However, none of these minor SNPs significantly altered COMT RNA abundance, protein expression or enzymatic activity (Nackley et al., 2009). A haplotype consisting of the minor alleles of rs737865 and rs4818 in the low activity haplotype are associated with increased thermal threshold variance, implicating a role for additional unobserved functional polymorphisms in the gene (Shibata et al., 2009). Furthermore, it has been suggested that effects of the intermediate and low activity haplotypes might be modified by epistatic interactions (i) occurring at gene loci nearby COMT or (ii) with mutations located in convergent molecular pathways (Nackley et al., 2009). Thorough re-sequencing of the COMT gene and impinging regions in order to identify additional SNPs would be of great interest. In addition, seven different mRNA splice variants exist for the COMT gene, which potentially exacerbates the complexity of COMT in biological mechanisms (Tunbridge et al., 2007). Finally, in future studies it would also be of great interest to look at the fetal contribution since placental COMT is likely to be of importance.
Our total preeclampsia cohort was selected based on registry data and is therefore likely to represent a broad continuum of pathogenesis, ranging from mild preeclampsia to severe preeclampsia with both maternal and fetal complications. In the present study, we demonstrated significant association between the low activity COMT haplotype and recurrent preeclampsia, but not non-recurrent preeclampsia. A possible explanation for this might be that the recurrent preeclampsia group represents an ‘end tail’ or extreme subgroup of the continuum, with a greater power to detect genetic variation underlying disease development (Terwilliger and Goring, 2000). Our result may suggest that this group, although smaller, has a greater power to detect genetic variation underlying disease development. Follow up studies in larger collections are warranted.
The aim of this study was to examine if COMT activity haplotypes observed in the Norwegian population were associated with preeclampsia. The two SNPs genotyped represent the four possible haplotypes that were independently tested. Since the outcomes of the tests performed for the four haplotypes are expected to be highly dependent, we have chosen not to include a conservative Bonferroni correction (dividing the P-value threshold with the number of tests performed, 0.05/4 = 0.0125). Using a conservative Bonferroni correction to adjust the P-value threshold due to testing two preeclampsia subgroups to 0.025 (0.05/2), the P-value for association between the recurrent preeclampsia group and the low activity haploype would still yield a significant result (P= 0.018). Although the evidence presented is not strong, our observation that a low COMT activity haplotype is associated with recurrent preeclampsia is consistent with other studies reporting low COMT protein expression (Kanasaki and Kalluri, 2009) and enzyme activity (Barnea et al., 1988) in preeclampsia. Larger studies are needed to further elucidate the hypothesis that genetic variation within the COMT gene could be an explanation for disruption of COMT and 2-ME homeostasis in preeclamptic women.
In conclusion, the available evidence makes COMT a likely and interesting candidate gene for preeclampsia development. The present study demonstrates that a low COMT activity haplotype contributes to the genetic liability of recurrent preeclampsia in our Norwegian HUNT2 cohort. Nonetheless, further genetic and functional studies are needed to validate our finding and clarify the role of the COMT enzyme in preeclampsia pathogenesis. Studies examining the expression and activity of the COMT enzyme throughout pregnancy are warranted.
Authors’ roles
L.T.R. and M.H.F. wrote the paper, contributed substantially to design, genotyping, statistical analyses and interpretation of results. L.T.R. and F.S. contributed substantially to conception of the study. M.P.J. and F.S. contributed substantially to the interpretation of data and drafting. S.F. contributed substantially to acquisition of epidemiological data. R.A. contributed substantially to acquisition of samples. M.P.J., E.K.M., S.F. and R.A. contributed substantially to revising and final approval of the manuscript.
Funding
This work was supported by grants from the Liaison Committee of NTNU and Central Norway Regional Health Authority (L.T.R. and M.H.F.). Funding to pay the Open Access publication charges for this article was provided by the Liaison Committee of NTNU and Central Norway Regional Health Authority.
Acknowledgements
The HUNT study is a collaboration between HUNT Research Centre, Faculty of Medicine at NTNU, the Norwegian Institute of Public Health and the Nord-Trøndelag County Council. We want to thank HUNT biobank, especially Kirsti Kvaløy, for performing the genotyping and supplying information on the quality control genotyping.
References
- Andersen S, Skorpen F. Variation in the COMT gene: implications for pain perception and pain treatment. Pharmacogenomics. 2009;10:669–684. doi: 10.2217/pgs.09.13. [DOI] [PubMed] [Google Scholar]
- Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol O-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism. 2008;57:708–711. doi: 10.1016/j.metabol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Azzam A, Mathews CA. Meta-analysis of the association between the catecholamine-O-methyl-transferase gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:64–69. doi: 10.1002/ajmg.b.20013. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Randeva H, Chambers AE. Mouse models for preeclampsia: disruption of redox-regulated signaling. Reprod Biol Endocrinol. 2009;7:4. doi: 10.1186/1477-7827-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-O-methyl transferase activity in the human term placenta. Am J Perinatol. 1988;5:121–127. doi: 10.1055/s-2007-999669. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O'Donovan MC. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton Pipkin F. What is the place of genetics in the pathogenesis of pre-eclampsia? Biol Neonate. 1999;76:325–330. doi: 10.1159/000014175. [DOI] [PubMed] [Google Scholar]
- Casey ML, MacDonald PC. Characterization of catechol-O-methyltransferase activity in human uterine decidua vera tissue. Am J Obstet Gynecol. 1983;145:453–457. doi: 10.1016/0002-9378(83)90316-2. [DOI] [PubMed] [Google Scholar]
- Chappell S, Morgan L. Searching for genetic clues to the causes of pre-eclampsia. Clin Sci (Lond) 2006;110:443–458. doi: 10.1042/CS20050323. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio M, Bellia C. Hyperhomocysteinemia and cardiovascular risk: effect of vitamin supplementation in risk reduction. Curr Clin Pharmacol. 2010;5:30–36. doi: 10.2174/157488410790410551. [DOI] [PubMed] [Google Scholar]
- Consortium G. Disentangling fetal and maternal susceptibility for pre-eclampsia: a British multicenter candidate-gene study. Am J Hum Genet. 2005;77:127–131. doi: 10.1086/431245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creveling CR. Reduced COMT activity as a possible environmental risk factor for breast cancer. Opin Neurotox Res. 2003;5:473–474. doi: 10.1007/BF03033157. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK. Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol Metab. 2009;20:374–379. doi: 10.1016/j.tem.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Jackson EK. 2-Methoxyestradiol: a potential treatment for multiple proliferative disorders. Endocrinology. 2007;148:4125–4127. doi: 10.1210/en.2007-0514. [DOI] [PubMed] [Google Scholar]
- Eriksson AL, Skrtic S, Niklason A, Hulten LM, Wiklund O, Hedner T, Ohlsson C. Association between the low activity genotype of catechol-O-methyltransferase and myocardial infarction in a hypertensive population. Eur Heart J. 2004;25:386–391. doi: 10.1016/j.ehj.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Fenstad MH, Johnson MP, Loset M, Mundal SB, Roten LT, Eide IP, Bjorge L, Sande RK, Johansson AK, Dyer TD, et al. STOX2 but not STOX1 is differentially expressed in decidua from pre-eclamptic women: data from the Second Nord-Trøndelag Health Study. Mol Hum Reprod. 2010;16:960–968. doi: 10.1093/molehr/gaq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gifford RW, August PA, Cunningham G, Green LA, Lindheimer MD, McNellis D, Roberts JM, Sibai BM, Taler SJ. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- Guven MA, Coskun A, Ertas IE, Aral M, Zencirci B, Oksuz H. Association of maternal serum CRP, IL-6, TNF-alpha, homocysteine, folic acid and vitamin B12 levels with the severity of preeclampsia and fetal birth weight. Hypertens Pregnancy. 2009;28:190–200. doi: 10.1080/10641950802601179. [DOI] [PubMed] [Google Scholar]
- Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, Zwart JA. High systolic blood pressure is associated with Val/Val genotype in the catechol-O-methyltransferase gene. The Nord-Trøndelag Health Study (HUNT) Am J Hypertens. 2007a;20:21–26. doi: 10.1016/j.amjhyper.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Hagen K, Stovner LJ, Skorpen F, Pettersen E, Zwart JA. The impact of the catechol-O-methyltransferase Val158Met polymorphism on survival in the general population—the HUNT study. BMC Med Genet. 2007b;8:34. doi: 10.1186/1471-2350-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleland H, Lundervold AJ, Halmoy A, Haavik J, Johansson S. Association between catechol O-methyltransferase (COMT) haplotypes and severity of hyperactivity symptoms in adults. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:403–410. doi: 10.1002/ajmg.b.30831. [DOI] [PubMed] [Google Scholar]
- Happonen P, Voutilainen S, Tuomainen TP, Salonen JT. Catechol-O-methyltransferase gene polymorphism modifies the effect of coffee intake on incidence of acute coronary events. PLoS ONE. 2006;1:e117. doi: 10.1371/journal.pone.0000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harirah H, Thota C, Wentz MJ, Zaman W, Al-Hendy A. Elevated expression of catechol-O-methyltransferase is associated with labor and increased prostaglandin E(2) production by human fetal membranes. Am J Obstet Gynecol. 2009;201:496. doi: 10.1016/j.ajog.2009.05.041. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7:220. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintsanen M, Elovainio M, Puttonen S, Kivimaki M, Lehtimaki T, Kahonen M, Juonala M, Rontu R, Viikari JS, Raitakari OT, et al. Val/Met polymorphism of the COMT gene moderates the association between job strain and early atherosclerosis in young men. J Occup Environ Med. 2008;50:649–657. doi: 10.1097/JOM.0b013e318165c7ec. [DOI] [PubMed] [Google Scholar]
- Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, Vatten L, Lund-Larsen PG. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Nor J Epidemiol. 2003;13:19–32. [Google Scholar]
- Holmen J, Kjelsaas M, Krüger Ø, Ellekjær H, Ross G, Holmen T, Midthjell K, Stavnås P, Krokstad S. Attitudes to genetic epidemiology—illustrated by questions for re-consent to 61,426 participants at HUNT. Nor J Epidemiol. 2004;14:27–31. [Google Scholar]
- Kanasaki K, Kalluri R. The biology of preeclampsia. Kidney Int. 2009;76:831–837. doi: 10.1038/ki.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- Lalouel JM, Rohrwasser A. Power and replication in case-control studies. Am J Hypertens. 2002;15:201–205. doi: 10.1016/s0895-7061(01)02285-3. [DOI] [PubMed] [Google Scholar]
- LaVallee TM, Zhan XH, Johnson MS, Herbstritt CJ, Swartz G, Williams MS, Hembrough WA, Green SJ, Pribluda VS. 2-Methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res. 2003;63:468–475. [PubMed] [Google Scholar]
- Lee SB, Wong AP, Kanasaki K, Xu Y, Shenoy VK, McElrath TF, Whitesides GM, Kalluri R. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176:710–720. doi: 10.2353/ajpath.2010.090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Kim SY, Kim DJ, Park SY, Han HW, Han JY, Lee SW, Yang JH, Ryu HM. Genetic polymorphism of catechol-O-methyltransferase and cytochrome P450c17 in preeclampsia. Pharmacogenet Genomics. 2010;20:605–610. doi: 10.1097/FPC.0b013e32833df033. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114:961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–848. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- Mignini LE, Latthe PM, Villar J, Kilby MD, Carroli G, Khan KS. Mapping the theories of preeclampsia: the role of homocysteine. Obstet Gynecol. 2005;105:411–425. doi: 10.1097/01.AOG.0000151117.52952.b6. [DOI] [PubMed] [Google Scholar]
- Moses EK, Fitzpatrick E, Freed KA, Dyer TD, Forrest S, Elliott K, Johnson MP, Blangero J, Brennecke SP. Objective prioritization of positional candidate genes at a quantitative trait locus for pre-eclampsia on 2q22. Mol Hum Reprod. 2006;12:505–512. doi: 10.1093/molehr/gal056. [DOI] [PubMed] [Google Scholar]
- Moses EK, Johnson MP, Tommerdal L, Forsmo S, Curran JE, Abraham LJ, Charlesworth JC, Brennecke SP, Blangero J, Austgulen R. Genetic association of preeclampsia to the inflammatory response gene SEPS1. Am J Obstet Gynecol. 2008;198:336. doi: 10.1016/j.ajog.2007.09.024. e1–5. [DOI] [PubMed] [Google Scholar]
- Mutze S, Rudnik-Schoneborn S, Zerres K, Rath W. Genes and the preeclampsia syndrome. J Perinat Med. 2008;36:38–58. doi: 10.1515/JPM.2008.004. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Lambert JE, Conrad MS, Gibson DG, Spiridonov AN, Satterfield SK, Diatchenko L. Low enzymatic activity haplotypes of the human catechol-O-methyltransferase gene: enrichment for marker SNPs. PLoS ONE. 2009;4:e5237. doi: 10.1371/journal.pone.0005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejatizadeh A, Stobdan T, Malhotra N, Pasha MA. The genetic aspects of pre-eclampsia: achievements and limitations. Biochem Genet. 2008;46:451–479. doi: 10.1007/s10528-008-9163-9. [DOI] [PubMed] [Google Scholar]
- Ntaios G, Savopoulos C, Grekas D, Hatzitolios A. The controversial role of B-vitamins in cardiovascular risk: an update. Arch Cardiovasc Dis. 2009;102:847–854. doi: 10.1016/j.acvd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96:950–955. [PubMed] [Google Scholar]
- Prasad SE, Howley S, Murphy KC. Candidate genes and the behavioral phenotype in 22q11.2 deletion syndrome. Dev Disabil Res Rev. 2008;14:26–34. doi: 10.1002/ddrr.5. [DOI] [PubMed] [Google Scholar]
- Rakvag TT, Ross JR, Sato H, Skorpen F, Kaasa S, Klepstad P. Genetic variation in the catechol-O-methyltransferase (COMT) gene and morphine requirements in cancer patients with pain. Mol Pain. 2008;4:64. doi: 10.1186/1744-8069-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta. 1999;20:519–529. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163:460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- Salonen Ros H, Lichtenstein P, Lipworth L, Cnattingius S. Genetic effects on the liability of developing pre-eclampsia and gestational hypertension. Am J Med Genet. 2000;91:256–260. [PubMed] [Google Scholar]
- Sanchez SE, Zhang C, Rene Malinow M, Ware-Jauregui S, Larrabure G, Williams MA. Plasma folate, vitamin B(12), and homocyst(e)ine concentrations in preeclamptic and normotensive Peruvian women. Am J Epidemiol. 2001;153:474–480. doi: 10.1093/aje/153.5.474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Shenoy V, Kanasaki K, Kalluri R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrinol Metab. 2010;21:529–536. doi: 10.1016/j.tem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Shibata K, Diatchenko L, Zaykin DV. Haplotype associations with quantitative traits in the presence of complex multilocus and heterogeneous effects. Genet Epidemiol. 2009;33:63–78. doi: 10.1002/gepi.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165:1408–1412. doi: 10.1016/0002-9378(91)90379-6. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger JD, Goring HH. Gene mapping in the 20th and 21st centuries: statistical methods, data analysis, and experimental design. Hum Biol. 2000;72:63–132. [PubMed] [Google Scholar]
- Tunbridge EM, Lane TA, Harrison PJ. Expression of multiple catechol-O-methyltransferase (COMT) mRNA variants in human brain. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:834–839. doi: 10.1002/ajmg.b.30539. [DOI] [PubMed] [Google Scholar]
- Voutilainen S, Tuomainen TP, Korhonen M, Mursu J, Virtanen JK, Happonen P, Alfthan G, Erlund I, North KE, Mosher MJ, et al. Functional COMT Val158Met polymorphism, risk of acute coronary events and serum homocysteine: the kuopio ischaemic heart disease risk factor study. PLoS ONE. 2007;2:e181. doi: 10.1371/journal.pone.0000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Yu BH. Catechol O-methyltransferase genetic polymorphism in panic disorder. Am J Psychiatry. 2002;159:1785–1787. doi: 10.1176/appi.ajp.159.10.1785. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]