Abstract
Na+, Ca2+- permeable acid-sensing ion channel 1a (ASIC1a) is involved in the pathophysiologic process of adult focal brain ischemia. However, little is known about its role in the pathogenesis of global cerebral ischemia or newborn hypoxia-ischemia (H-I). Here, using a newborn piglet model of asphyxia-induced cardiac arrest, we investigated the effect of ASIC1a-specific blocker psalmotoxin-1 on neuronal injury. During asphyxia and the first 30 mins of recovery, brain tissue pH fell below 7.0, the approximate activation pH of ASIC1a. Psalmotoxin-1 injection at 20 mins before hypoxia, but not at 20 mins of recovery, partially protected the striatonigral and striatopallidal neurons in putamen. Psalmotoxin-1 pretreatment largely attenuated the increased protein kinase A-dependent phosphorylation of DARPP-32 and N-methyl-D-aspartate (NMDA) receptor NR1 subunit and decreased nitrative and oxidative damage to proteins at 3 h of recovery. Pretreatment with NMDA receptor antagonist MK-801 also provided partial neuroprotection in putamen, and combined pretreatment with psalmotoxin-1 and MK-801 yielded additive neuroprotection. These results indicate that ASIC1a activation contributes to neuronal death in newborn putamen after H-I through mechanisms that may involve protein kinase A-dependent phosphorylation of NMDA receptor and nitrative and oxidative stress.
Introduction
Varieties of homomeric and heteromeric acid-sensing ion channel (ASIC) subunits can form sodium-permeable channels that are expressed in neurons throughout the central and peripheral nervous system (Krishtal, 2003). Among these channels, the homomeric ASIC1a allows not only Na+ but also Ca2+ influx into cells after it is activated by a reduction in extracellular pH (Yermolaieva et al., 2004). Physiologically, ASIC1a is involved in synaptic plasticity and learning and memory (Wemmie et al., 2002). However, studies have shown that it acts as a prominent mediator in acidosis-induced neuronal injury in adult brain ischemia (Gao et al., 2005; Pignataro et al., 2007; Xiong et al., 2004).
Extracellular and intracellular pH in the brain are maintained physiologically at approximately 7.3 and 7.1, respectively (Nedergaard et al., 1991; Tomlinson et al., 1993). Cerebral ischemia induces substantial tissue acidosis, although the pattern and magnitude of intracellular and extracellular pH varies with ischemic severity and duration (Simon and Xiong, 2006; Yao and Haddad, 2004). Lactate accumulation from anaerobic glycolysis and increased H+ release from ATP hydrolysis reduce brain pH to 6.5 or lower during severe ischemia (Laptook et al., 1992; Nedergaard et al., 1991). This pH reduction is capable of activating ASIC1a channels and allowing Ca2+ entry into neurons in a glutamate receptor-independent manner (Xiong et al., 2004; Yermolaieva et al., 2004). Pharmacologic intervention with psalmotoxin-1 (PcTX), a specific ASIC1a blocker, or ASIC1 gene ablation significantly reduces infarct volume in adult stroke models (Pignataro et al., 2007; Xiong et al., 2004).
Complex interactions may exist between ASIC1a and the N-methyl-D-aspartate (NMDA) receptor in ischemic injury. On the one hand, NMDA receptors can modulate ASIC1a channel function through Ca2+/calmodulin protein kinase II-dependent ASIC1a phosphorylation in ischemic adult brain (Gao et al., 2005). On the other hand, some studies have suggested that ASIC1a may change NMDA receptor function. ASIC1 knockout mice show reduced excitatory postsynaptic potentials and NMDA receptor activation (Wemmie et al., 2002). PcTX can significantly reduce NMDA-induced cell death (Gao et al., 2005), and the neuroprotective effect of an NMDA receptor antagonist is less prominent in ischemic ASIC1 knockout mice than in wild-type mice (Xiong et al., 2004). Moreover, combined treatment with an ASIC1a blocker and an NMDA receptor antagonist resulted in a further reduction of brain damage and extended the therapeutic time window for NMDA receptor blockade (Pignataro et al., 2007). All of these findings open the possibility that ASIC1a-mediated acidotoxicity aggravates NMDA receptor-mediated excitotoxicity in ischemic brain and may help to explain why glutamate receptor antagonists have failed in clinical trials. Considering that ASIC1 promotes dopamine signaling (Voglis and Tavernarakis, 2008) and that dopamine receptor activity can modulate NMDA receptor function in striatal neurons, ASIC1a may bear resemblance to dopamine receptors that modulate NMDA receptor activity through dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32)-dependent phosphorylation of NMDA receptor subunit NR1 (Svenningsson et al., 2004; Yang et al., 2007).
We have shown previously that dopamine receptor activation participates in the mechanisms of striatal neurodegeneration in neonatal hypoxia-ischemia (H-I) by modulating NMDA receptor phosphorylation (Yang et al., 2007); however, the contribution of ASIC1a to the mechanisms of neonatal brain damage is unknown. Here, we explored the possibility that ASIC1a is a target for neuroprotection in a piglet model of neonatal H-I. We investigated whether PcTX modulates the severity of brain damage, the phosphorylation state of the NMDA receptor subunit NR1, and the level of oxidative and nitrative stress after neonatal H-I. Piglets were selected partly because a period of rapid brain growth occurs soon after birth and the level of development is more analogous to term human newborns than to many rodents with a postnatal growth spurt (Dobbing and Sands, 1979). Previous work (Yang et al., 2010) has also indicated distinct anatomical and biochemical differences in basal ganglia between the newborn and adult swine. Moreover, regions of selective vulnerability to H-I in piglet brain resemble those in term human newborns, and physiological monitoring is more easily performed in piglets than in postanat rodents (Martin et al., 1997). We focused on putamen because neurodegeneration in this region is rapid and resembles that occurring with excitotoxic lesions (Martin et al., 2000).
Materials and Methods
Experimental protocol
Animal protocols were approved by the Animal Care and Use Committee of the Johns Hopkins University and performed in accordance with National Institutes of Health guidelines. As described previously (Yang et al., 2010; Yang et al., 2007), 86 4- to 7-day-old male piglets (2–2.5 kg) were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal) and intubated. Catheters were placed into a femoral artery and vein under aseptic conditions. To induce H-I, inspired O2 was decreased to 10.0 ± 0.2% for 40 mins, followed by ventilation with 21% O2 for 5 mins (required for cardiac resuscitation) and airway occlusion (asphyxia) for 7 mins (to produce cardiac arrest). Piglets were resuscitated by ventilation with 50% O2, manual chest compressions, and/or intravenous injection of epinephrine until return of spontaneous circulation. After resuscitation, inspired O2 was gradually reduced to 30% to maintain arterial O2 saturation. Arterial blood pressure, blood gases, and glucose were monitored until the animal regained consciousness. Sham-operated animals were subjected to catheterization but not hypoxia or asphyxia. All animals were treated with the cefazolin (20 mg/kg, i.v., q.d.).
PcTX was obtained from tarantula Psalmopoeus cambridgei venom (Spider Pharm Inc., Yarnell, AZ). Small drill holes were made in the skull bilaterally at 8 mm anterior and 3 mm lateral from bregma under aseptic conditions, and sterile stainless steel cannulae (0.5 mm diameter) were inserted 14 mm below the dura and into the lateral ventricles. One microgram of venom protein in 50 μl of artificial cerebrospinal fluid (aCSF) was injected into each lateral ventricle at 20 mins before hypoxia or at 20 mins of recovery. The microsyringe's cannulae were removed 10 min after the injection, the holes were sealed with bone wax, and the incisions were closed with suture. Control animals received only aCSF. To compare the neuroprotective effect of PcTX with that of NMDA receptor antagonist, some H-I piglets were administered 1 mg/kg MK-801 (Ascent Scientific; Princeton, NJ; iv. 20 mins before hypoxia) or a pretreatment combination of PcTX and MK-801.
To study the diffusion range of PcTX, 0.25 nM FITC-PcTX (Phoenix Pharmaceuticals, Inc. Burlingame, CA) in 50 μl of aCSF was injected into each lateral ventricle of two piglets 20 mins before induction of hypoxia. Animals experienced H-I and brains were harvested at 30 mins of recovery.
We focused on putamen because this region is the most vulnerable to H-I in this model (Martin et al., 1997). Neuronal cell death progresses between 3 and 24 h after reoxygenation in this region. Therefore, we made biochemical measurements at 3 h before the loss of neurons would affect the measurements. To allow for a possible delay in the maturation of histological injury with PcTX treatment, H-I neuronal damage was observed at 4 days of recovery (Martin et al., 2000).
Cerebral pH measurement
An optic pH sensor probe (Polestar Technologies, MA) with a detection range of pH 5.5 to pH 8.5 was used for continuous measurement of brain tissue pH in a cohort of 4 piglets. The probe was stereotaxically implanted into the sensorimotor cortex 20 mins before H-I (bregma level -10 mm, 8 mm lateral, and 3 mm depth), and brain pH was continually measured until 3 h of recovery. The probe was checked with buffer solutions (pH 6 and 7.6) in the absence of light before and after the experiment.
Neurobehavioral assessment
A neurologic deficit score (0 = best outcome, 154 = worst outcome) (Agnew et al., 2003; Yang et al., 2010; Yang et al., 2007) was used to quantify overall neurologic function in an observer-blinded fashion based on seven different components: 1) level of consciousness; 2) brain stem function; 3) sensory responses; 4) motor function; 5) behavioral activities; 6) spatial orientation; and 7) excitability.
Immunohistochemical and double immunofluorescence staining
The anesthetized piglets were perfused transcardially with ice-cold phosphate-buffered saline and 4% paraformaldehyde at 30 mins, 3 h, or 4 days of recovery. Brains were removed, bisected mid-sagittally, and cut into 1-cm slabs. The left forebrain was embedded in paraffin for histology with hematoxylin and eosin (H&E) staining and neuropathological assessment of damaged neurons. The right forebrain was cryoprotected, frozen, and cut into serial 40-μm coronal sections through the putamen.
Double immunofluorescence staining was used to assess the neuronal localization of ASIC1; the relationship among ASIC1, phospho-NR1 Ser897, and 3-nitrotyrosine; the relationship between dopamine D1 receptor and early B cell factor 1 (Ebf1); the relationship between dopamine D2 receptor and G-protein-coupled receptor 6 (Gpr6); and the relationship between ASIC1 and Ebf1 or Gpr6 in striatum. Sections were blocked in 10% normal horse serum and incubated with primary antibodies overnight at 4°C. Antibody binding was visualized by incubating sections with Cy3-conjugated (1:500, Jackson ImmunoResearch, West Grove, PA) or Alexa Fluor 488-conjugated (1:500, Invitrogen, Eugene, OR) secondary antibodies. The following primary antibodies were used in this study: rabbit anti-ASIC1 (1:500, Sigma-Aldrich, St. Louis, MO) or goat anti-ASIC1 (1:50, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-NeuN (1:500, Chemicon, Temecula, CA), mouse anti-NR1 (1:200, BD Pharmingen, San Jose, CA), rabbit anti-phospho-NR1Ser897 (1:200, Upstate, Lake Placid, NY), mouse anti-nitrotyrosine (1:500, Upstate), rabbit anti-D1R (1:300, Chemicon), goat anti-D2R (Santa Cruz), mouse anti-Ebf1 (1:100, Santa Cruz), and rabbit anti-Gpr6 (1:100, MBL International, Woburn, MA).
Immunohistochemical assessments were made on Ebf1 and Gpr6 in sham-operated and H-I piglet brain. Free-floating sections were blocked in 10% normal horse serum and incubated with anti-Ebf1 or anti-Gpr6 antibody followed with biotinylated IgG (1:200, Vector Laboratories, Burlingame, CA) and VECTASTAIN Elite ABC reagent (Vector). Immunostaining was developed with diaminobenzidine (Vector) as a chromogen. Negative controls were treated without primary antibodies and showed no positive signals. For quantification of immunoreactivity, seven 200× optical fields per section were randomly selected in anterior, median, and posterior putamen sections (bregma level: +15 mm, +9 mm, and +5 mm) (Salinas-Zeballos et al., 1986), and the number of immunopositive stained cells was quantified with ImageJ software (version 1.42q, NIH).
Western Blotting
Brain putamen tissues obtained from H-I piglets at 3 h of recovery and time-matched sham-operated animals were homogenized and fractionated into membrane-enriched and cytosol-enriched fractions, as described previously (Yang et al., 2010; Yang et al., 2007). Twenty-microgram protein samples were separated by 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were probed with the following primary antibodies: rabbit anti-ASIC1 (1:2000), mouse anti-Ebf1 (1:1000), rabbit anti-Gpr6 (1:1000), mouse anti-NR1 (1:2000), rabbit anti-phospho-NR1 Ser896, rabbit anti-phospho-NR1 Ser897 (1:2000, Upstate), mouse anti-synaptophysin (1:20,000, Chemicon), rabbit anti-DARPP-32 or anti-phospho-DARPP-32 Thr34 (1:1500, Cell Signaling Technology, Danvers, MA), or mouse anti-nitrotyrosine (1:40,000). Synaptophysin was used as a protein loading standard for membrane-enriched fractions. Resulting protein bands were scanned and analyzed with Quantity One software (Bio-Rad, Hercules, CA). Optical density values were normalized to the value of a sham-operated, aCSF-treated animal on each gel. Each experiment was performed in quadruplicate using tissue from four to six piglets per group.
Data and statistical analysis
Profile counting was used to estimate ischemic neuronal damage in mid-coronal sections of putamen on H&E-stained paraffin sections. In level-matched sections, the number of ischemic and non-ischemic neuronal profiles was counted randomly in an observer-blinded fashion in seven non-overlapping microscopic fields at 1000× power in anterior, median, and posterior putamen sections (bregma level: +15 mm, +9 mm, and +5 mm). The values were averaged to obtain a single value of viable neurons per square millimeter for each piglet to be used in the statistical analysis.
All values are expressed as means ± SD. The neurologic deficit score was analyzed by two-way analysis of variance (ANOVA) with repeated measures. Other measurements were analyzed with one-way ANOVA followed by the Student-Newman-Keuls multiple range test. P < 0.05 was considered statistically different.
Results
Alteration of arterial and cerebral cortical tissue pH during and after H-I
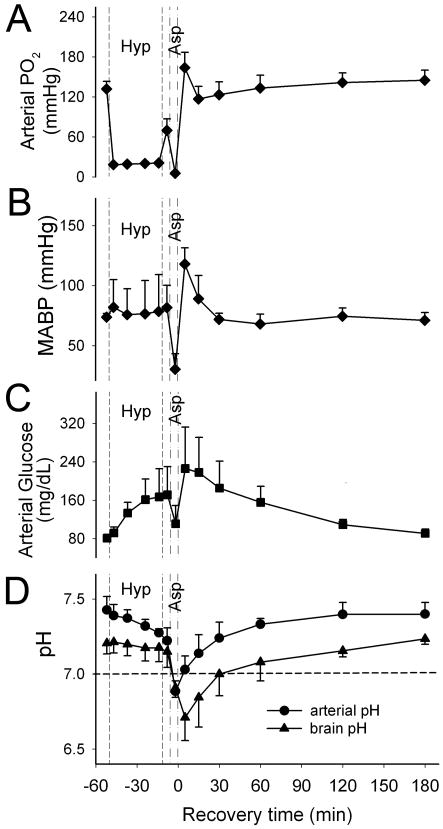
During the 40 mins of hypoxia, arterial PO2 was reduced to approximately 20 mmHg (Figure 1A), and mean arterial blood pressure (MABP) was maintained near the baseline level. Severe bradycardia and hypotension occurred during the 7-min period of asphyxia. After successful cardiac resuscitation, MABP increased to above baseline and then gradually returned to baseline level at 30 mins of recovery (Figure 1B). H-I induced hyperglycemia, which gradually resolved after resuscitation (Figure 1C). Arterial blood pH gradually decreased from 7.43 ± 0.09 to 7.28 ± 0.03 during hypoxia, dropped sharply during asphyxia, and reached a level of 6.89 ± 0.07 at 5 mins of asphyxia (Figure 1D).
Figure 1.
Arterial PO2 (A), mean arterial blood pressure (MABP, B), arterial glucose (C) and arterial and brain tissue pH (D) of four piglets (means ± SD) during baseline, hypoxia (Hyp), asphyxia (Asp), and 3 h of recovery. The dashed line provides a reference pH of 7.0, which represents the approximate pH threshold below which ASIC1a begins to open.
Cerebral cortical tissue pH fell slightly from 7.21 ± 0.07 to 7.17 ± 0.09 by the end of hypoxia and reached a minimum value of 6.71 ± 0.15 at 5 mins after resuscitation (n = 4). Notably, brain pH remained acidic (<7.0) between the asphyxia period and 30 mins of recovery but was nearly normal at 2 h of recovery.
Localization of ASIC1 in piglet neurons
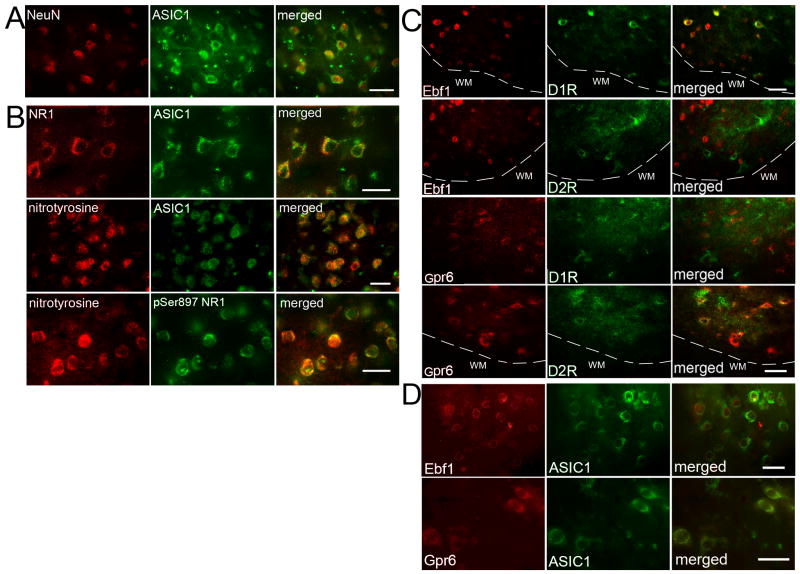
The ASIC1 antibody reacted with a ∼60 kDa protein in piglet and mouse striatum extracts (Supplemental Figure 1). Immunohistochemical results showed ASIC1 immunoreactivity to be located mainly in neuronal bodies and primary dendrites of newborn piglet striatum (Figure 2A). This result is consistent with a previous report that ASIC1a is enriched in medium spiny neurons of striatum in rodents (Jiang et al., 2009).
Figure 2.
Double immunofluorescence of ASIC1 and NeuN in normal pig striatum (A); ASIC1 and NMDA receptor NR1 subunit or nitrotyrosine, and nitrotyrosine and phosphorylated NR1 Ser897 (pSer897 NR1) in pig putamen at 3 h of recovery after H-I (B); Ebf1 or Gpr6 and dopamine D1 receptor (D1R) or dopamine D2 receptor (D2R) in pig putamen at 3 h of recovery after H-I (C); ASIC1 and Ebf1 or Gpr6 in normal pig striatum (D). White dash line indicates the boundary between grey matter and white matter. White matter (WM). Scale bar = 20 μm.
Neuronal damage in putamen after H-I
To determine if PcTX penetrates into the brain parenchyma after intracerebroventricular injection, we tracked PcTX-FITC. Compared to the weak background autofluorescence observed in caudate and putamen of animals treated with non-conjugated PcTX, injection of PcTX-FITC into lateral ventricle 20 mins before hypoxia produced fluorescent signals in septum, caudate, and putamen (anterior, median, and posterior) at 30 mins of recovery. Within these regions, PcTX-FITC was detected in the extracellular space and neuropil and was associated with cell bodies of presumptive neurons (Supplemental Figure 2).
PcTX was administered with or without the NMDA receptor antagonist MK-801 to study the role of ASIC1a channels and NMDA receptors in striatal neuronal injury after H-I. No significant difference in MABP or rectal temperature was observed among groups that received aCSF infusion, PcTX pretreatment, PcTX post-treatment, MK-801 treatment, or combined treatment during H-I and 3 h of recovery (Supplemental Table). Successful resuscitation was achieved in 58 of 68 piglets (85%). The amount of epinephrine [86 ± 115 μg/kg, 56 ± 85 μg/kg, 88 ± 137 μg/kg, 89 ± 118 μg/kg, and 70 ± 97 μg/kg in the aCSF, PcTX pretreatment, PcTX post-treatment, MK-801, and combined treatment groups, respectively] did not show any significant difference among treatment groups. In addition, no significant difference was observed in arterial PO2, PCO2, or pH among H-I groups. Subsequent mortality during the 4-day recovery period was 1 of 8 piglets in the aCSF group, 1 of 9 piglets in the PcTX pretreatment group, 0 of 8 piglets in the PcTX post-treatment group, 2 of 9 piglets in the MK-801 treatment group, and 3 of 10 piglets in the combined treatment group. Mortality rates include piglets that were euthanized because of inability to extubate within 10 h of recovery or severe generalized seizures [1/0 (no extubation/seizures), 0/1, 0/0, 2/0, and 2/1 in the aCSF, PcTX pretreatment, PcTX post-treatment, MK-801, and combined treatment groups, respectively].
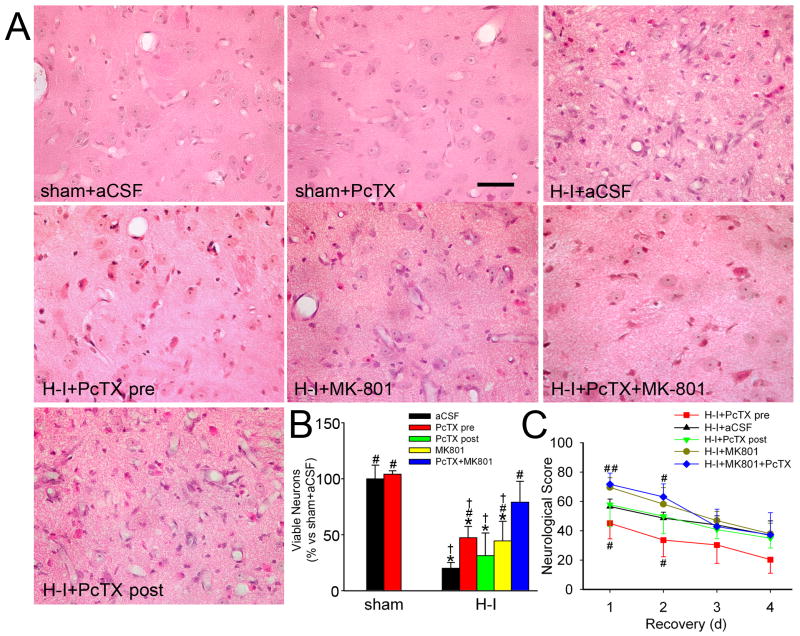
At 4 days of recovery, sham-operated piglets treated with aCSF and PcTX exhibited normal cellular morphology and cytoarchitecture in the striatum (Figure 3A). Consistent with previously published work (Martin et al., 1997; Yang et al., 2010; Yang et al., 2007), H-I piglets treated with aCSF showed selectively distributed ischemic neurons in caudate and putamen, with consistent neuronal death in the central putamen. Most putaminal neurons exhibited ischemic morphology that consisted of cytoplasmic microvacuolation, eosinophilia, and nuclear pyknosis, or no longer had distinct structure based on H&E staining. The number of viable neurons in the putamen of the H-I aCSF group was 20.1 ± 5.0% (n = 7) of the number in the sham-operated, aCSF-injected piglets. Ischemic neuronal injury was significantly less in groups pretreated with PcTX, MK-801, or the combination. The density of viable neurons was significantly increased by PcTX pretreatment (47.4 ± 10%, n = 8). This protective effect was equivalent to that of MK-801 pretreatment (44.5 ± 17.5%, n = 7). Combined treatment with PcTX and MK-801 further protected putamen neurons from H-I injury (79.2 ± 18.5%, n = 7). However, delaying the intraventricular administration of PcTX until 20 mins of recovery diminished its protective effect (Figure 3B). The piglets post-treated with PcTX displayed 31.4 ± 17.5% (n = 7) viable putamen neurons, a value not significantly different from that of the H-I aCSF group. Neuronal injury was less severe and more variable in caudate than in putamen, and pretreatment with PcTX, MK-801, or both drugs did not show a significantly protective effect. The percentage of neurons with ischemic cytopathology was 62 ± 24% in the aCSF group, 68 ± 20% in the PcTX pretreatment group, 69 ± 23% in the MK-801 group, and 80 ± 21% in the combined treatment group.
Figure 3.
Effects of PcTX on neuronal damage and neurologic deficits in piglets subjected to H-I. Piglets exposed to H-I or sham surgery received aCSF, PcTX pretreatment, PcTX post-treatment, MK-801, or PcTX and MK-801 in combination. (A) Representative photographs of H&E-stained sections at 4 days of recovery show normal cellular morphology and cytoarchitecture in putamen of sham-operated animals treated with aCSF and PcTX. All H-I groups contained putaminal neurons that exhibited ischemic morphology (cytoplasmic microvacuolation, eosinophilia, and nuclear pyknosis) or that had lost distinct structure. Scale bar = 40 μm. (B) Quantitative results for viable putamen neurons (expressed as a percentage of the mean value of the sham+aCSF group). (C) Neurologic scores during the 4-day recovery. Data are shown as means ± SD (n = 4 to 8 per group). *P < 0.05 versus sham-operated aCSF group; #P < 0.05 versus H-I aCSF group; † P < 0.05 versus H-I PcTX+MK-801group; ANOVA followed by the Student-Newman-Keuls test. H-I+PcTX pre, H-I piglets pretreated with PcTX; H-I+PcTX post, H-I piglets post-treated with PcTX.
Neurobehavioral deficits were the most severe on the first day of recovery from H-I. At this time point, most H-I piglets treated with aCSF exhibited impaired consciousness, no light and/or no auditory reflexes, and low muscle tone; some showed no response to pain stimulation. Two-way repeated measures ANOVA indicated an overall protective effect of PcTX pretreatment (P<0.001) and time (P<0.001) (Figure 3C). PcTX pretreatment significantly improved neurobehavioral recovery of H-I piglets at 24 h and 48 h of recovery compared with aCSF infusion. Interestingly, the neurobehavioral deficits in the MK-801-treated group were significantly worse than those in the aCSF piglets at 24 h, and the deficits remained worse at 24 h and 48 h in those pretreated with MK-801 and PcTX. Sham-operated piglets administered aCSF or PcTX infusion did not show neurologic deficit after recovery from anesthesia.
Protein kinase A-dependent phosphorylation of NMDA receptor NR1 subunit and DARPP-32 after H-I
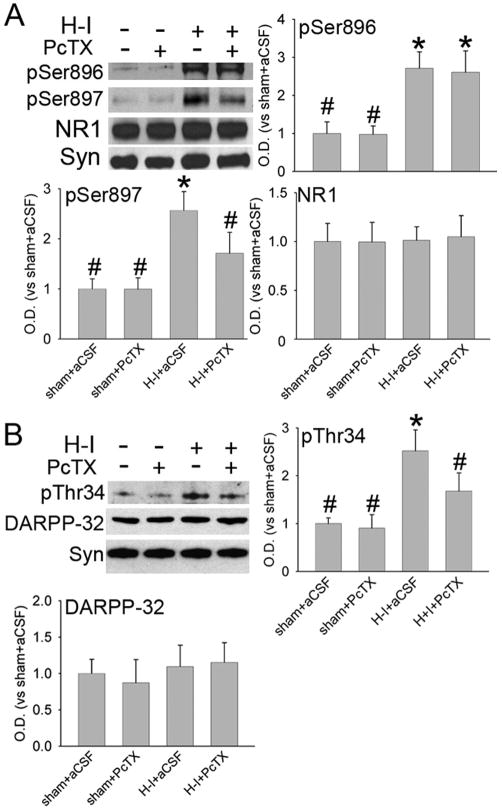
The NMDA receptor NR1 subunit can be phosphorylated by protein kinase A (PKA) at Ser897 or by protein kinase C at Ser896 soon after resuscitation. This phosphorylation plays an important role in neuronal injury in piglet striatum (Guerguerian et al., 2002; Yang et al., 2007). Double immunofluorescence staining indicated that most ASIC1 and NR1 immunoreactivities were co-localized in putamen of sham-operated piglet brains (Figure 2B). Here, we tested whether PcTX alters the level of phosphorylated NR1 after H-I. Western blot results (Figure 4A) showed that PcTX did not change the basal level of phospho-NR1 Ser896 or phospho-NR1 Ser897 in sham-operated piglets (n=4 each). H-I increased phosphorylation of NR1 at Ser897 and Ser896 in putamen at 3 h of recovery (n=6). PcTX pretreatment (n=6) inhibited the increase in phosphorylation of Ser897 but not Ser896. The levels of total NR1 protein remained unchanged after H-I and PcTX treatment.
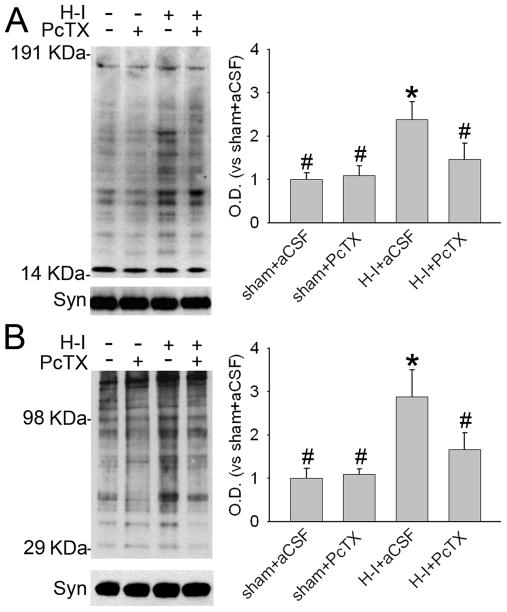
Figure 4.
Western blot analysis showing effects of PcTX pretreatment on levels of phosphorylated and total NMDA receptor NR1 subunit (A) and DARPP-32 (B) in a membrane-enriched fraction of putamen at 3 h of recovery (n = 4 to 6 per group). Synaptophysin (Syn) was used as a loading control. Data (means ± SD) were normalized to the sham+aCSF value. *P < 0.05 versus sham+aCSF group; #P < 0.05 versus H-I+aCSF group; one-way ANOVA followed by the Student-Newman-Keuls test.
Immunoblotting was used to examine the levels of total DARPP-32 protein and PKA-dependent phosphorylation of DARPP-32 at Thr34 in putamen of sham-operated and H-I piglets. Phosphorylation of DARPP-32 at Thr34 is known to inhibit protein phosphatase 1 activity (Svenningsson et al., 2004). PcTX did not change the basal level of phospho-DARPP-32 Thr34 in sham piglets. However, the level of phospho-DARPP-32 Thr34 was significantly induced in H-I piglets at 3 h of recovery (Figure 4B). This increase was significantly attenuated by PcTX pretreatment. In contrast to the change in Thr34 phosphorylation, the level of total DARPP-32 protein was unchanged with H-I and PcTX pretreatment.
Nitrative and oxidative stress after H-I
Peroxynitrite-mediated protein damage is another important feature in H-I–damaged piglet putamen (Martin et al., 2000; Yang et al., 2010; Yang et al., 2007). 3-Nitrotyrosine immunoreactivity has been widely used as a marker for peroxynitrite-induced neurotoxicity. Here, double immunofluorescence staining showed that most 3-nitrotyrosine immunoreactivity co-localized with ASIC1 and phospho-NR1 Ser897 signals in putamen at 3 h after H-I (Figure 2B). Immunoblotting of membrane-enriched extracts from putamen at 3 h after H-I indicated that PcTX pretreatment did not alter the basal level of 3-nitrotyrosine immunoreactivity integrated over multiple protein bands in sham-operated piglets (Figure 5A). With H-I, the total amount of 3-nitrotyrosine immunoreactivity increased in the aCSF group, but this increase was significantly reduced by PcTX treatment. Oxyblot analysis also identified carbonyl groups, a hallmark of the oxidative alteration of proteins. The results indicate significant oxidative alteration to membrane-enriched proteins at 3 h of recovery (Figure 5B). Proteins between 29 and 98 kDa appeared to be prominent targets of oxidative stress. The intensity of these bands was elevated at 3 h of recovery. Likewise, PcTX significantly attenuated carbonyl formation at 3 h of recovery.
Figure 5.
The effect of PcTX pretreatment on H-I–induced nitrative and oxidative stress in putamen at 3 h of recovery. Western blot analysis showed that PcTX decreased H-I–induced 3-nitrotyrosine (A) and carbonyl (B) immunoreactivity on multiple protein bands in putamen of H-I piglets at 3 h of recovery. All data are shown as means ± SD. *P < 0.05 versus sham+aCSF group; #P < 0.05 versus H-I+aCSF group; one-way ANOVA followed by the Student-Newman-Keuls test.
Ebf1 and Gpr6 immunoreactivity at 4 days of recovery in H-I piglets
Dopamine D1 and D2 receptors are expressed on striatonigral and striatopallidal neurons, respectively (Gerfen, 2000; Hersch et al., 1995). We wondered if PcTX selectively rescues striatonigral or striatopallidal neuronal subpopulations. However, it is difficult to determine surviving cell bodies with D1 receptor and D2 receptor immunoreactivity because most of the labelings are dendritic in the neuropil. Ebf1 and Gpr6 are thought to be selectively located in striatal neurons that project to nigra and globus pallidus, respectively (Lobo et al., 2007; Lobo et al., 2006). Antibodies to Ebf1 and Gpr6 reacted specifically with proteins corresponding to these proteins in piglet and mouse striatal extracts (Supplemental Figure 1). We also confirmed this pattern of striatonigral and striatopallidal segregation in piglet striatum by immunohistochemistry. Most piglet putamen cell bodies with D1 receptor signals co-expressed Ebf1 rather than Gpr6, and most D2 receptor-positive cell bodies immunostained with Gpr6 but not Ebf1 (Figure 2C). In addition, Ebf1- and Gpr6-positive cells co-immunostained with ASIC1 (Figure 2D).
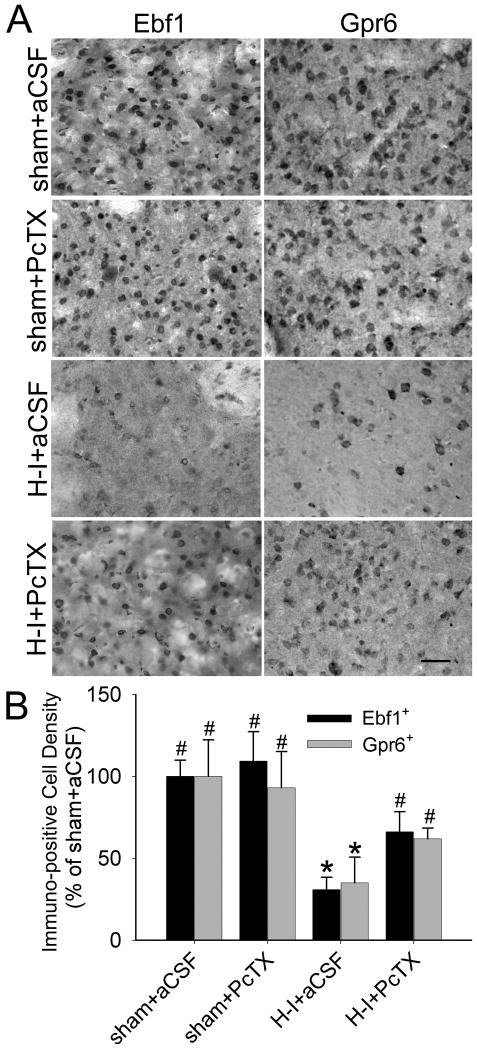
Immunohistochemical results revealed widespread Ebf1- and Gpr6-positive cells throughout putamen of sham-operated piglets (Figure 6). PcTX pretreatment did not change the distribution pattern of Ebf1 and Gpr6 immunoreactivity or the number of Ebf1- and Gpr6-positive cells in putamen of sham-operated animals. H-I significantly decreased the number of Ebf1- and Gpr6-positive cells in putamen at 4 days of recovery. Ebf1-positive cells decreased to 30 ± 8% and Gpr6-positive cells decreased to 35 ± 16% that of the sham-operated, aCSF-treated control group (n = 7 each). PcTX pretreatment significantly attenuated the decrease of Ebf1-positive (66 ± 12%, n = 7) and Gpr6-positive (62 ± 6%, n = 7) cells compared with aCSF infusion (Figure 6B).
Figure 6.
PcTX treatment attenuates the loss of Ebf1- and Gpr6-positive cells in putamen at 4 days of recovery after H-I. Representative immunohistochemical staining shows Ebf1 or Gpr6 signals (A) in putamen of piglets at 4 days of recovery after sham surgery or H-I. Scale bar = 50 μm. (B) Quantitative results indicate that H-I induced a loss of Ebf1- and Gpr6-positive cells in putamen at 4 days of recovery; both were attenuated by PcTX treatment. All data are shown as means ± SD. *P < 0.05 versus sham+aCSFgroup; #P < 0.05 versus H-I+aCSF group; one-way ANOVA followed by the Student-Newman-Keuls test.
Discussion
The present study reveals several findings that help to clarify the evolution and mechanisms of striatal neuronal degeneration in a clinically relevant animal model of pediatric H-I encephalopathy. The results show that 1) brain tissue pH drops markedly during asphyxia and remains below the approximate ASIC1a activation threshold through 30 mins of recovery; 2) PcTx can protect the newborn brain from H-I injury; 3) PcTX has a relatively narrow therapeutic window for protecting striatal neurons from H-I injury; 4) pretreatment with PcTX rescues Ebf1-positive and Gpr6-positive neurons equivalently, thereby implying that striatonigral and striatopallidal neurons are equally protected; 5) pretreatment with PcTX reduces PKA-dependent phosphorylation of DARPP-32 and NMDA receptor subunit NR1 and alleviates nitrative and oxidative damage to proteins after H-I; 6) the partial neuroprotection provided by PcTX pre-treatment in putamen is at least as important as that of an NMDA receptor antagonist; and 7) the protective effect of PcTX was additive with that of an NMDA receptor antagonist.
The brain has increased reliance on anaerobic metabolism during H-I (Vannucci et al., 2005). The glycolysis-induced lactate accumulation combined with increased H+ release from ATP hydrolysis causes a reduction in brain pH. Here, we found that brain parenchymal pH dropped markedly during asphyxia and reached a nadir of 6.71 in sensorimotor cortex at 5 mins of recovery. This pH is sufficient to activate ASIC1a, as it is below the activation threshold of pH 7.0 (Pignataro et al., 2007; Xiong et al., 2004). The decrease in brain tissue pH and subsequent recovery time depend on ischemic severity and duration (Hurn et al., 1991). The relatively short duration of the ischemic period and the full pH recovery during reperfusion may explain why the decrease in tissue pH in this model was not as great and did not last as long as that observed in penumbra regions of animals with focal brain ischemia (Back et al., 2000). In addition, during the early recovery period, immature brain can metabolize the elevated lactate during reperfusion and reoxygenation or rapidly export it into the venous circulation with its unique lactate transport ability (Vannucci and Hagberg, 2004). Thus, it was not unexpected that the brain could recover to a pH above 7.0 by 30 mins.
The demonstrated transient acidic milieu in the piglet brain during asphyxia provided the rationale that PcTX could be protective in this model. PcTX injection into lateral ventricle 20 mins before hypoxia partially protected putamen neurons from H-I. In contrast, PcTX injection at 20 mins of recovery did not produce significant neuroprotection in putamen. The dose and diffusion time need to be considered in interpreting this lack of effect of post-treatment. Xiong et al (Xiong et al., 2004) used an effective dose of 0.2 ng of PcTX (0.4 μl of 500 ng/ml) in mouse brain. Because the piglet brain is about 100 times the weight of a mouse brain, a dose of 20 ng would be expected to give a similar concentration in the CSF. However, because the diffusion distance from the lateral ventricle ependymal surface to the most distant edge of the putamen is nearly 10 mm, we increased the dose of PcTX to 1 μg to accelerate diffusion into a reasonable time frame before much of the PcTX would be cleared by CSF absorption into the venous system. Thus, the dose should have been adequate when administered 20 mins before hypoxia (65 mins before asphyxia), but the diffusion time may have been too long for therapeutic efficacy when injected at 20 mins of recovery and when the brain pH has largely recovered by 30 mins (assuming that tissue pH recovery in putamen paralleled that measured in neocortex). Consequently, PcTX has a relatively short therapeutic window in this model, a finding that contrasts with the prolonged effectiveness of PcTX in focal brain ischemia of adult rodents (Pignataro et al., 2007).
NMDA receptor-mediated excitotoxicity has been implicated in neonatal ischemic damage (Ferriero, 2004; Johnston, 2005), and the NMDA receptor antagonist MK-801 has shown neuroprotective effects in rodent neonatal H-I models (Johnston, 2005). However, NMDA receptor-mediated synaptic activity promotes neuronal survival. MK-801 can cause reversible vacuolization in cortical neurons even in a dose of 0.1mg/kg (Allen and Iversen, 1990) and can trigger widespread apoptosis in the developing brain (Ikonomidou et al., 1999). In addition, NMDA receptors mediate brain stem activity in response to hypoxia and help sustain ventilation in the newborn (Panigrahy et al., 2000). In contrast to the effects in immature rodents, intravenous injection of 3 mg/kg MK-801 provides no neuroprotection and worsens neurologic outcomes in a piglet model of carotid artery ligation (LeBlanc et al., 1991). In the present model, a lower dose of 1 mg/kg MK-801 protected putaminal neurons from H-I injury but aggravated neurologic deficit during the first day of recovery. The greater neurological deficit after treatment with MK-801 or MK-801 plus PcTX, despite improved neuronal viability, presumably reflects the adverse behavioral side effects that have limited the clinical use of NMDA antagonists. Although the purpose of this study was not to find an optimal dose, it is possible that lower doses of MK-801 would minimize the augmented early functional deficits and still provide neuroprotection.
ASIC1a and NMDA receptors are activated in ischemic brain and likely cross-talk to enhance ischemic neuronal death. Previous work suggested that ASIC1a channels may act on NMDA receptors and modulate their function. ASIC1 knockout mice have decreased NMDA receptor activation during high-frequency stimulation (Wemmie et al., 2002). Furthermore, the neuroprotective effect of an NMDA receptor antagonist is not as prominent in ASIC1 knockout mice as in wild-type mice (Xiong et al., 2004). PcTX significantly reduces NMDA-induced cell death (Gao et al., 2005). Moreover, combined therapy of ASIC1a blockade and NMDA receptor antagonism results in a further reduction of brain damage and extends the therapeutic time window for NMDA receptor blockade (Pignataro et al., 2007). However, no work has shown how ASIC1a modulates the activity of the NMDA receptor. Considering ASIC-1 promotion of dopamine signaling (Voglis and Tavernarakis, 2008) and the integrated relationship between dopamine and NMDA receptors in striatal neurons, we hypothesized that ASIC1a modulates the activity of the NMDA receptor through DARPP-32-dependent phosphorylation of NMDA receptor subunit NR1 (Svenningsson et al., 2004; Yang et al., 2007).
Phosphorylation is an important mechanism for the regulation of NMDA receptor function. Although it is not fully understood how phosphorylation of NR1 at Ser897 modulates NMDA receptor activity, PKA-dependent phosphorylation of NR1 enhances Ca2+ influx (Skeberdis et al., 2006) and promotes forward trafficking of the receptor from the endoplasmic reticulum to the synapse (Scott et al., 2003). Therefore, increased NR1 phosphorylation in H-I piglet striatum may signify increased activation or potentiation of NMDA receptors. This process is relevant to the pathophysiology of striatal neuron death (Guerguerian et al., 2002; Yang et al., 2007). In the present study, ASIC1, phosphorylated NR1, and 3-nitrotyrosine co-localized in ischemic striatum. PcTX pretreatment attenuated PKA-dependent phosphorylation of NR1 Ser897 and decreased nitrative and oxidative stress in H-I piglets. These results suggest that the neuroprotective effect of PcTX is, at least in part, associated with attenuated PKA-dependent NR1 phosphorylation.
Many factors are involved in the PKA-dependent phosphorylation of NR1 after H-I. For example, agonists for dopamine D1 receptors but not D2 receptors increase overall phosphorylation of NR1 (Snyder et al., 1998). Stimulation of D1 receptors leads to phosphorylation of NR1 Ser897 (Dunah and Standaert, 2001). In addition, D1 receptor activation increases PKA-dependent phosphorylation of Thr34 on DARPP-32, which permits sustained phosphorylation of serine and threonine sites on NR1 by inhibition of protein phosphatase-1; D2 receptor activation has the opposite effect (Svenningsson et al., 2004). Our results show that PcTX attenuates phosphorylation of both DARPP-32 Thr34 and NR1 Ser897 and suggest that the D1 receptor/PKA/DARPP-32 cascade may be involved in ASIC1a-neuronal injury after H-I. If so, PcTX may selectively protect striatonigral neurons with D1 receptors rather than striatopallidal neurons with D2 receptors. However, the D1 and D2 receptors are diffusely distributed throughout the neuropil with less localization in striatal cell bodies (Hersch et al., 1995; Yang et al., 2007), thereby making it difficult to quantify selective neuronal loss. In our current work, Ebf1 and Gpr6 serve as alternative cell markers for D1- and D2-receptor-positive cells, respectively. We found that PcTX pretreatment protected Ebf1- and Gpr6-positive cells equivalently, as ASIC1 immunoreactivity was evident in most striatal neurons and co-localized with Ebf1 and Gpr6. Therefore, ASIC1 expression does not distinguish between D1- and D2-receptor-positive neurons. This observation is consistent with the finding that PcTX protected striatonigral and striatopallidal neurons. The fact that PcTX protected striatal neurons independent of dopamine receptor subtype suggests that neurotoxic ASIC1a channel activation has an indirect relationship with postsynaptic dopamine receptor signaling or that ASIC1a channels are involved in presynaptic mechanisms of dopamine release.
Differences in timing of ASIC1a and NMDA receptor activation may contribute to the observed additive protection afforded by PcTX and MK-801. ASIC1a is presumably activated during asphyxia and early recovery when parenchymal pH is <7.0. However, currents through NMDA-activated channels are known to be suppressed at pH levels achieved during ischemia (Giffard et al., 1990). Thus, NMDA receptor-mediated excitotoxicity is likely to be more prominent at later times of recovery, when ASIC1a is less active. Blocking Na+ and Ca2+ entry in different phases of ischemia/reperfusion may provide additive protection. However, this explanation is probably an oversimplification because of the ability of PcTX to reduce NR1 phosphorylation at 3 h, long after parenchymal pH has recovered. One possibility is that early oxidative stress initiated by ASIC1a activation has long-lasting effects on DARPP-32 Thr 34 phosphorylation, which is known to inhibit protein phosphatase-1. Inhibition of protein phosphatase-1 would, in turn, maintain NR1 in a phosphorylated state and presumably prolong excitotoxicity and consequent oxidative stress. Analogously, pretreatment with a D1 receptor antagonist reduces DARPP-32 Thr34 and NR1 Ser897 phosphorylation at 3 h of recovery, yet dopamine in microdialysates largely recovers by 30 mins after reoxygenation. Thus, promotion of excitotoxic stress at later times of recovery may be dependent on dopaminergic and acidic stimulation that occur earlier during ischemia/reperfusion.
One limitation of this study is that the solution of PcTX contains multiple proteins. Although we cannot exclude off-target effects in piglet brain, the observation that mice with ASIC1a gene deletion have the same ischemic neuroprotection as PcTX treatment of wild-type mice provides some assurance that the effects of PcTX are largely mediated by ASIC1a effects (Pignataro et al., 2007; Xiong et al., 2004).
In conclusion, this study demonstrates that treatment with ASIC1a blocker PcTX before hypoxia reduces neuronal injury in selectively vulnerable putamen and accelerates neurologic recovery in a large-animal model of neonatal H-I encephalopathy. This protection was associated with a marked reduction in nitrative and oxidative damage to proteins. These effects may be mediated, at least in part, by modulation of NMDA receptor phosphorylation. Combined inhibition of ASIC1a channels and NMDA receptors had a greater neuroprotective effect than each individual treatment. This additive effect implies that ASIC1a and NMDA receptors also act independently in damaged neurons. Therefore, ASIC1a is another important channel that contributes to oxidative stress and neurodegeneration in global cerebral ischemia in immature brain.
Supplementary Material
Research Highlights.
Brain tissue pH drops during asphyxia and early recovery in neonatal H-I pig; PcTX pretreatment reduces oxidative damages and protects striatal neurons; this effect is as important as and additive with that of MK-801; PcTX reduces PKA-dependent phosphorylation of DARPP-32 and NMDA receptor subunit NR1; striatonigral and striatopallidal neurons are equally protected.
Acknowledgments
This work was supported by NIH grant NS060703 (R.C.K.) and by an American Heart Association-Phillips Resuscitation Research Fellow Award (Z.-J.Y.). We are grateful to Claire Levine for her editorial assistance of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnew DM, Koehler RC, Guerguerian AM, Shaffner DH, Traystman RJ, Martin LJ, Ichord RN. Hypothermia for 24 hours after asphyxic cardiac arrest in piglets provides striatal neuroprotection that is sustained 10 days after rewarming. Pediatr Res. 2003;54:253–62. doi: 10.1203/01.PDR.0000072783.22373.FF. [DOI] [PubMed] [Google Scholar]
- Allen HL, Iversen LL. Phencyclidine, dizocilpine, and cerebrocortical neurons. Science. 1990;247:221. doi: 10.1126/science.2403696. [DOI] [PubMed] [Google Scholar]
- Back T, Hoehn M, Mies G, Busch E, Schmitz B, Kohno K, Hossmann KA. Penumbral tissue alkalosis in focal cerebral ischemia: relationship to energy metabolism, blood flow, and steady potential. Ann Neurol. 2000;47:485–92. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–58. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–46. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res. 1990;506:339–42. doi: 10.1016/0006-8993(90)91276-m. [DOI] [PubMed] [Google Scholar]
- Guerguerian AM, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia-ischemia. Brain Res Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–37. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Koehler RC, Norris SE, Blizzard KK, Traystman RJ. Dependence of cerebral energy phosphate and evoked potential recovery on end-ischemic pH. Am J Physiol. 1991;260:H532–41. doi: 10.1152/ajpheart.1991.260.2.H532. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009;162:55–66. doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–40. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–83. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Laptook AR, Corbett RJ, Arencibia-Mireles O, Ruley J. Glucose-associated alterations in ischemic brain metabolism of neonatal piglets. Stroke. 1992;23:1504–11. doi: 10.1161/01.str.23.10.1504. [DOI] [PubMed] [Google Scholar]
- LeBlanc MH, Vig V, Smith B, Parker CC, Evans OB, Smith EE. MK-801 does not protect against hypoxic-ischemic brain injury in piglets. Stroke. 1991;22:1270–5. doi: 10.1161/01.str.22.10.1270. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Cui Y, Ostlund SB, Balleine BW, Yang XW. Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat Neurosci. 2007;10:1395–7. doi: 10.1038/nn1987. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–52. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–85. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Price AC, Kaiser A, Agnew DM, Ichord RN, Traystman RJ. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol Dis. 2000;7:169–91. doi: 10.1006/nbdi.2000.0282. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–8. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy A, Rosenberg PA, Assmann S, Foley EC, Kinney HC. Differential expression of glutamate receptor subtypes in human brainstem sites involved in perinatal hypoxia-ischemia. J Comp Neurol. 2000;427:196–208. doi: 10.1002/1096-9861(20001113)427:2<196::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–8. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- Salinas-Zeballos M, Zeballos GA, Gootman PM. A sterotaxic atlas of the developing swine (Sus scrofa) forebrain. New York: Plenum Press; 1986. [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–67. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Simon R, Xiong Z. Acidotoxicity in brain ischaemia. Biochem Soc Trans. 2006;34:1356–61. doi: 10.1042/BST0341356. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–10. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–96. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tomlinson FH, Anderson RE, Meyer FB. Acidic foci within the ischemic penumbra of the New Zealand white rabbit. Stroke. 1993;24:2030–9. doi: 10.1161/01.str.24.12.2030. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Brucklacher RM, Vannucci SJ. Glycolysis and perinatal hypoxic-ischemic brain damage. Dev Neurosci. 2005;27:185–90. doi: 10.1159/000085991. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–54. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. Embo J. 2008;27:3288–99. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–77. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Carter EL, Torbey M, Martin LJ, Koehler RC. Sigma receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates neuronal nitric oxide synthase/postsynaptic density-95 coupling mechanisms and protects against neonatal ischemic degeneration of striatal neurons. Exp Neurol. 2010;221:166–174. doi: 10.1016/j.expneurol.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, Koehler RC. Dopamine receptor modulation of hypoxic-ischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metab. 2007;27:1339–51. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium. 2004;36:247–55. doi: 10.1016/j.ceca.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–7. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.