Abstract
Background
Causes of autism are unknown. Associations with maternal nutritional factors and their interactions with gene variants have not been reported.
Methods
Northern California families were enrolled from 2003–2009 in the CHARGE (CHildhood Autism Risks from Genetics and Environment) population-based case-control study. Children aged 24–60 months were evaluated and confirmed to have autism (n = 288), autism spectrum disorder (n = 141), or typical development (n = 278) at the University of California–Davis Medical Investigation of Neurodevelopmental Disorders Institute using standardized clinical assessments. We calculated adjusted odds ratios (ORs) for associations between autism and retrospectively collected maternal vitamin intake before and during pregnancy. We explored interaction effects with functional genetic variants involved in one-carbon metabolism (MTHFR, COMT, MTRR, BHMT, FOLR2, CBS, and TCN2) as carried by the mother or child.
Results
Mothers of children with autism were less likely than those of typically developing children to report having taken prenatal vitamins during the three months before pregnancy or the first month of pregnancy (OR = 0.62 [95% confidence interval = 0.42–0.93]). Significant interaction effects were observed for maternal MTHFR 677 TT, CBS rs234715 GT+TT, and child COMT 472 AA genotypes, with greater risk for autism when mothers did not report taking prenatal vitamins periconceptionally (4.5 [1.4–14.6]; 2.6 [1.2–5.4]; and 7.2 [2.3–22.4], respectively). Greater risk was also observed for children whose mothers had other one-carbon metabolism pathway gene variants and reported no maternal prenatal vitamin intake.
Conclusions
Periconceptional use of prenatal vitamins may reduce the risk of having children with autism, especially for genetically susceptible mothers and children. Replication and mechanistic investigations are warranted.
Autism is a neurodevelopmental disorder defined by the presence of impaired social reciprocity, abnormal communication, and restricted interests or repetitive behavior, with symptoms apparent by three years of age. Prevalence of autism spectrum disorders is approximately one in 110 children in the United States,1 and incidence appears to be rising.2 Although genetic contributions to autism etiology are widely accepted, the rising incidence, inconsistent findings from genetic studies, and less-than-100% concordance in monozygotic twins suggest a role for multiple interactions between susceptibility genes and environmental factors.3 A recently reported association between short intervals between pregnancies and autism suggests that nutritional factors could play a role.4 Additional research demonstrates that valproic acid, which disrupts folate metabolism, can induce autism.5 The neuropathology may originate early in pregnancy5—a time when folate and other B vitamins are known to be critical to neurodevelopment, with potential to prevent up to 70% of neural tube defects.6
Previous studies suggest that some children with autism have altered B vitamin metabolism and reduced methylation capacity.7 A few studies have reported independent associations between autism and functional gene variants within the B vitamin-dependent folate, methionine, and transmethylation pathways (eFigure, http://links.lww.com), including variants in 5,10-methylenetetrahydrofolate reductase (MTHFR), dihydrofolate reductase (DHFR), solute carrier family 19, member 1 (SLC19A1, RFC1), 5-methyltetrahydrofolate-homocysteine methyltransferase (MTRR), transcobalamin II (TCN2), and catechol-O-methyltransferase (COMT),8–11 with findings replicated only for MTHFR.9–10 These reports did not address maternal vitamin intake, and only one considered maternal genotypes,11 even though maternal polymorphisms can influence fetal health.12
Our objective was to examine associations between autism and maternal vitamin supplement intake in the preconception and prenatal periods both—alone and in combination with common functional maternal and child gene polymorphisms in folate, methionine, and transmethylation pathways.
METHODS
Participants, Eligibility and Diagnostic Criteria
Families were recruited through the large, population-based, case-control CHARGE (CHildhood Autism Risks from Genetics and Environment) Study.13 Eligible children were between 24 and 60 months, living with at least one biologic parent who spoke English or Spanish, born in California, and residing in the catchment areas of a specified group of Regional Centers (primarily in Northern California) that coordinate services for persons with developmental disabilities. Children with autism or with intellectual disability or developmental delay were identified through the state’s Department of Developmental Services, provider and clinic referrals, general public outreach, and self-referrals. General population controls were identified from state birth files and were frequency-matched to the projected age, sex and catchment area distribution of the autism cases. All children were assessed for cognitive function using the Mullen Scales of Early Learning and for adaptive function using the Vineland Adaptive Behavior Scales, as described previously.13 Autism cases were assessed using the Autism Diagnostic Interview–Revised14 and the Autism Diagnostic Observation Schedule–Generic15 to confirm child’s diagnostic group. The children recruited from the general population or with developmental delays were screened for evidence of autism spectrum disorder using the Social Communication Questionnaire. Those who scored above 15 were further evaluated for autism. Assessments were carried out at the Medical Investigation of Neurodevelopmental Disorders (M.I.N.D.) Institute clinic by trained staff in English or in Spanish. Final autism case status was defined as meeting criteria on the Communication, Social, and Repetitive Behavior domains of the ADI–R and scoring at or above the total cutoff for autistic disorder on the ADOS Module 1 or 2. Autism spectrum disorder was defined using the criteria of Risi et al.16 Study protocols were approved by the University of California Davis Institutional Review Board and the State of California Committee for the Protection of Human Subjects. No data were collected without informed parental consent. Children were included in these analyses if they were the first child within the family recruited into the study, they were born after 1999, and their parents completed the study interview from 2003 through July 2009.
Data Collection
Mothers were asked in telephone interviews whether they consumed prenatal vitamins, multivitamins, selected nutrient-specific vitamins, cereals, and other supplements at any time during the period three months before conception through pregnancy and the breastfeeding period. If they had, mothers were further asked the brand, dose and frequency of consumption, and in which months they consumed the item (eAppendix, http://links.lww.com). When given, the brand of the vitamin was used to check the appropriateness of the vitamin category. The periconceptional period was defined as the period three months before pregnancy through the first month of pregnancy.
Genotyping Methods
Blood was collected from all family members as part of the study protocol, and genomic DNA was isolated from peripheral blood leukocytes using standard procedures (Puregene kit; Gentra Inc). MTHFR 677C>T (rs1801133), MTHFR A1298C (rs1801131), COMT 472G>A (rs4680), MTRR A66G (rs1801394), BHMT G716A (rs3733890), TCN2 C776G (rs1801198), CBS rs234715, MTR 2756A>G (rs1805087), FOLR1 rs2071010, and FOLR2 rs2298444 variants were genotyped in maternal, paternal, and child samples using the TaqMan system from Applied Biosystems, Inc. (ABI, Foster City, CA). Selection of these common, functional, one-carbon metabolism gene variants was based on previous findings for associations with higher homocysteine levels, neural tube defects, and autism, and the availability of a genotyping assay through ABI’s “Assays on Demand” database. Maternal and child MTHFR 677C>T, COMT 472G>A, MTRR A66G, BHMT G716A, FOLR2 rs229844, CBS rs234715, and TCN2 C776G variants were selected for evaluation of interaction effects based on previous literature and adequate numbers for stratification.
Statistical Analysis
We constructed logistic regression models adjusted for confounding variables to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for vitamin intake and maternal, paternal, and infant genotypes using SAS, version 9.2 software (SAS Institute Inc., Cary, North Carolina). We examined the following variables as potential confounders of the association between prenatal vitamin intake and autism: child’s sex, birth year, parent-reported race/ethnicity, family history of mental health conditions, paternal age at child’s birth, maternal age at child’s birth, education, pre-pregnancy body mass index (BMI) category, cereal intake from three months before through the first month of pregnancy, cigarette smoking, alcohol consumption, and residence with a smoker during the period three months before pregnancy to delivery. We retained covariates causing a 10% or greater change in the estimated log of the exposure odds ratio. Only maternal education and the child’s year of birth met this criterion. We used stratified analyses and interaction terms to examine effect modification by child sex and race/ethnicity, and maternal age, education and pre-pregnancy BMI categories. We also examined effect modification for vitamin intake by genotype. Population structure bias was assessed for each gene variant using case trios and parents of typically developing children in a hybrid log-linear model by adding an interaction term between case status and mating-type parameters, and by testing the improvement in fit with both a 5-degree-of-freedom likelihood-ratio-test and a more sensitive 1-degree-of-freedom test-for-trend across mating-type parameters.17 To ensure that the results represented the study base, we used survey research methods to estimate the logistic regression models, with participants assigned weights equal to the inverse of the estimated response probability in strata defined by the entry case group and demographic factors.18 Sensitivity analyses assessed the impact of missing data, using multiple imputation via the Markov Chain Monte Carlo algorithm.19
RESULTS
Participant Characteristics
Of the families contacted and deemed eligible, 26% of children from the general population and 30% of children from the autism pool were enrolled. Of the participants included in the vitamin analyses who were the first child enrolled in their family and whose parents had completed an exposure questionnaire by July 2009, 278 children met diagnostic criteria for typical development, 288 met the criteria for autism, and 144 met the criteria for autism spectrum disorder. Children with autism differed somewhat from typically developing children in racial distribution, with a higher percentage Asian and a lower percentage mixed or other (Table 1). Mothers of children with autism were sociodemographically similar to mothers of typically developing children (Table 1).
TABLE 1.
Characteristics of Children with Autism and with Typical Development and their Mothers in the Childhood Autism Risks from Genetics and the Environment Study, Northern California, 2003–2009
| Characteristics | Eligible Families
|
Genotyped Families
|
||
|---|---|---|---|---|
| Autism (n = 288) | Typical Development (n = 278) | Autism (n = 238) | Typical Development (n = 232) | |
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Child sex | ||||
| Boy | 251 (87) | 228 (82) | 206 (87) | 192 (83) |
| Girl | 37 (13) | 50 (18) | 32 (13) | 40 (17) |
| Child race/ethnicity | ||||
| Non-Hispanic white | 146 (51) | 141 (51) | 126 (53) | 117 (50) |
| Hispanic | 82 (28) | 82 (30) | 60 (25) | 66 (28) |
| Non-Hispanic black | 6 (2) | 6 (2) | 6 (3) | 4 (2) |
| Asian | 24 (8) | 8 (3) | 20 (8) | 7 (3) |
| Mixed and other | 30 (10) | 41 (15) | 26 (11) | 38 (16) |
| Maternal age at child’s birth (years) | ||||
| ≤ 19 | 9 (3) | 14 (5) | 7 (3) | 11 (5) |
| 20–29 | 125 (45) | 106 (38) | 106 (45) | 89 (38) |
| 30–34 | 95 (34) | 107 (39) | 81 (34) | 95 (41) |
| 35 + | 51 (18) | 49 (18) | 44 (18) | 37 (16) |
| Maternal birthplace | ||||
| United States | 214 (74) | 223 (80) | 183 (77) | 187 (81) |
| Mexico | 21 (7) | 18 (6) | 12 (5) | 15 (6) |
| Other | 53 (18) | 37 (13) | 43 (18) | 30 (13) |
| Maternal education | ||||
| High school graduate or less | 44 (15) | 44 (16) | 35 (15) | 41 (18) |
| Some college, technical, vocational, associate degree | 104 (36) | 86 (31) | 85 (36) | 69 (30) |
| Bachelor, masters, professional, doctorate degree | 139 (48) | 148 (53) | 117 (49) | 122 (53) |
| Insurance delivery type | ||||
| Private | 240 (83) | 243 (87) | 203 (85) | 201 (87) |
| Government program | 48 (17) | 35 (13) | 35 (15) | 31 (13) |
| Intention to become pregnant | ||||
| Intended to become pregnant when she did | 181 (67) | 185 (70) | 159 (69) | 155 (69) |
| Indifferent about becoming pregnant at that time | 33 (12) | 34 (13) | 28 (12) | 32 (14) |
| Intended to become pregnant later | 39 (14) | 32 (12) | 33 (14) | 25 (11) |
| Did not intend to become pregnant at all | 18 (7) | 14 (5) | 12 (5) | 13 (6) |
| Maternal cigarette smokinga | ||||
| No | 230 (87) | 236 (89) | 196 (86) | 199 (89) |
| Yes | 35 (13) | 28 (11) | 32 (14) | 24 (11) |
| Maternal alcohol consumptiona | ||||
| No | 147 (55) | 124 (49) | 121 (52) | 105 (49) |
| Yes | 121 (45) | 131 (51) | 110 (48) | 111 (51) |
| Periconceptional maternal prenatal vitaminb | ||||
| No | 111 (44) | 70 (31) | 89 (42) | 63 (32) |
| Yes | 143 (56) | 159 (69) | 125 (58) | 133 (68) |
| Periconceptional maternal multivitaminb,c | ||||
| No | 106 (82) | 91 (83) | 85 (83) | 78 (84) |
| Yes | 23 (18) | 18 (17) | 18 (17) | 15 (16) |
Any reported for the period three months before pregnancy through the end of pregnancy.
Any reported for the three months before pregnancy and the first month of pregnancy.
Mothers who reported intake of prenatal vitamins during this period were excluded.
We obtained biologic samples and genotyped 232 families (83%) of children with typical development and 238 families (83%) of children with autism disorder. Genotyped families closely resembled all eligible families for both cases and controls, including their vitamin intake (Table 1).
Maternal Prenatal Vitamin Intake
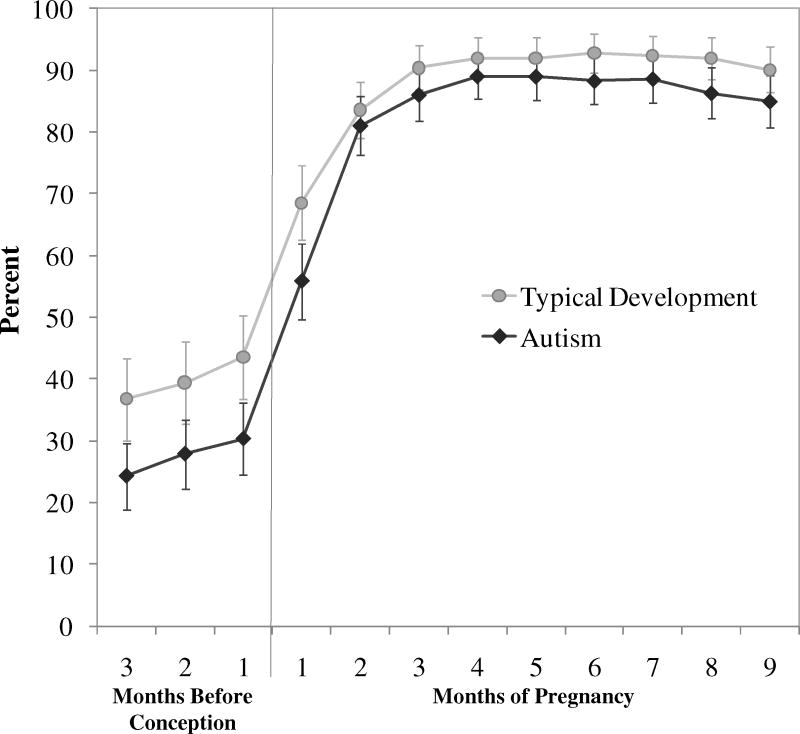
Maternal prenatal vitamin consumption for the three months before pregnancy through delivery was reported for 269 children (97%) with typical development and 276 children (96%) with autism. The percentage of mothers who reported consuming prenatal vitamins increased after the first month of pregnancy, and by the end of the first trimester, approximately 90% of mothers with children in both groups reported consuming prenatal vitamins (Figure). Mothers of children with autism were less likely to report having consumed prenatal vitamins than mothers of typically developing children during all months examined, especially during the three months before and the first month of pregnancy (Figure). After adjustment for maternal education and child’s birth year, prenatal vitamin use during that period was associated with lower risk for autism (unweighted OR = 0.62 [95% CI = 0.42–0.93]) (Table 2). No associations were observed for intake during months two through nine of pregnancy. Increased frequency of prenatal vitamin intake during the periconceptional period was associated with further reductions in autism risk (2-sided test for trend, P = 0.002) (Table 2). Reported use of ordinary multivitamins around the time of conception was not associated with decreased risk of autism compared with those taking no vitamins (1.1 [0.57–2.3]) (Table 2).
Figure. Prenatal vitamin use by perinatal month.
Percent (95% confidence interval) of mothers of children with autism and mothers of typically developing children reporting prenatal vitamin intake for each month from three months before and throughout pregnancy.
TABLE 2.
Associations between Maternal Vitamin Intake Three Months Before Pregnancy through the First Month of Pregnancy and Risk for Autism
| Periconceptional Vitamin Intakea | Autism (n = 288) No. (%) |
Typical Development (n = 278) No. (%) |
OR (95% CI)b | Weighted OR (95% CI)b |
|---|---|---|---|---|
| Prenatal Vitamin | ||||
| Nonec | 111 (44) | 70 (31) | 1.0 | 1.0 |
| Any | 143 (56) | 159 (69) | 0.62 (0.42–0.93) | 0.61 (0.39–0.97) |
| Prenatal Vitamin Frequencyd | ||||
| Nonec | 111 (44) | 70 (31) | 1.0 | 1.0 |
| Irregular or < 4 days/week | 4 (2) | 3 (1) | 0.76 (0.16–3.6) | 0.96 (0.18–5.0) |
| 4 Days/week daily | 130 (51) | 142 (62) | 0.64 (0.43–0.95) | 0.61 (0.38–0.98) |
| > Daily | 9 (4) | 14 (6) | 0.47 (0.19–1.2) | 0.51 (0.19–1.4) |
| Test for trende | P = 0.002 | P = 0.002 | ||
| Multivitaminf | ||||
| Nonec | 109 (82) | 91 (83) | 1.0 | 1.0 |
| Any | 24 (18) | 18 (17) | 1.1 (0.57–2.3) | 1.2 (0.51–2.6) |
Any reported for the three months before pregnancy and the first month of pregnancy.
Odds ratios adjusted for maternal education and child’s birth year.
Reference category.
Frequency categories determined a priori based on similar vitamin metabolite levels for those taking supplements regularly (most days/week) and those taking them daily. If those with 4–6 days/week are moved to the second category, the test for trend remains similar (P=.003).
Two-sided p value for test of trend.
Mothers who reported intake of prenatal vitamins during this period were excluded.
Non-Hispanic white, college-educated mothers, and those not born in Mexico were more likely to report taking prenatal vitamins and multivitamins during the three months before and first month of pregnancy (eTable 1, http://links.lww.com). Adjusted ORs for reported periconceptional prenatal vitamin intake varied little by maternal education and race/ethnicity (data not shown). Women who are not planning a pregnancy are unlikely to take prenatal supplements before conceiving. We therefore conducted an analysis restricted to women who intended to become pregnant (66% of all women), finding similar results (0.53 [0.32–0.87]). Age of the child (representing duration of recall period) had little influence on the OR: for mothers with children under the age of 4 years at the time of interview, OR=0.63 (CI = 0.39–1.0), and for those with children age 4 and older, OR = 0.69 (0.35–1.4). Sensitivity analyses with imputed values for missing prenatal vitamin intake showed the association was robust and held even when people with missing data were intentionally assigned such that no association was present in this group.
Interaction Effects between Relevant Genotypes and Periconceptional Prenatal Vitamin Intake
All polymorphisms had genotype distributions consistent with expected Hardy-Weinberg equilibrium proportions (P>.05) among control families. We found no evidence for population structure bias by either test for any of the variants. Genotyping success rates were above 95% for mothers and children in both diagnostic groups (data not shown). Allele frequencies for MTHFR 677T (35%), COMT 472A (46%), MTRR 66G (52%), BHMT 716A (32%), FOLR2 rs2298444 (16%), CBS rs234715 (24%), and TCN2 776G (47%) in non-Hispanic white parents of typically developing children were similar to those reported in other white populations (33–37%,7,9,19 43–52%,19 44–55%,19 28–34%,19 0–22%,19 22–30%,19 and 45%,19 respectively).
Interactions were observed for autism between lack of reported periconceptional maternal prenatal vitamin intake and both maternal MTHFR 677 TT (test for interaction P = 0.04) and CBS rs234715 GT+TT (test for interaction P = 0.05) genotypes, with combined ORs of 4.5 (CI = 1.4–14.6) and 2.6 (1.2–5.4), respectively (Table 3). The child variants for both of these genes were not associated with increased risk for autism, and child MTHFR 677 TT tended toward an inverse association. However, children with the COMT 472 AA genotype were at increased risk for autism if their mothers reported having taken periconceptional prenatal supplements (OR = 1.8 [CI = 0.99–3.5]), and were at substantially higher risk if their mothers did not (7.2 [2.3–22.4]; test for interaction P = .05) (Table 3). Interaction effects were not statistically significant for other gene variants, although risk for autism associated with most maternal gene variants tended to be higher than predicted by additive or multiplicative interaction models when mothers did not report taking prenatal vitamins during the periconceptional period (Table 3).
TABLE 3.
Maternal and Child Genotypes by Maternal Prenatal Vitamin Intake Three Months Before Pregnancy and the First Month of Pregnancy and Risk for Autism
| Gene Variant | Periconceptional Prenatal Vitamin | Genotype | Autism No. (%) | Typical Development No. (%) | OR(95% CI)a | Expected Joint OR: Additive Model | Expected Joint OR: Multiplicative Model | |
|---|---|---|---|---|---|---|---|---|
| MTHFR 667 | Maternal | Yes | CC+CTb | 105 (51) | 106 (57) | 1.0 | ||
| TT | 16 (8) | 22 (12) | 0.74 (0.36–1.5) | |||||
| No | CC+CT | 68 (33) | 53 (29) | 1.2 (0.77–2.0) | ||||
| TT | 15 (7) | 4 (2) | 4.5 (1.4–14.6) | 0.97 | 0.91 | |||
| Child | Yes | CC+CTb | 109 (54) | 104 (58) | 1.0 | |||
| TT | 10 (5) | 18 (10) | 0.59 (0.26–1.35) | |||||
| No | CC+CT | 73 (36) | 47 (26) | 1.4 (0.88–2.4) | ||||
| TT | 9 (4) | 11 (6) | 0.63 (0.23–1.7) | 1.0 | 0.84 | |||
| COMT 472 | Maternal | Yes | GG+GAb | 89 (45) | 109 (58) | 1.0 | ||
| AA | 26 (13) | 20 (11) | 1.6 (0.75–3.4) | |||||
| No | GG+GA | 70 (35) | 50 (27) | 1.8 (1.0–3.0) | ||||
| AA | 13 (7) | 8 (4) | 1.6 (0.53–4.7) | 2.2 | 2.6 | |||
| Child | Yes | GG+GAb | 89 (43) | 100 (56) | 1.0 | |||
| AA | 32 (16) | 22 (12) | 1.8 (0.99–3.5) | |||||
| No | GG+GA | 59 (30) | 54 (30) | 1.3 (0.76–2.1) | ||||
| AA | 22 (11) | 4 (2) | 7.2 (2.3–22.4) | 2.1 | 2.3 | |||
| CBS | Maternal | Yes | GGb | 84 (42) | 88 (47) | 1.0 | ||
| GT+TT | 35 (17) | 42 (22) | 0.99 (0.52–1.9) | |||||
| No | GG | 46 (23) | 40 (21) | 1.0 (0.58–1.8) | ||||
| GT+TT | 37 (18) | 18 (10) | 2.6 (1.2–5.4) | 1.0 | 1.0 | |||
| Child | Yes | GGb | 84 (42) | 79 (43) | 1.0 | |||
| GT+TT | 34 (17) | 44 (24) | 0.61 (0.32–1.2) | |||||
| No | GG | 53 (27) | 40 (22) | 1.2 (0.67–2.0) | ||||
| GT+TT | 29 (15) | 20 (11) | 1.2 (0.57–2.7) | 0.78 | 0.71 | |||
| MTRR 66 | Maternal | Yes | AAb | 41 (19) | 36 (19) | 1.0 | ||
| AG+GG | 83 (39) | 97 (50) | 0.85 (0.49–1.5) | |||||
| No | AA | 26 (12) | 26 (13) | 0.88 (0.43–1.8) | ||||
| AG+GG | 61 (29) | 35 (18) | 1.6 (0.84–3.0) | 0.73 | 0.75 | |||
| Child | Yes | AAb | 34 (17) | 40 (23) | 1.0 | |||
| AG+GG | 83 (42) | 82 (46) | 1.3 (0.72–2.2) | |||||
| No | AA | 26 (13) | 19 (11) | 1.6 (0.74–3.5) | ||||
| AG+GG | 55 (28) | 36 (20) | 1.8 (0.96–3.5) | 1.9 | 2.0 | |||
| BHMT 716 | Maternal | Yes | GGb | 63 (31) | 62 (33) | 1.0 | ||
| GA+AA | 58 (28) | 67 (36) | 0.94 (0.54–1.6) | |||||
| No | GG | 39 (19) | 30 (16) | 1.1 (0.55–2.0) | ||||
| GA+AA | 45 (22) | 28 (15) | 1.7 (0.89–3.3) | 0.99 | 0.99 | |||
| Child | Yes | GGb | 58 (29) | 57 (31) | 1.0 | |||
| GA+AA | 62 (31) | 65 (36) | 0.86 (0.50–1.5) | |||||
| No | GG | 42 (21) | 33 (18) | 1.2 (0.66–2.3) | ||||
| GA+AA | 39 (19) | 26 (14) | 1.4 (0.71–2.8) | 1.1 | 1.1 | |||
| FOLR2 | Maternal | Yes | GG+GAb | 40 (21) | 43 (24) | 1.0 | ||
| AA | 76 (39) | 82 (46) | 1.0 (0.58–1.7) | |||||
| No | GG+GA | 24 (12) | 23 (13) | 1.1 (0.51–2.2) | ||||
| AA | 53 (27) | 29 (16) | 1.9 (1.0–3.7) | 1.1 | 1.1 | |||
| Child | Yes | GG+GAb | 44 (23) | 46 (26) | 1.0 | |||
| AA | 72 (37) | 75 (42) | 1.1 (0.62–1.8) | |||||
| No | GG+GA | 30 (15) | 19 (11) | 1.7 (0.80–3.5) | ||||
| AA | 49 (25) | 39 (22) | 1.3 (0.70–2.4) | 1.7 | 1.8 | |||
| TCN2 | Maternal | Yes | CCb | 38 (19) | 41 (22) | 1.0 | ||
| CG+GG | 80 (40) | 88 (47) | 0.91 (0.49–1.7) | |||||
| No | CC | 26 (13) | 12 (6) | 2.2 (0.91–5.3) | ||||
| CG+GG | 57 (28) | 46 (25) | 1.1 (0.56–2.2) | 2.1 | 2.0 | |||
| Child | Yes | CCb | 32 (16) | 45 (25) | 1.0 | |||
| CG+GG | 85 (43) | 74 (42) | 1.9 (1.0–3.5) | |||||
| No | CC | 23 (12) | 15 (8) | 2.3 (0.96–5.3) | ||||
| CG+GG | 58 (29) | 43 (24) | 2.2 (1.1–4.4) | 3.1 | 4.2 |
Odds ratios adjusted for maternal education and child’s birth year. ORs for maternal COMT 472 adjusted for child genotype. Maternal and child CBS adjusted for one another. Maternal and child BHMT adjusted for one another. Maternal and child TCN2 adjusted for one another.
Reference category.
Results were similar when we expanded analyses to include families of an additional 141 children with autism spectrum disorder and in weighted analyses (Table 2, last column; eTables 2–4, http://links.lww.com). The decreased risk associated with prenatal vitamins and the interaction effects with maternal MTHFR 677 and child COMT 472 were similar, although attenuated, when analyses were restricted to non-Hispanic white families (eTables 5, http://links.lww.com).
DISCUSSION
In this population-based case-control study, reported maternal periconceptional prenatal vitamin intake was associated with reduced risk for autism. In addition, the combination of specific gene variants within one-carbon metabolism pathways and no reported periconceptional prenatal vitamin intake was associated with significantly greater risk for autism in the child compared with either exposure alone.
Maternal Prenatal Vitamin Intake
We know of no previous studies that have linked the reported maternal intake of prenatal vitamins to a lower risk of autism. The apparent protective relationship was strong and robust to sensitivity analysis and adjustment for confounders. Moreover, findings did not differ when limited to women with planned pregnancies. Nonetheless, confounding from other unmeasured factors is possible.
No association was observed for reported multivitamin intake during the same period. We had lower power, however, to evaluate multivitamin intake during this period, as only 18 women were taking them; these women did not differ sociodemographically from those reporting prenatal vitamin use (eTable 1, http://links.lww.com). In support of a causal interpretation, prenatal vitamins typically contain more iron, vitamins B6 and B12 and twice as much folic acid (800 μg) as multivitamins (400 μg), to ensure levels adequate to protect against neural tube defects. Our findings could therefore reflect the higher concentrations of certain nutrients in prenatal vitamins, which might be needed to substantially reduce risk.
Folate and other B vitamins are critical to neurodevelopment. Cohort studies have shown that mothers who took folic acid-containing supplements before/during the first trimester have children with fewer behavioral problems at 18 months of age,21 improved scores on verbal, verbal-executive function, social competence, and attention measures at four years,22 and reduced hyperactivity and peer problems at 8 years.23 Iron or B vitamins other than folic acid are also essential for neurodevelopment, and are present at higher levels in prenatal vitamins as compared with multivitamins. These too could have contributed to the observed associations in our data and the findings of others.
With about 90% of mothers reporting prenatal vitamin supplementation by the third month of pregnancy, the power to distinguish exposure differences later in pregnancy was limited. Still, the periconceptional period aligns with evidence placing origins of at least some autism cases around the time of embryonic neural tube closure,5 i.e., four weeks postconception in humans. During this critical window, preimplantation mammalian embryos undergo extensive DNA de-methylation, followed by reestablishment of methylation patterns after implantation.24 Folate and other dietary methyl donors (betaine, choline, vitamin B12, and methionine) are vital for methylation of DNA, proteins, phospholipids, and neurotransmitters (eFigure, http://links.lww.com). Animal studies demonstrate that supplementing the periconceptional maternal diet with methyl donors alters epigenetic regulation of gene expression in their offspring in a manner that affects their long-term health.25 Moreover, a recent study provides direct evidence that maternal periconceptional folic acid increases methylation in specific gene regions of the child.26 Children with autism and their mothers have impaired methylation capacity compared with children who are typically developing and their mothers,10–11 and epigenetic mechanisms could contribute to the etiology of autism spectrum disorders.27 Periconceptional folic acid and other B vitamins may protect against neural tube defects,28 and possibly autism, through methylation mechanisms. Iron deficiency during critical periods of neurodevelopment can also lead to altered gene transcription and long-lasting changes in brain structure and function, with cognitive and behavioral consequences.29 Thus, the specific time period in which we observed a protective effect has biologic plausibility.
Interaction Effects between Genotypes and Periconceptional Prenatal Vitamin Intake
Our data suggest gene-by-environment interaction in autism etiology. All maternal gene variants that were associated with greater autism risk in the child in the absence of periconceptional prenatal vitamin supplementation are known to confer less efficient one-carbon metabolism and higher homocysteine levels. The MTHFR enzyme regulates folate availability and the MTHFR 677 TT genotype is associated with 60% reduced enzyme activity, inefficient folate metabolism, decreased blood folate, elevated plasma homocysteine levels, and reduced methylation capacity, particularly in individuals with low B vitamins levels.30 With vitamin B6 as a cofactor, the CBS enzyme catalyzes a reaction that permanently removes homocysteine from the methionine pathway by diverting it to the transsulfuration pathway (eFigure, http://links.lww.com). CBS gene mutations associated with decreased CBS activity also lead to elevated plasma homocysteine. FOLR2 helps transport folate (and folate derivatives) into cells and TCN2 facilitates transport of cobalamin (vitamin B12). MTRR and BHMT assist the folate/B12-dependent and choline/betaine-dependent conversions of homocysteine to methionine (eFigure, http://links.lww.com). Maternal gene variants in folate-related pathways and maternal homocysteine levels correlate with fetal homocysteine.31 Supplemental folic acid and cobalamin can reduce homocysteine levels and eliminate differences in risk for neural tube defects otherwise observed across genotypes, including MTHFR 677 TT, CBS rs234715, and MTRR 66 GG.32–34 Thus, the increased risk for autism in children whose mothers had these genotypes and did not report consuming prenatal vitamins in periconception has mechanistic support.
The interaction effect between reported maternal periconceptional prenatal vitamin intake and child COMT 472 AA genotype suggests that the maternal-fetal environment could magnify effects of a child susceptibility gene involved in methylation. Sufficient levels of folate and other B vitamins are required for the methionine cycle to provide methyl groups for methylation reactions, including those of COMT (eFigure, http://links.lww.com). High levels of homocysteine and S-adenosyl-L-homocysteine inhibit COMT activity and other methyltransferase reactions,35 and can be lowered by supplemental folic acid and vitamins B12 and B6. COMT degrades catecholamine neurotransmitters by catalyzing the transfer of a methyl group to them from S-adenosylmethionine. Though COMT’s role in development is not well-understood, animal experiments show that COMT activity initiates and peaks during early embryogenesis and the development of the sympathetic nervous system.36 Structural and functional brain differences have been described across COMT genotypes, particularly in the hippocampal and prefrontal cortex,37 brain regions that are implicated in autism pathology and where COMT plays a key role in dopamine degradation.38 Excess extracellular dopamine can be neurotoxic38 and can inhibit expression of brain-derived neurotrophic factor,39 which is essential for normal neuronal development and has been implicated in autism pathology.40 The common COMT 472G>A (val158met) gene variant alters the enzyme’s thermostability and reduces its activity 3- to 4-fold.41 The low-activity genotype has been inconsistently associated with obsessive-compulsive disorder, and other psychiatric conditions.42 Our findings suggest that child COMT 472G>A confers genetic susceptibility to autism, especially in combination with certain nutrient environments.
Because COMT is located in a region that is susceptible to copy number variation and that has been associated with autism,43 COMT 472G>A could be in linkage disequilibrium with other genetic variants that are responsible for the observed interaction effect. The fact that the other selected child gene variants were not associated with increased risk may imply a stronger role for maternal than child one-carbon metabolism. Findings recently reported by James and colleagues11 implicating genetic and metabolic alterations within the same pathways for mothers of autistic children, support this notion. The reason for the association with the child MTHFR 677 TT variant to be shifted in the opposite direction remains unclear; if not due to chance, any advantage to the child would likely occur through alternate mechanisms, as the reduced OR was independent of reported maternal vitamin intake.
Study Strengths and Limitations
Typical development and all autism diagnoses were clinically confirmed. Numerous known or suspected potential confounders were assessed. A large sample size allowed investigation of gene-by-environment interaction effects. Genotyping success rates were high, genotype frequencies did not deviate from HWE proportions, and tests provided evidence against population structure bias that can result from population stratification in case-control genetic studies.17
The retrospective reporting of vitamin and supplement information (whereby mothers were asked, after the child’s developmental status was known, to recall a period several years before the interview) raises the issues of recall accuracy and bias. The percentage of mothers of typically developing children taking prenatal vitamins three months prior to pregnancy in our study (37%), resembled percentages reported for women of childbearing age in the United States (33%).44 In addition, the differences we observed among racial groups and by maternal education levels were similar to those previously described,44 lending general support to the validity and accuracy of prenatal vitamin use reported by our study population. Although a slightly greater proportion of typically developing children were missing data on maternal periconceptional prenatal vitamin intake, sensitivity analyses demonstrated that our findings were robust.
A scenario in which recall bias explained part of the association between prenatal vitamins and autism would involve case mothers under-reporting or control mothers over-reporting their intake of prenatal vitamins during the months before pregnancy and the first month of pregnancy. The literature on recall bias indicates that mothers of control children are more likely to underreport exposures during pregnancy than mothers of cases, although these reports focus on exposures generally deemed harmful.45 Use of over-the-counter medications and multivitamins during pregnancy, including initiation and duration of use, are recalled equivalently and accurately for up to five years by mothers of case and control children.45–46 Notably, the association was similar (slightly stronger) for women recalling the information for a more recent vs. less recent pregnancy. While differential recall across case status cannot be ruled out, the interactions we observed with genotypes are difficult to attribute to recall bias, as recall differences between cases and controls would have to be concentrated primarily in those with high risk genotypes.
Our study did not collect complete diet information for the pregnancy, precluding a full assessment of nutrient intake. Having this information might have provided valuable information for discriminating between specific nutrient effects, and it could have allowed control for possible confounding effects of diet.
We recognize that certain gene-environment interaction results were based on cell sizes as small as n = 4. However, our result was surprising especially for COMT, as the expected number of children with the AA genotype whose mothers did not report taking supplements was 12; similarly the expected number of children whose mother did not supplement and had the MTHFR 677 TT genotype was 8.5. One explanation is that the combination of no supplementation with the high risk genotypes could be relatively incompatible with typical development in the child, depending on other modifying factors.
Our data suggest that supplementation with prenatal vitamins before pregnancy and during the first month of pregnancy might protect against autism, particularly in genetically susceptible individuals. Additionally, COMT genotype may contribute to an elevated risk for autism, especially in offspring of unsupplemented mothers. This evidence for gene-by-environment interaction effects in autism etiology could help explain variations in previous findings across genetic studies. Whether similar interactions exist for susceptibility genes in other pathways, particularly those epigenetically regulated through methylation, remains to be explored. More research is warranted to replicate the findings, explicate potential mechanisms, and explore interactions with other autism candidate genes.
Supplementary Material
Acknowledgments
FUNDING: Supported by grants 1R01-ES015359, P01-11269, and T32-MH073124 from the National Institutes of Health; grants #R-829388 & R-833292 from the U.S. EPA STAR program; and by the UC Davis Medical Investigations of Neurodevelopmental Disorders Institute.
We acknowledge the investigators and staff of the Childhood Autism Risk from Genetics and Environment (CHARGE) Study for their valuable contributions. We also express our gratitude to the families participating in the CHARGE Study.
References
- 1.Prevalence of autism spectrum disorders --- autism and developmental disabilities monitoring network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20. [PubMed] [Google Scholar]
- 2.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 4.Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics. 2011;127(2):246–53. doi: 10.1542/peds.2010-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247–61. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–7. [PubMed] [Google Scholar]
- 7.Main PA, Angley MT, Thomas P, O’Doherty CE, Fenech M. Folate and methionine metabolism in autism: a systematic review. Am J Clin Nutr. 2010;91(6):1598–620. doi: 10.3945/ajcn.2009.29002. [DOI] [PubMed] [Google Scholar]
- 8.Adams M, Lucock M, Stuart J, Fardell S, Baker K, Ng X. Preliminary evidence for involvement of the folate gene polymorphism 19bp deletion-DHFR in occurrence of autism. Neurosci Lett. 2007;422(1):24–9. doi: 10.1016/j.neulet.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Boris M, Goldblatt A, Galanko J, James SJ. Association of MTHFR gene variants with autism. J Am Phys Surg. 2004;9:106–8. [Google Scholar]
- 10.James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141(8):947–56. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SJ, Melnyk S, Jernigan S, Pavliv O, Trusty T, Lehman S, Seidel L, Gaylor DW, Cleves MA. A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(6):1209–20. doi: 10.1002/ajmg.b.31094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy AM, Mills JL, McPartlin J, Kirke PN, Scott JM, Daly S. Maternal and fetal plasma homocysteine concentrations at birth: the influence of folate, vitamin B12, and the 5,10-methylenetetrahydrofolate reductase 677C-->T variant. Am J Obstet Gynecol. 2002;186(3):499–503. doi: 10.1067/mob.2002.121105. [DOI] [PubMed] [Google Scholar]
- 13.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–25. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 15.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–23. [PubMed] [Google Scholar]
- 16.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH, Jr, Leventhal BL, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg CR, Umbach DM. A hybrid design for studying genetic influences on risk of diseases with onset early in life. American Journal of Human Genetics. 2005;77(4):627–36. doi: 10.1086/496900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalton G, Piesse A. Survey research methods in evaluation and case-control studies. Stat Med. 2007;26(8):1675–87. doi: 10.1002/sim.2796. [DOI] [PubMed] [Google Scholar]
- 19.Schafer JL, editor. Analysis of Incomplete Multivariate Data. London: Chapman & Hall; 1997. [Google Scholar]
- 20.Anonymous. Applied Biosystems TaqMan SNP Genotyping Assays. Assay Search. 2007;2007 https://products.appliedbiosystems.com/ab/en/US/adirect/ab?cmd=ABGTKeywordSearch&catID=601283.
- 21.Roza SJ, van Batenburg-Eddes T, Steegers EA, Jaddoe VW, Mackenbach JP, Hofman A, Verhulst FC, Tiemeier H. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br J Nutr. 2009:1–8. doi: 10.1017/S0007114509991954. [DOI] [PubMed] [Google Scholar]
- 22.Julvez J, Fortuny J, Mendez M, Torrent M, Ribas-Fito N, Sunyer J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol. 2009;23(3):199–206. doi: 10.1111/j.1365-3016.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 23.Schlotz W, Jones A, Phillips DI, Gale CR, Robinson SM, Godfrey KM. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry. 2010;51(5):594–602. doi: 10.1111/j.1469-7610.2009.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2(1):21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 25.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132(8 Suppl):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 26.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4(11):e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15(Spec No 2):R138–50. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- 28.Blom HJ. Folic acid, methylation and neural tube closure in humans. Birth Defects Res A Clin Mol Teratol. 2009;85(4):295–302. doi: 10.1002/bdra.20581. [DOI] [PubMed] [Google Scholar]
- 29.Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36(Pt 6):1267–71. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hustad S, Midttun O, Schneede J, Vollset SE, Grotmol T, Ueland PM. The methylenetetrahydrofolate reductase 677C-->T polymorphism as a modulator of a B vitamin network with major effects on homocysteine metabolism. Am J Hum Genet. 2007;80(5):846–55. doi: 10.1086/513520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopreato FR, Stabler SP, Carvalho FR, Hirata RD, Hirata MH, Robi DL, Sampaio-Neto LF, Allen RH, Guerra-Shinohara EM. Relationships between gene polymorphisms of folate-related proteins and vitamins and metabolites in pregnant women and neonates. Clin Chim Acta. 2008;398(1–2):134–9. doi: 10.1016/j.cca.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Boyles AL, Billups AV, Deak KL, Siegel DG, Mehltretter L, Slifer SH, Bassuk AG, Kessler JA, Reed MC, Nijhout HF, George TM, Enterline DS, Gilbert JR, Speer MC. Neural tube defects and folate pathway genes: family-based association tests of gene-gene and gene-environment interactions. Environ Health Perspect. 2006;114(10):1547–52. doi: 10.1289/ehp.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen B, Arbour L, Tran P, Leclerc D, Sabbaghian N, Platt R, Gilfix BM, Rosenblatt DS, Gravel RA, Forbes P, Rozen R. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet. 1999;84(2):151–7. doi: 10.1002/(sici)1096-8628(19990521)84:2<151::aid-ajmg12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Wilson A, Platt R, Wu Q, Leclerc D, Christensen B, Yang H, Gravel RA, Rozen R. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab. 1999;67(4):317–23. doi: 10.1006/mgme.1999.2879. [DOI] [PubMed] [Google Scholar]
- 35.Schatz RA, Wilens TE, Sellinger OZ. Decreased transmethylation of biogenic amines after in vivo elevation of brain S-adenosyl-l-homocysteine. J Neurochem. 1981;36(5):1739–48. doi: 10.1111/j.1471-4159.1981.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 36.Ignarro LJ, Shideman FE. Catechol-O-methyl transferase and monoamine oxidase activities in the heart and liver of the embryonic and developing chick. J Pharmacol Exp Ther. 1968;159(1):29–37. [PubMed] [Google Scholar]
- 37.Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45(1):44–51. doi: 10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol Psychiatry. 2003;8(11):898–9. doi: 10.1038/sj.mp.4001370. [DOI] [PubMed] [Google Scholar]
- 40.Correia CT, Coutinho AM, Sequeira AF, Sousa IG, Lourenco Venda L, Almeida JP, Abreu RL, Lobo C, Miguel TS, Conroy J, Cochrane L, Gallagher L, Gill M, Ennis S, Oliveira GG, Vicente AM. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 2010;9(7):841–8. doi: 10.1111/j.1601-183X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- 41.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Craddock N, Owen MJ, O’Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry. 2006;11(5):446–58. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- 43.Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1104–13. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Use of Dietary Supplements Containing Folic Acid Among Women of Childbearing Age: United States, 2005. MMWR -Morbidity & Mortality Weekly Report. 2005;54(38):955–958. [PubMed] [Google Scholar]
- 45.Werler MM, Pober BR, Nelson K, Holmes LB. Reporting accuracy among mothers of malformed and nonmalformed infants. Am J Epidemiol. 1989;129(2):415–21. doi: 10.1093/oxfordjournals.aje.a115145. [DOI] [PubMed] [Google Scholar]
- 46.Bosco JL, Tseng M, Spector LG, Olshan AF, Bunin GR. Reproducibility of reported nutrient intake and supplement use during a past pregnancy: a report from the Children’s Oncology Group. Paediatr Perinat Epidemiol. 2010;24(1):93–101. doi: 10.1111/j.1365-3016.2009.01070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.