Abstract
Background
Recent series of incidental thyroid activity on fluorodeoxyglucose positron emission tomography (FDG-PET) in patients evaluated for nonthyroidal malignancy, which we refer to as a “PEToma,” have suggested that such lesions are associated with a significant incidence of primary thyroid cancer.
Methods
We retrospectively reviewed 6457 FDG-PET scans performed on 4726 patients from May 2004 to March 2007. We reviewed the cases of patients whose PET or computed tomography (CT) radiology reports described PET uptake within the thyroid to identify incidence and malignant potential of PETomas and evaluate their clinical and histopathologic features.
Results
We found that 160 patients (3.4%) had incidental, abnormal FDG uptake in the thyroid gland, 103 of whom had focal uptake (the PEToma group). Of these patients, 50 (48%) underwent further investigations, including ultrasonography in 48, fine-needle aspiration cytology in 38 and computed tomography in 3. Ten patients underwent surgery, and papillary thyroid cancer was identified in 9. The remaining 53 patients with PETomas underwent no further investigation. Interestingly, 5 patients who had focal uptake within the thyroid showed either spontaneous resolution on repeat FDG-PET (self-resolving) or no focal lesion on subsequent ultrasonography (false-positive).
Conclusion
The incidence of papillary thyroid cancer in the present series is similar to that in the literature. Although some patients will show self-resolving or false-positive focal thyroid uptake on FDG-PET, we believe that, if the patient’s clinical status permits, the evaluation of patients with incidental thyroid PEToma should include ultrasonographic confirmation and fine-needle aspiration cytology.
Abstract
Contexte
Des séries récentes sur l’activité thyroïdienne découverte fortuitement par tomographie par émission de positrons au fluorodésoxyglucose (TEP-FDG) chez des patients évalués pour une tumeur maligne non thyroïdienne, que nous appelons « PETome » (de l’acronyme anglais de la TEP), ont indiqué que ces lésions pour-raient être associées à une incidence importante de cancer primitif de la thyroïde.
Méthodes
Nous avons procédé à un examen rétrospectif de 6457 examens TEP-FDG effectués sur 4726 patients de mai 2004 à mars 2007. Nous avons revu les cas des patients dont les rapports de TEP ou de tomodensitométrie (TDM) décrivaient la fixation de TEP dans la thyroïde pour déterminer l’incidence et le potentiel cancéreux des PETomes et pour évaluer leurs caractéristiques cliniques et histopathologiques.
Résultats
Nous avons découvert que 160 patients (3,4 %) présentaient une fixation fortuite anormale de FDG dans la thyroïde, dont 103 présentaient une fixation focale (le groupe PETome). Parmi ces patients, 50 (48 %) ont subi des examens plus poussés, y compris une échographie dans 48 cas, une cytologie par aspiration à l’aiguille fine dans 38 cas et une TDM dans 3 cas. Dix patients ont subi une intervention chirurgicale, et on a repéré un cancer papillaire de la thyroïde dans 9 cas. Les 53 autres patients qui avaient un PETome n’ont pas subi d’autres examens. Chez 5 patients qui présentaient une fixation focale dans la thyroïde, il y a eu résorption spontanée à la suite d’une TEP-FDG répétitive (auto-absorption) ou une échographie ultérieure n’a révélé aucune lésion focale (faux positif).
Conclusion
L’incidence du cancer papillaire de la thyroïde dans la présente série ressemble à celle qu’indiquent les publications. Même si, chez certains patients, il y aura fixation focale dans la thyroïde autorésorbée ou faussement positive révélée par la TEP-FDG, nous croyons que si l’état clinique du patient le permet, l’évaluation de PETomes thyroïdiens découverts fortuitement devrait inclure une confirmation par échographie et une cytologie par aspiration à l’aiguille fine.
Although thyroid cancer is relatively uncommon, its incidence in North America has increased in recent decades.1,2 The increased incidence of small, micropapillary thyroid cancer is especially remarkable. Although there is no definitive explanation, it seems that the more frequent use of diagnostic imaging and fine-needle aspiration cytology (FNAC) has led to increased detection, which is in part responsible for this increasing incidence. Despite increasing incidence, mortality from thyroid cancer has not increased over recent decades.1
The evolution of diagnostic imaging modalities has led to an increase in the discovery of subclinical, incidentally detected thyroid nodules. These constitute the so-called thyroid “incidentalomas.”3 Traditionally, thyroid incidentalomas have been detected by ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI). With the advent of fluorodeoxyglucose positron emission tomography (FDG-PET), another source of thyroid incidentalomas has been identified. Fluorodeoxyglucose-PET is commonly used for the staging and monitoring of patients with cancer. The normal thyroid gland shows very low-grade FDG uptake and is usually not visualized on the whole-body FDG-PET scan. Several reports have described incidentally detected focal thyroid lesions on FDG-PET in patients with no history of thyroid pathology.3–11 We refer to these incidentalomas as “PETomas.” The overall reported incidence of FDG-PET thyroid PETomas varies from 1.1% to 4.0%,3–11 whereas the prevalence of thyroid cancer in these series is remarkably high, ranging from 14% to 56%.3–11
Adequate management of thyroid incidentalomas is not well established. Because most thyroid incidentalomas are small, observation is a reasonable option for patients with benign cytology and no obvious risk factors. Thyroid PETomas, however, exhibit a high prevalence of malignancy compared with incidentalomas detected by ultra-sonography or CT.4 The current American Thyroid Association and National Comprehensive Cancer Network guidelines recommend further investigations for all incidentally identified focal PET positive lesions in the thyroid.12,13
The aim of the present study was to identify the incidence and malignant potential of thyroid PETomas, to evaluate the clinical and histopathologic features of malignant PETomas and to clarify the particulars of self-resolving or false-positive FDG uptake in the thyroid to ultimately suggest an appropriate management of PETomas.
Methods
We identified our study sample through a retrospective evaluation of a computerized database of 6457 FDG-PET scans performed at the McGill University Health Centre (MUHC) in 4726 consecutive patients from May 2004, when PET/CT scanning became available at our institution, to March 2007.
Fluorodeoxyglucose-PET scanning was performed for the staging or monitoring of nonthyroid cancer. At the MUHC, CT without contrast was performed before FDG-PET for attenuation correction of the PET data and for localization purposes. Fluorodeoxyglucose was administered intravenously 1 hour before the scan, and imaging was performed from the base of the skull to the upper thighs. Patients whose PET/CT reports, as generated by 1 of 4 experienced radiologists, described PET uptake within the thyroid gland were reviewed.
We analyzed all data using the Mann–Whitney U test. We considered p < 0.05 to indicate a statistically significant difference.
Results
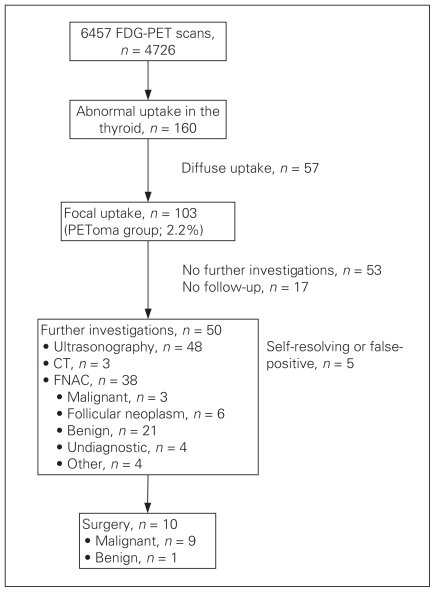
Of 6457 FDG-PET/CT scans in 4726 patients, abnormal FDG uptake in the thyroid was incidentally indentified in 160 (3.4%) patients. A summary of the results is presented in Figure 1. Focal as opposed to diffuse FDG uptake within the thyroid was identified in 103 patients (2.2%, 117 lesions). This represents the so-called PEToma group. We identified diffuse FDG uptake in 57 patients (1.2%). Fifty of the 103 patients with PETomas underwent further investigation, including ultrasonography in 48, CT in 3 and FNAC in 38 patients. Of those who underwent FNAC, the abnormalities were reported to be malignant in 3 patients, benign in 21 and consistent with a follicular neoplasm in 6 patients; the cytology was unsatisfactory in 8 patients. Ten patients underwent thyroidectomy, 9 of whom were found to have cancer on final histology (Table 1). Two other patients with follicular neoplasms identified by FNAC have been closely followed and managed nonsurgically because of the progression of their primary disease. All 9 patients with cancer had papillary thyroid carcinomas, of which 2 were micropapillary thyroid cancers. Extrathyroidal extension was identified in 2 patients with papillary thyroid carcinomas, and multi-focal involvement was identified in 4 patients. One patient had over 50% poorly differentiated papillary thyroid carcinoma. Thus, of the 30 PETomas adequately evaluated with cytology and/or histology, these 9 primary thyroid cancers represent a prevalence of 30%.
Fig. 1.
Out of 4726 patients, incidental thyroid fluorodeoxyglucose uptake was identified in 160 patients, and PETomas were identified in 103 patients (2.2%). Fifty (48%) of 103 patients underwent further investigations, and 9 (30%) of the 30 patients with PETomas that were adequately evaluated with cytology/histology had confirmed primary thyroid cancer. CT = computed tomography; FDG-PET = fluorodeoxyglucose positron emission tomography; FNAC = fine-needle aspiration cytology.
Table 1.
Clinical and pathologic features of malignant PETomas
| Pt. no. | Age, yr/sex | Primary cancer | SUV | FNAC result | Pathology | Size, cm | Extrathyroid extention | Vascular invasion | Multifocal | Lymph node status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41/M | Melanoma | 10.1 | PTC | PTC | 1.0 | + | — | + | NA |
| 2 | 53/F | Gastric cancer | 4.7 | Follicular neoplasm | PTC | 1.0 | + | + | + | NA |
| 3 | 52/F | Sarcoma | 4.2 | Benign | microPTC | 0.3 | — | — | — | NA |
| 4 | 33/F | Sarcoma | 5.3 | PTC | PTC | 1.7 | — | — | + | 0/2 |
| 5 | 57/F | Renal cell carcinoma | 5.8 | Follicular neoplasm | PTC | 2.3 | — | — | + | 0/5 |
| 6 | 73/M | Lung cancer | 3.0 | Follicular neoplasm | microPTC | 0.3 | — | — | — | 0/1 |
| 7 | 74/F | Lung cancer | 6.0 | Follicular neoplasm | PTC* | 2.5 | — | — | — | NA |
| 8 | 59/M | Melanoma | 25.6 | PTC | PTC | NA | — | — | — | NA |
| 9 | 69/M | Metastatic colorectal cancer | 8.5 | NA | PTC | NA | — | — | — | NA |
F = female; FNAC = fine-needle aspiration cytology; M = male; NA = not available; PTC = papillary thyroid carcinoma; SUV = standardized uptake value.
Poorly differentiated.
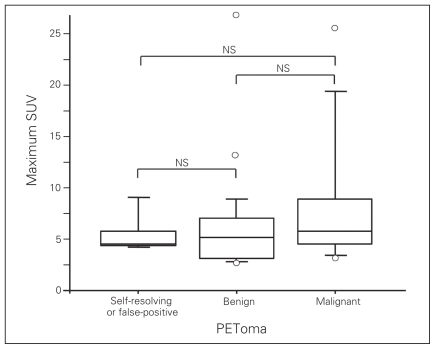
There were no documented instances of metastatic disease to the thyroid. The mean size of malignant PETomas was 1.54 cm in diameter. The maximum standardized uptake value (SUV) in malignant PETomas was 5.8, which was not significantly different from the maximum SUV of 5.2 recorded for benign PETomas (p = 0.36; Fig. 2).
Fig. 2.
Median maximum standardized uptake value (SUV) was 5.2 in benign and 5.8 in malignant PETomas, p = 0.36. Maximum SUV could not differentiate benign from malignant PETomas. NS = not significant.
There were 5 of 103 PETomas classified as either self-resolving or false-positive FDG uptakes (Table 2). Three patients who underwent repeat FDG-PET scans showed spontaneous resolution of focal activity within the thyroid (self-resolving). In 2 patients, no focal lesion was identified by subsequent ultrasonography (false-positive). The mean size of PETomas in these patients was 1.32 cm, and the median maximum SUV was 4.5, which was not significant compared with either benign or malignant PETomas. One patient underwent chemotherapy between 2 FDG-PET scans.
Table 2.
Data on patients with self-resolving or false-positive FDG-PET results
| Pt. no. | Age, yr/sex | Primary cancer | Status | SUV | Size, cm | Time to repeat imaging, mo |
|---|---|---|---|---|---|---|
| 1 | 83/M | Lymphoma | Relapse after chemotherapy | 4.2 | 1.1 | 6* |
| 2 | 60/F | Lymphoma | Partial response after chemotherapy | 9.1 | 2.4 | 6† |
| 3 | 50/F | Sarcoma | Disease-free after surgery | 4.5 | 1.0 | 1* |
| 4 | 75/F | Lung cancer | Staging | 4.6 | 1.1 | 10† |
| 5 | 75/M | Lymphoma | Complete remission after chemotherapy/stem cell transplant | 4.5 | 1.0 | 6† |
F = female; FDG-PET = fluorodeoxyglucose positron emission tomography; M = male; SUV = standardized uptake value.
Ultrasonography.
Positron emission tomography.
Fifty-three patients underwent no further investigation of their PETomas. Thirty-six patients did not have further investigations owing to the progression of their primary cancers. In the remaining 17 patients, the reasons that their PETomas were not investigated could not be ascertained.
Case presentations of self-resolving or false-positive FDG-PET
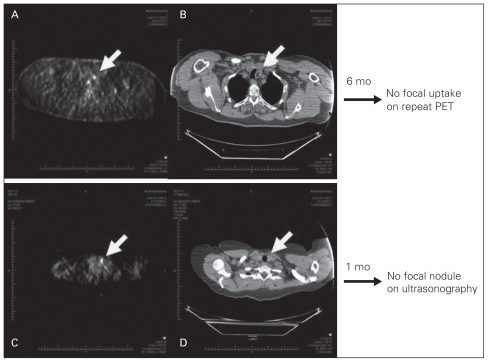
Case 1: A 75-year-old man with lymphoma that was in clinical remission following chemotherapy underwent a follow-up FDG-PET scan, which incidentally revealed focal uptake in the left lobe of the thyroid measuring 1.1 cm with a maximum SUV of 4.2 (Fig. 3A and B). The patient was clinically and biochemically euthyroid at the time. Six months later, a repeat scan showed no focal uptake within the thyroid, demonstrating spontaneous self-resolution.
Fig. 3.
(A) Follow-up fluorodeoxyglucose positron emission tomography (FDG-PET) scan demonstrated 1.0 cm, 4.2 maximum standardized uptake value (SUV), uptake in the left lobe of the thyroid in a 75-year-old man with lymphoma. (B) Computed tomography also showed incidental thyroid nodule corresponding to FDG-PET. Repeat FDG-PET performed 6 months later, however, failed to show any uptake in the thyroid. (C) Follow-up FDG-PET scan demonstrated 1.0 cm, 4.5 maximum SUV, uptake in the left lobe of the thyroid in a 50-year-old woman with sarcoma. (D) Computed tomography also showed incidental thyroid nodule corresponding to FDG-PET. Subsequent ultrasonography 1 month later, however, did not show any focal lesion in the thyroid.
Case 2: A 50-year-old woman treated for sarcoma underwent a follow-up FDG-PET scan, which detected focal uptake in the left lobe of the thyroid measuring 1.0 cm with an SUV of 4.5 (Fig. 3C and D). She was clinically and biochemically euthyroid at the time. One month later, she underwent ultrasonography, which failed to detect any focal lesions within the gland, implying a false-positive PEToma.
Discussion
With the development of diagnostic imaging modalities, clinicians are being called on to evaluate an increasing number of small incidentalomas of the thyroid. Studies have shown the incidence of such thyroid incidentalomas to be between 19% and 46% for ultrasonography and about 16% for CT and MRI.8,14 The risk of malignancy in these lesions is relatively low, ranging from 1.5% to 10.0%.15
With the increasing use of FDG-PET in the evaluation and surveillance of patients with cancer, a substantial number of patients will present with incidentally identified focal uptake within the thyroid,1 which we refer to as a “PEToma.” According to earlier reports, the incidence of PEToma varies from 1.1% to 4.0% of all PET scans.3–11 Moreover, the prevalence of malignancy within these PETomas is remarkably high at 14%–56%.3–11 In contrast, diffuse, bilateral FDG-PET uptake is usually associated with benign disease (Table 3).4
Table 3.
Literature review on PEToma
| Study | No. patients | Focal, no. (%) | Diffuse | Further examination | FNAC/operation | Cancer, no. (%) | Pathology (micro) | SUV |
|---|---|---|---|---|---|---|---|---|
| Cohen et al.6 | 4 525 | 71 (1.6) | 31 | NA | 15/NA | 7 (47.0) | 3 PTC 2 poorly differentiated PTC |
Useful |
| Kim et al.9 | 4 136 | 45 (1.1) | 45 | NA | 32/7 | 18 (56.0) | 14 PTC 2 metastatic 2 follicular thyroid cancer |
NS |
| Chen et al.7 | 4 803 | 60 (1.2) | — | 50 | — | 7 (14.0) | 7 PTC | Useful |
| Choi et al.8 | 1 763 | 70 (4.0) | — | 44 | 29/NA | 17 (38.6) | 16 PTC | Useful |
| Are et al.5 | 8 800 | 101 (1.1) | 162 | 57 | 57/27 | 24 (42.0) | 19 PTC | NS |
| Chu et al.11 | 6 241 | 76 (1.2) | — | — | 14/1 | 4 (28.6) | 4 PTC | NA |
| Bogsrud et al.10 | 7 347 | 79 (1.1) | — | 48 | NA/11 | 15 (31.3) | 12 PTC 2 metastatic |
NS |
| King et al.16 | 15 711 | 22 (0.1) | — | 21 | 21/14 | 7 (32.0) | 6 PTC (4) 1 MTC |
NA |
| Nishimori et al.* | 4 726 | 103 (2.2) | 57 | 50 | 30/10 | 9 (30.0) | 8 PTC (2) 1 poorly differentiated PTC |
NS |
FNAC = fine-needle aspiration cytology; MCT = medullary thyroid cancer; NA = not available; NS = not significant; PTC = papillary thyroid cancer; SUV = standardized uptake value.
Unpublished data, 2008.
In the current study, the prevalence of cancer was 30% (9/30) for those in whom a confirmed cytologic or histologic diagnosis was obtained via FNAC or surgery, and this incidence was similar to that reported by others.3–11 The overall incidence of cancer in the current series was relatively low at 8.7% (9 of 103). Because more than half of the patients with PETomas (53 of 103) did not undergo further evaluation with either ultrasonography, FNAC or surgery, the true prevalence of cancer is arguably underestimated. Given their relative risk, all PETomas should be considered suspicious for a primary thyroid cancer until fully evaluated.
All 9 malignant PETomas were classified as papillary thyroid cancer by pathologic examination; 2 of them were micropapillary thyroid cancers. There were no documented instances of metastasis to the thyroid from an unrelated organ. Extrathyroidal extension was identified in 2 patients, whereas multifocal involvement was observed in 4 patients. Are and colleagues5 have suggested that incidentally detected thyroid cancers have poorer prognosis owing to a high prevalence of unfavourable pathologic features, including tall-cell variant, poorly differentiated cancer and extrathyroidal extension. In the present study, 3 patients (30%) had such poor prognostic features on final pathologic review. All 3 received postoperative radioiodine remnant ablation therapy and continue to be followed closely.
The clinical application and usefulness of maximum SUV in patients with PETomas is still controversial. A recent systematic review reported that the maximum SUV of malignant lesions was significantly higher than that of benign lesions (mean 6.8, SD 4.6 v. 4.6, SD 2.1; p < 0.001).17 Mitchell and colleagues18 described the sensitivity and specificity of FDG-PET for known thyroid cancer to be 60% and 91%, respectively, using an SUV of 5.0 as a cut-off value. Most previous studies, however, failed to show the benefit of SUV to differentiate benign from malignant PETomas.3,9,10 Although maximum SUV is sometimes clinically useful in differentiating some malignancies, it is subject to variability because of the patient’s body composition, period of uptake, lesion size, partial volume averaging and plasma glucose.11 Also, many PETomas are subcentimetre, and thus maximum SUV may not be accurately calculated. Kim and colleagues9 reported a significant correlation between maximum SUV and maximum diameter of papillary thyroid cancer. Comparison of maximum SUV did not differentiate benign from malignant PETomas in the current study. However, the present series included only 9 patients with malignant PETomas, and further study is necessary to elucidate the role of maximum SUV.
Interestingly, there were 5 (4.9%) patients with either self-resolving or false-positive FDG uptake identified in this study. Three PETomas subsequently self-resolved on repeat FDG-PET. To date, there are no reports describing self-resolved PET–positive incidentalomas on repeat PET scans. The remaining 2 PETomas were not identified by subsequent ultrasonography. Our data suggest that these 5 patients likely represent a subgroup of false-positive PETomas. All patients were clinically euthyroid at the time of the initial FDG-PET scan. There are several putative causes of false-positive PETomas. Physiologic uptake in brown fat, skeletal muscle, lymphoid tissue and thymus have previously been reported.19 Benign inflammatory conditions and infection can also lead to a false-positive result. Because FDG-PET indirectly assesses metabolic alterations in tissues and is less sensitive in defining a given lesion’s morphologic features, we feel that ultrasonography can adequately exclude the presence of an anatomic lesion, whereas a good-quality FNAC can guide the need for surgical intervention. In our practice, if the clinical status of the patient permits, we recommend thyroidectomy to patients with PETomas who have a FNAC report that is positive for cancer. In cases of nondiagnostic cytology, repeat FNAC should be considered.
Conclusion
The incidental finding of focal thyroid uptake on FDG-PET scanning, a so-called PEToma, warrants careful evaluation as a substantial number will harbour a primary thyroid cancer. Use of maximum SUV values alone is a poor predictor of malignancy in these patients. At a minimum, these patients require further evaluation with ultrasonography and FNAC.
Acknowledgements
We thank Richard J. Payne, MD, of the Department of Otolaryngology, Royal Victoria Hospital, Montréal, for his scientific advice and Shinichi Hisasue, MD, of the Department of Psychology, University of Massachusetts, for his assistance in the statistical analysis.
Footnotes
Competing interests: None declared.
Contributors: Drs. Tabah and How designed the study. Drs. Nishimori and How wrote the article, which Drs. Tabah, Hickeson and How critically reviewed. All authors acquired and analyzed the data and approved publication of the article.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.How J, Tabah R. Explaining the increasing incidence of differentiated thyroid cancer. CMAJ. 2007;177:1383–4. doi: 10.1503/cmaj.071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Are C, Hsu JF, Schoder H, et al. FDG-PET detected thyroid incidentalomas: Need for further investigation. Ann Surg Oncol. 2007;14:239–47. doi: 10.1245/s10434-006-9181-y. [DOI] [PubMed] [Google Scholar]
- 4.Salvatori M, Melis L, Castaldi P, et al. Clinical significance of focal and diffuse thyroid disease identified by 18F-fluorodeoxyglucose positron emission tomography. Biomed Pharmacother. 2007;61:477–81. doi: 10.1016/j.biopha.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Are C, Hsu JF, Chossein RA, et al. Histological aggressiveness of fluorodeoxyglucose positron-emission tomogram (FDG-PET)-detected incidental thyroid carcinomas. Ann Surg Oncol. 2007;14:3210–5. doi: 10.1245/s10434-007-9531-4. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Arslan N, Dehdashti F, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery. 2001;130:941–6. doi: 10.1067/msy.2001.118265. [DOI] [PubMed] [Google Scholar]
- 7.Chen YK, Ding HJ, Chen KT, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421–6. [PubMed] [Google Scholar]
- 8.Choi JY, Lee KS, Kim HJ, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609–15. [PubMed] [Google Scholar]
- 9.Kim TY, Kim WB, Ryu JS, et al. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope. 2005;115:1074–8. doi: 10.1097/01.MLG.0000163098.01398.79. [DOI] [PubMed] [Google Scholar]
- 10.Bogsrud TV, Karantanis D, Nathan MA, et al. The value of quantifying 18F-FDG uptake in thyroid nodules found incidentally on whole-body PET-CT. Nucl Med Commun. 2007;28:373–81. doi: 10.1097/MNM.0b013e3280964eae. [DOI] [PubMed] [Google Scholar]
- 11.Chu QD, Connor MS, Lilien DL, et al. Positron emission tomography (PET) positive thyroid incidentaloma: the risk of malignancy observed in a tertiary referral center. Am Surg. 2006;72:272–5. [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 13.NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network website. [accessed 2010 Dec. 8]. Available: www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 14.Kang HW, No JH, Chung JH, et al. Prevalence, clinical and ultra-sonographic characteristics of thyroid incidentalomas. Thyroid. 2004;14:29–33. doi: 10.1089/105072504322783812. [DOI] [PubMed] [Google Scholar]
- 15.Burguera B, Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinol Metab Clin North Am. 2000;29:187–203. doi: 10.1016/s0889-8529(05)70123-7. [DOI] [PubMed] [Google Scholar]
- 16.King DL, Stack BC, Jr, Spring PM, et al. Incidence of thyroid carcinoma in fluorodeoxyglucose positron emission tomography-positive thyroid incidentalomas. Otolaryngol Head Neck Surg. 2007;137:400–4. doi: 10.1016/j.otohns.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Shie P, Cardarelli R, Sprawls K, et al. Systematic review: prevalence of malignant incidental thyroid nodules identified on fluorine-18 fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2009;30:742–8. doi: 10.1097/MNM.0b013e32832ee09d. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JC, Grant F, Evenson AR, et al. Preoperative evaluation of thyroid nodules with 18FDG-PET/CT. Surgery. 2005;138:1166–74. doi: 10.1016/j.surg.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum SJ, Lind T, Antoch G, et al. False-positive FDG PET uptake — the role of PET/CT. Eur Radiol. 2006;16:1054–65. doi: 10.1007/s00330-005-0088-y. [DOI] [PubMed] [Google Scholar]