Abstract
Background
Most patients with hepatocellular carcinoma (HCC) have advanced-stage disease at diagnosis. The prognosis for patients with HCC is very poor, especially for those with portal vein tumour thrombi (PVTT). The purpose of our study was to observe the prognostic value of PVTT and tumour-infiltrating regulatory T cells (Tregs) and the correlation between them.
Methods
We examined 76 HCC specimens by immunohistochemistry for CD4+, CD8+ T cells and Foxp3+ Tregs. The survival of patients was prospectively followed up. Patients with HCC were grouped according to the formation of PVTT or Treg infiltration status. We performed a Kaplan–Meier survival analysis to observe the difference in prognosis between the groups. We analyzed the correlation of Treg expression with clinical and pathologic features.
Results
Survival analysis revealed that both the disease-free survival rate and total survival rate after hepatic resection were significantly lower in patients in the PVTT group than those in the non-PVTT group (p = 0.026 and p = 0.022, respectively). Likewise, both the disease-free survival rate and the total survival rate were significantly lower in patients in the high Treg group than those in the low Treg group (p = 0.012 and p = 0.023, respectively). We found that intratumoural Tregs were associated with PVTT formation (p = 0.001) and that patients with high Treg infiltration had a higher percentage of PVTT formation.
Conclusion
Patients with PVTT formation or high intratumoural Treg infiltration tended to have a poor prognosis. Intratumoural Treg was associated with formation of PVTT in patients with HCC.
Abstract
Contexte
Chez la plupart des patients atteints de carcinome hépatocellulaire (CHC), la maladie est au stade avancé au moment du diagnostic. Le pronostic de ces patients est très médiocre, en particulier en présence de thrombose tumorale de la veine porte (TTVP). Notre étude visait à observer la valeur pronostique des TTVP et des lymphocytes T régulateurs (Tregs) infiltrant la tumeur, ainsi que le lien entre eux.
Méthodes
Nous avons examiné 76 spécimens de CHC par immunohistochimie pour déterminer la présence de lymphocytes CD4+, CD8+ T et Foxp3+ Tregs. Nous avons suivi de façon prospective la survie des patients. Les patients atteints de CHC ont été regroupés en fonction de la formation de TTVP ou de l’état d’infiltration par les lymphocytes Treg. Nous avons procédé à une analyse de Kaplan–Meier de la survie pour observer la différence au niveau du pronostic entre les groupes. Nous avons analysé le lien entre l’expression des lymphocytes Treg et les caractéristiques cliniques et pathologiques.
Résultats
L’analyse de la survie a révélé que le taux de survie exempte de maladie et le taux de survie total après une résection hépatique étaient beaucoup moins élevés dans les groupes des patients qui avaient une TTVP que dans le groupe de ceux qui n’en avaient pas (p = 0,026 et p = 0,022 respectivement). De même, le taux de survie exempte de maladie et le taux de survie total étaient tous deux beaucoup moins élevés chez les patients où l’expression des lymphocytes Treg était élevée que chez ceux où elle était faible (p = 0,012 et p = 0,023 respectivement). Nous avons trouvé un lien entre les lymphocytes Treg intratumoraux et la formation de TTVP (p = 0,001) et constaté que le pourcentage de TTVP était plus élevé chez les patients chez lesquels l’infiltration par les lymphocytes Treg était importante.
Conclusion
Le pronostic des patients qui ont une TTVP ou une infiltration importante par des Treg intratumoraux avait tendance à être médiocre. On a établi un lien entre la présence de lymphocytes Treg intratumoraux et la présence de TTVP chez les patients atteints de CHC.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world.1 Despite diagnostic and therapeutic progress, most patients with HCC have advanced-stage disease at diagnosis. The prognosis for patients with HCC is very poor, especially for those with portal vein tumour thrombi (PVTT). Without treatment, few patients live for more than 3 months after diagnosis.2
The presence of PVTT is reported in as many as 12.5%–39.7% of patients with HCC and correlates negatively with prognosis. As for the mechanism of formation of PVTT, several theories have been put forward. It has been suggested that 2 vascular-specific growth factors, VEGF and Ang2, and endocrine gland–derived vascular endothelial growth factor might play a role in carcinogenesis of PVTT formation in patients with HCC by modulating angiogenesis.3 Conversely, the preoperative plasma thrombomodulin levels of patients with HCC has been reported to be significantly higher than that in patients with benign liver-occupying lesions and that in healthy controls.4 In addition, decreased expression of plasma thrombomodulin has been found to be correlated with portal vein tumour thrombus formation and might work as a biomarker for PVTT formation.4 Likewise, concentrations of thrombus precursor protein have been found to be significantly higher in patients with HCC than in healthy controls, and significantly higher in patients with tumour thrombosis than those without tumour thrombosis. By multivariate logistic analysis, positive thrombus precursor protein level has been found to be a significant predictor of the presence of tumour thrombosis.5 Through a comparative proteomic approach, annexin-v has been shown to be upregulated in tumour thrombus samples and might serve as a biomarker for early detection and therapeutic targets of HCC with PVTT.6 Portal venous hemodynamics in HCC has been investigated using a duplex system consisting of an electronic sector scanner and a pulsed Doppler velocitometer.7 The authors’ observations suggested that the portal venous flow was kept relatively constant in patients with advanced HCC until the tumour invaded into the portal trunk, and that portal venous flow increased when the hepatic arterial flow was occluded. In some patients, hepatofugal flow in the portal trunk owing to arterioportal shunts indicated that tumour cells might migrate into the portal vein in this way.7
In spite of all these possibilities, few studies have correlated the formation of PVTT with the immune status of the tumour. The tumour immune microenvironment has been found to play an important role in the establishment and progression of tumours, with special emphasis on the infiltration of T lymphocytes within tumours. Like the existence of PVTT, the compromised tumour infiltrating T lymphocytes have also been connected with poor prognosis in patients with HCC. Extremely marked infiltration of T cells, including predominant CD8+ T cells, has been generally shown to be closely associated with a low recurrence rate and good prognosis,8 whereas infiltration by a subpopulation of CD4+ T cells with immunosuppressive properties (Foxp3+ regulatory T cells; Treg) has been reported to counteract the beneficial effect of CD8+ T cells and has been negatively correlated with patient survival.9,10
In fact, both circulating Tregs and infiltration of Tregs within HCC have been correlated with disease progression and bad prognosis. Low concentrations of intratumoural Tregs in combination with high concentrations of intratumoural activated CD8+ cytotoxic T lymphocyte (CTL) infiltration have been found to be independent prognostic factors for both improved disease-free survival and overall survival. Most infiltrating CD8+ lymphocytes were found to be quiescent and did not express either perforin or granzyme B, which suggested that they were incapable of cytotoxic function.9 Furthermore, Tregs of patients with HCC have been found to significantly impair the CTL effect of CD8+ T cells through inhibition of proliferation, activation, degranulation and production of granzyme A, granzyme B and perforin.11
Although both the existence of PVTT and the compromised T lymphocyte infiltration, especially increased Tregs, have been correlated with poor prognosis in patients with HCC, the possibility of a correlation between them has not been well studied.
In this study, through investigation of tumour specimens by immunohistochemistry, we found that infiltration of Tregs, but not CD8+ or CD4+ lymphocytes, correlated with the formation of PVTT. We proposed that the compromised immune microenvironment might be one of the mechanisms involved in the formation of PVTT.
Methods
Patients and specimens
Between Feburary 2008 and December 2008 at the Department of Hepatobiliary Surgery, the First Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China), we prospectively collected specimens from patients with HCC after obtaining informed consent. All patients underwent liver resection with curative intent without distant metastasis or any prior anticancer treatment. The histological grade of tumour differentiation was assigned by the Edmondson grading system. Liver function was assessed by the Child–Pugh score system. Ethical approval was obtained from the ethics committee of the hospital.
All patients were regularly followed up and prospectively monitored for recurrence by assessment of serum α-fetoprotein (AFP) and by ultrasonography or contrast computed tomography every 1–3 months.
Immunohistochemistry
Paraffin-embedded formalin-fixed liver tissue was cut as 6 μm sections onto polylysine-coated slides. Sections were deparaffinized and rehydrated, and antigen retrieval was achieved by microwave antigen retrieval in citrate buffer (pH 8.0). The sections were then immersed in a 3% solution of H2O2 for 15 minutes at room temperature to block endogenous peroxidase activity. After blocking with 5% bovine serum albumin for 30 minutes at 37°C, sections were incubated with primary antibody (overnight at 4°C) and then rinsed. We used the following antibodies: mouse monoclonal, CD4, CD8 (all Novo Castra) and rabbit anti-Foxp3 (Abcam Ltd.). Detection of bound antibody was accomplished with the DAKO EnVision + System. The sections were incubated for 30 minutes at room temperature with secondary antibodies conjugated to the HRP–labelled polymer (DAKO) and rinsed with phosphate-buffered saline solution. Sections were then developed with 3,3′-diaminobenzidine for 5 minutes, counterstained with hematoxylin and covered with slides. We obtained negative controls by subjecting representative sections to the same protocol, with the primary antibody substituted by phosphate-buffered saline.
We counted the number of tumour-infiltrating lymphocytes (TILs), including CD4+ and CD8+, using the method described by Gao and colleagues.10 In brief, under light microscopy (× 400), 5 random high-power fields of a section representing the densest lymphocyte infiltration were selected for each patient sample. The results were expressed as the mean number of cells for each microscopic field. We then counted the number of Tregs in each of the high-power fields, and we added them to represent the Treg counts for each patient.
Statistical analysis
We assessed between-group differences using 1-way analysis of variance (ANOVA). Disease-free and overall survival rates were calculated according to the Kaplan–Meier method, and differences between groups were tested for significance using the log-rank test. We considered differences to be significant at p < 0.05.
Results
Patient characteristics
We collected specimens from a total of 76 patients: 70 men and 6 women with a mean age of 49 years at the time of hepatic resection. Hepatitis B surface antigen (HBsAg) was positive in 70 patients (92.1%), and we found an increased level of AFP in 57 patients (75.0%). According to Child–Pugh classifications, liver function grade A was observed in 74 patients and grade B in 2 patients. Histologically, 62 (81.6%) patients were shown to have cirrhosis, and invasion into the portal vein was observed in 31 (40.8%) patients. The mean tumour size was 92.2 mm (range 20.0–194.0 mm).
Analyses of survival after liver resection according to formation of PVTT and intratumoural Tregs
By September 2009, 41 patients had died of HCC, and 2 patients were lost to follow-up. Fourteen patients were alive but with recurrent HCC, and 19 were alive without recurrence. According to the presence of PVTT, we classified 76 patients with HCC into 2 groups: the PVTT group (31 patients) and the non-PVTT group (45 patients). As shown in Table 1, these 2 groups were well balanced regarding the clinical and pathologic characteristics except for tumour size.
Table 1.
Clinical and pathologic characteristics of patients
| Characteristic | No. patients | Group; no.* | p value | |
|---|---|---|---|---|
| Non-PVTT | PVTT | |||
| Sex | ||||
| Male | 70 | 41 | 29 | 0.70 |
| Female | 6 | 4 | 2 | |
| Mean age, yr | 48.67 | 49.71 | 0.71 | |
| Tumour size, mean cm | 8.29 | 10.11 | 0.049 | |
| Preoperative blood test value | ||||
| White blood cell count | 76 | 7.75 | 6.79 | 0.32 |
| γ-Glutamyl transferase | 76 | 138.92 | 189.79 | 0.15 |
| α-Fetoprotein | 76 | 15 397 | 17 720 | 0.71 |
| Albumin | 76 | 40.64 | 40.36 | 0.78 |
| Alanine transaminase | 76 | 46.14 | 56.14 | 0.26 |
| Total bilirubin | 76 | 20.51 | 27.34 | 0.52 |
| Hepatitis B | ||||
| Negative | 6 | 2 | 4 | 0.18 |
| Positive | 70 | 43 | 27 | |
| Background liver disease | ||||
| No cirrhosis | 14 | 8 | 6 | 0.86 |
| Cirrhosis | 62 | 37 | 25 | |
| Child–Pugh score | ||||
| A | 74 | 44 | 30 | 0.79 |
| B | 2 | 1 | 1 | |
| Tumour differentiation | ||||
| I–II | 51 | 32 | 19 | 0.38 |
| III–IV | 25 | 13 | 12 | |
| Tumour number | ||||
| Single | 54 | 33 | 21 | 0.60 |
| Multiple | 22 | 12 | 10 | |
| Capsulation | ||||
| Capsulated | 42 | 29 | 13 | 0.07 |
| Noncaspulated | 34 | 16 | 18 | |
PVTT = portal vein tumour thrombus.
Unless otherwise indicated.
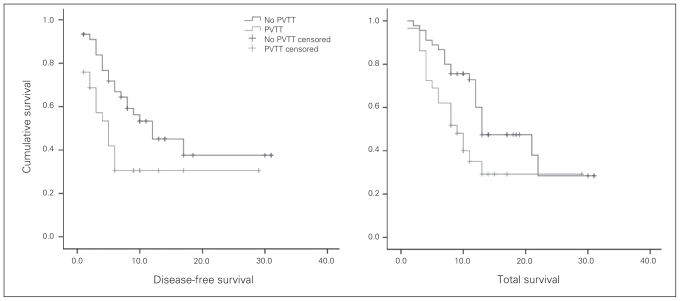
Survival analysis revealed that both the disease-free survival rate and total survival rate after hepatic resection was significantly lower in patients in the PVTT group than those in the non-PVTT group (p = 0.026 and 0.022, respectively; Fig. 1). The 6-month, 1-year and 2-year disease-free survival was 72%, 51%, 36%, respectively, in the non-PVTT group and 43%, 28%, 28%, respectively, in the PVTT group. The corresponding 6-month, 1-year and 2-year total survival were 89%, 71%, 29%, respectively, in the non-PVTT group and 69%, 33%, 25%, respectively, in the PVTT group.
Fig. 1.
Disease-free survival and total survival curves after hepatic resection according to the presence of portal vein tumour thrombus (PVTT). Both the disease-free survival rate (p = 0.026) and the total survival rate (p = 0.022) were significantly lower in patients in the PVTT group (n = 31) than in patients in the non-PVTT group (n = 45).
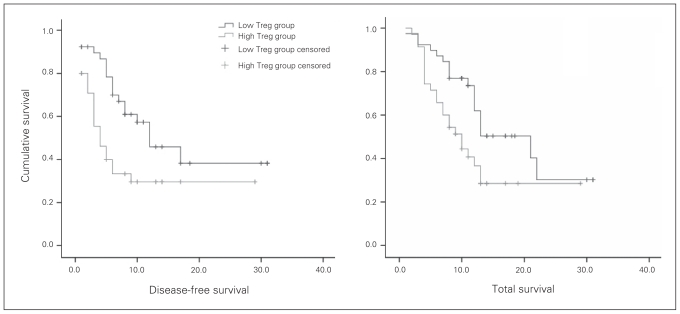
Patients with HCC were classified into 1 of 2 groups according to the median number of tumour-infiltrating Tregs: the low Treg group (< 27 per 5 high-powered fields; 41 patients) and the high Treg group (> 27 per 5 high-powered fields; 35 patients) respectively. Univariate analysis revealed that both the disease-free survival rate and the total survival after hepatic resection was significantly lower in patients in the high Treg group than in those in the low Treg group (p = 0.012 and 0.023, respectively; Fig. 2). The 6-month, 1-year and 2-year disease-free survival was 79%, 54%, 35%, respectively, in the low Treg group and 40%, 29%, 29%, respectively, in the high Treg group. The corresponding 6-month, 1-year and 2-year total survival were 90%, 72%, 30%, respectively in the low Treg group and 71%, 40%, 24%, respectively, in the high Treg group.
Fig. 2.
Disease-free survival and total survival curves after hepatic resection according to the number of intratumour Foxp3+ regulatory T cells (Tregs). Both the disease-free survival rate (p = 0.012) and the total survival rate (p = 0.023) were significantly lower in patients in the high Treg group (n = 35) than in patients in the low Treg group (n = 41).
Correlation between formation of PVTT and infiltration of Tregs
Intratumoural Tregs, but not peritumoural Tregs, were found to be associated with PVTT formation (p = 0.001). In addition, no correlation was found between both intratumoural and peritumoural CD4+ and CD8+ T cells with PVTT formation (all p > 0.05; Table 2). Furthermore, a χ2 analysis of patient distribution showed that patients with low Treg infiltration tended to have a lower percentage of PVTT formation (12 of 41, 29.3%), whereas patients with high Treg infiltration had a higher pencentage of PVTT formation (19 of 35, 54.3%; χ2 = 8.316, p = 0.040).
Table 2.
Correlation between CD4+ T cells, CD8+ T cells, Foxp3+ Treg and formation of portal vein tumour thrombus
| Variable | No. of patients | Group; value | p value | |
|---|---|---|---|---|
| Non-PVTT, n = 45 | PVTT, n = 31 | |||
| CD4+ T cells | ||||
| Intratumour | 76 | 34.73 | 36.26 | 0.49 |
| Peritumour | 76 | 51.13 | 52.32 | 0.69 |
| CD8+ T cells | ||||
| Intratumour | 76 | 28.38 | 29.68 | 0.41 |
| Peritumour | 76 | 62.76 | 66.1 | 0.21 |
| Foxp3+ Tregs | ||||
| Intratumour | 76 | 25.02 | 31.54 | 0.001 |
| Peritumour | 76 | 32.96 | 31.81 | 0.62 |
PVTT = portal vein tumour thrombus; Treg = regulatory T cells.
Discussion
Few studies have examined the role of host immune responses in the course of formation of PVTT, although various arguments suggest that T lymphocytes may play a role in the control of the development of this type of cancer.10 In patients with HCC, both compromised T lymphocytes and formation of PVTT have been shown to be negatively correlated with the prognosis of patients.2,10–11 Our study supported this finding, so there might be a correlation between compromised T lymphocytes and the formation of PVTT.
In fact, compromised T lymphocytes and increased tumour invasion have been reported in other types of tumours. In cervical cancer, analysis of the local anti-tumour immunity has been carried out by enumerating tumour-infiltrating dendritic cells and CD4+, CD8+ and regulatory T cells, as well as the ratio of CD8+/CD4+ T cells and CD8+/regulatory T cells. It was found that a high number of intra-epithelial CD8+ tumour-infiltrating lymphocytes was associated with the absence of lymph node metastases.12 Epstein-Barr virus (EBV)–positive gastric carcinoma has been found to have a better prognosis owing to a lower incidence of lymph node metastases (LN+). In comparison with patients with EBV–negative gastric carcinoma, patients with EBV–positive gastric carcinoma had higher expression of major histocompatibility complex (MHC) class II on the tumour cells, more extensive infiltration of activated granzyme B+ CD8+ T cells and higher numbers of mature dendritic cells, indicating that local triggering of cellular immune responses in EBV–positive gastric carcinoma prevents formation of lymph node metastasis.13
In patients with colorectal cancer with hepatic metastasis, the peripheral blood T cell subsets and natural killer (NK) cell activity, along with the production of interleukin (IL)-2 and prostaglandin E2 (PGE2) of peripheral blood mononuclear cells were investigated. No significant difference between T cell subsets and NK cell activity were found between patients with or without hepatic metastasis and healthy controls, whereas a significant decrease of IL-2 and increase of PGE2 were observed in patients with hepatic metastasis. It was concluded that patients with colorectal cancer with hepatic metastasis tended to have decreased cell-mediated immune status.14
Therefore, the immune status might be one of the mechanisms leading to tumour metastasis. In our study, tumour specimens from patients with PVTT were found to have increased Tregs compared with those from patients without PVTT. Further analysis showed that patients with low Treg infiltration tended to have a lower percentage of PVTT formation, whereas patients with high Treg infiltration had a higher pencentage of PVTT formation. On the other hand, no significant differences were observed between CD8+ and CD4+ T lymphocyte infiltration.
Consistent with our results, Gao and colleagues10 found that high-density Treg infiltration within tumour tissues correlated positively with vascular invasion and correlated negatively with tumour encapsulation, both of which are putative clinicopathologic markers of HCC invasiveness. These results sustain the hypothesis that Tregs could modify HCC cells in ways that potentiate their invasiveness, such as PVTT formation.
If the Tregs are deleted or suppressed, what will be the prognosis? In animal models, removal of Tregs improved immune-mediated tumour clearance and enhanced the response to immunotherapy. It was found that cyclophosphamide (CTX) could be used in combination with chemotherapy to achieve regulatory T cell depletion. In the mouse mesothelioma model, combined use of normal chemotherapeutic drugs and immunopotentiating CTX could eradicate established tumours, and this effect could be negated by the adoptive transfer of Tregs. Ghiringhelli and colleagues15 demonstrated that oral administration of metronomic CTX in patients with advanced cancer induced a profound and selective reduction of circulating regulatory T cells, associated with a suppression of their inhibitory functions on conventional T cells and NK cells, leading to a restoration of peripheral T cell proliferation and innate killing activities. They concluded that administration of CTX favours a better control of tumour progression and should precede anticancer immunotherapy.
We have previously shown that hepatectomy plus portal thrombectomy in combination with postoperative adjuvant immunochemotherapy of PIAF (5-fluorouracil, doxorubicin, cisplatin and IFN-α) via the portal vein improves the prognoses of patients with HCC and PVTT, as assessed by both the median tumour-free survival time and median total survival time, which were significantly prolonged in the postoperative adjuvent immunochemotherapy group compared with the control group. Our results indicated that immunochemotherapy might represent a promising method for patients with HCC and PVTT.16 Inhibition of Tregs might further improve this effect and needs further investigation in clinical trials.
Conclusion
In conclusion, our study demonstrated that intratumoural Tregs were associated with formation of PVTT in patients with HCC and that compromised immune microenvironment might account for this finding. Combined with our previous research,16 we propose that the combination of depletion of Tregs and concomitant chemotherapy might be an effective strategy to reduce recurrence and prolong the survival of patients with HCC.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 30672052, 30872486) and the Natural Science Foundation of Guangdong Province (8151008901000161).
Footnotes
Competing interests: None declared.
Contributors: All authors helped design the study, analyzed the data, critically reviewed the article and approved its publication. Drs. Shen, Kuang and Lai acquired the data. Drs. Shen, Peng, He, Kuang and Lai wrote the article.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Xu B, Fu L, et al. Correlation of four vascular specific growth factors with carcinogenesis and portal vein tumor thrombus formation in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2006;25:403–9. [PubMed] [Google Scholar]
- 4.Zhou J, Tang ZY, Fan J, et al. The potential of plasma thrombomodulin as a biomarker of portal vein tumor thrombus in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2001;127:559–64. doi: 10.1007/s004320100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HK, Lee KR, Yang JH, et al. Plasma levels of D-dimer and soluble fibrin polymer in patients with hepatocellular carcinoma: a possible predictor of tumor thrombosis. Thromb Res. 2003;109:125–9. doi: 10.1016/s0049-3848(03)00183-x. [DOI] [PubMed] [Google Scholar]
- 6.Guo WX, Man XB, Yuan HX, et al. [Proteomic analysis on portal vein tumor thrombus-associated proteins for hepatocellular carcinoma] [Article in Chinese] Zhonghua Yi Xue Za Zhi. 2007;87:2094–7. [PubMed] [Google Scholar]
- 7.Ohnishi K, Sato S, Tsunoda T, et al. Portal venous hemodynamics in hepatocellular carcinoma. Effects of hepatic artery embolization. Gastroenterology. 1987;93:591–6. doi: 10.1016/0016-5085(87)90923-1. [DOI] [PubMed] [Google Scholar]
- 8.Wada Y, Nakashima O, Kutami R, et al. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–14. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 9.Unitt E, Rushbrook SM, Marshall A, et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–30. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 10.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 12.Piersma SJ, Jordanova ES, van Poelgeest MI, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–61. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 13.van Beek J, zur Hausen A, Snel SN, et al. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. Am J Surg Pathol. 2006;30:59–65. doi: 10.1097/01.pas.0000176428.06629.1e. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu H, Ito H, Kimura F, et al. Decreased cell-mediated immune status in colorectal cancer patients with hepatic metastasis. Hepatogastroenterology. 2005;52:1106–9. [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang LJ, Hu WJ, Yin XY, et al. Adjuvant intraportal venous chemotherapy for patients with hepatocellular carcinoma and portal vein tumor thrombi following hepatectomy plus portal thrombectomy. World J Surg. 2008;32:627–31. doi: 10.1007/s00268-007-9364-0. [DOI] [PubMed] [Google Scholar]