Abstract
Objective
The objective of this study was to investigate the role of vascular ABCG1 in atherogenesis without a confounding difference in macrophage ABCG1 expression. The ATP Binding Cassette Transporter G1 (ABCG1) is highly expressed in macrophages and endothelial cells. ABCG1 preserves endothelial function by maintaining endothelial nitric oxide synthase (eNOS) activity and by reducing adhesion molecule expression and monocyte adhesion.
Methods and Results
To investigate the role of vascular ABCG1 expression in atherosclerosis in vivo, Abcg1−/−Ldlr−/− and Ldlr−/− mice were transplanted with wild-type bone marrow and fed a Western type diet for 12 or 23 weeks. The atherosclerotic lesion area was similar in both groups after 12 weeks, but was increased in Abcg1−/−Ldlr−/− recipients after 23 weeks, especially in the aortic arch (2.2-fold; P<0.01). ENOS-mediated vascular relaxation was impaired in male Abcg1−/−Ldlr−/− recipients.
Conclusion
Our data show an athero-protective role of vascular ABCG1 especially in the aortic arch, likely related to its role in preservation of eNOS activity.
Keywords: ABCG1, HDL, atherosclerosis, eNOS, endothelium
The cholesterol efflux promoting ATP-Binding Cassette Transporter G1 (ABCG1) is highly expressed in macrophages and endothelial cells (ECs)1,2 where it helps to preserve eNOS activity1 and likely reduces expression of vascular adhesion molecules, chemokines, and monocyte adhesion.3 Large high density lipoproteins (HDLs) that result from treatment with niacin or cholesteryl ester transfer protein inhibitors efficiently promote cholesterol efflux from ABCG1-expressing cells,4 suggesting that endothelial ABCG1 might promote beneficial effects of HDL raising therapies on vascular functions.
Reduced eNOS activity and increased expression of adhesion molecules are features of atherosclerotic lesions in humans.5 ENOS deficiency accelerates atherogenesis in apolipoprotein E deficient (Apoe−/−) mice, whereas hypomorphic vascular cell adhesion molecule-1 (VCAM-1) mice show decreased atherogenesis.6,7 These studies suggest the hypothesis that vascular ABCG1 expression is anti-atherogenic. To investigate the role of vascular ABCG1 in atherosclerosis in vivo, we transplanted Abcg1−/−Ldlr−/− and Ldlr−/− mice with wild-type bone marrow (BM).
Methods
Ldlr−/− and Abcg1−/−Ldlr−/− mice were transplanted with wild-type BM and fed a Western type diet (WTD) for 12 or 23 weeks. Atherosclerotic lesion area was assessed. The supplementary data contain additional information (available online at http://www.atvb.ahajournals.org).
Results
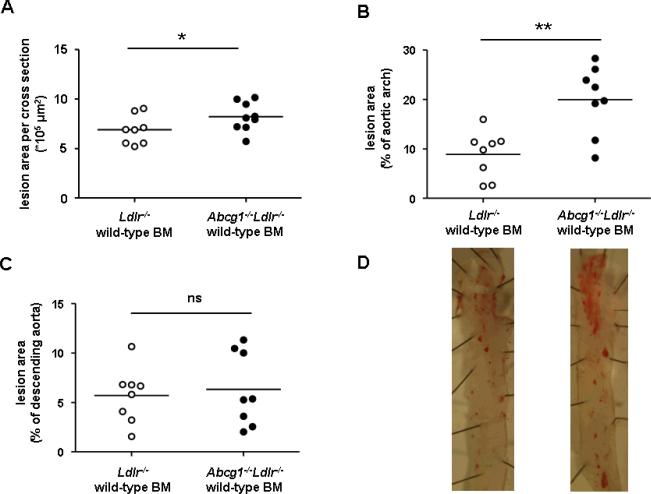
To investigate the role of vascular ABCG1 in atherogenesis, we transplanted Ldlr−/− and Abcg1−/−Ldlr−/− mice with wild-type BM. The efficiency of BM reconstitution was greater than 90%. Five weeks after transplantation, animals were fed a WTD for 12 weeks. Cholesterol levels did not differ between the two groups (Supplemental Table I). The atherosclerotic lesion area in the aortic root was similar in the 2 groups; there was minimal lesion formation and no difference in the aortic arch and descending aorta (Supplemental Figure IA and B). However, after feeding the WTD for 23 weeks, Abcg1−/−Ldlr−/− recipients showed a 20% increase in atherosclerosis in the aortic root (Figure 1A, Supplemental Figure II) (P<0.05), a 2.2-fold increase in the aortic arch (P<0.01) (Figure 1B and D), and no difference in the descending aorta (Figure 1C and D). Combined data from the aortic arch and descending aorta showed a 1.9-fold increase in lesions in Abcg1−/−Ldlr−/− recipients (P<0.05) (Supplemental Figure IIIA). The difference was significant in males and females (2.2-fold and 1.6-fold, respectively; P<0.05 for both) (Supplemental Figure IIIB and C).
Figure 1.
Atherosclerosis in Ldlr−/− (n=8) and Abcg1−/−Ldlr−/− mice (n=9) transplanted with wild-type BM (23 weeks WTD). Haematoxylin-eosin staining was performed on paraffin sections of the aortic root and Oil Red O staining on the aortic arch and descending aorta. Atherosclerotic lesion area in (A) the aortic root, (B) the aortic arch, and (C) the descending aorta. D. Representative pictures of Oil Red O staining in the aortic arch and descending aorta. In A–C, each point represents an individual mouse. *P<0.05, **P<0.01.
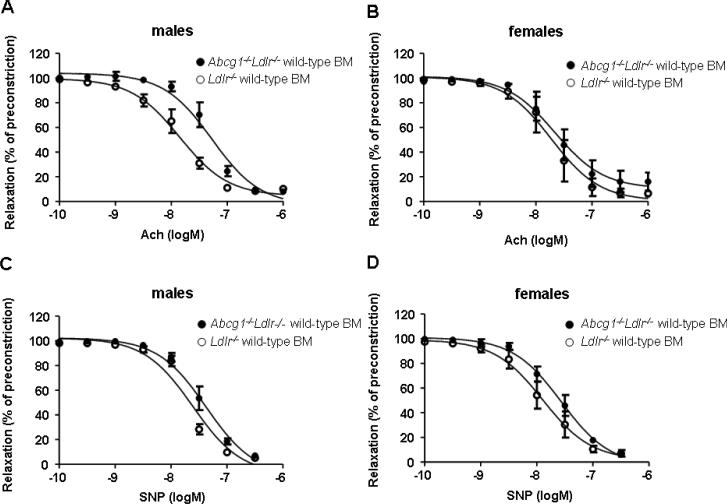
To investigate vascular function in Abcg1−/−Ldlr−/− and Ldlr−/− recipients a WTD, femoral arteries were preconstricted with phenylephrine, and responses to endothelium-dependent acetylcholine (ACh) and smooth muscle cell (SMC)-dependent sodium nitroprusside (SNP) vasodilating agents were measured. Male Abcg1−/−Ldlr−/− recipients showed reduced vasorelaxation in response to ACh (Figure 2A), reflected by an increased EC50 value compared to Ldlr−/− recipients (EC50, 57.3±14.8 nM versus 15.4±5.0 nM) (P<0.05). ACh-induced vasorelaxation was similar in female Abcg1−/−Ldlr−/− and Ldlr−/− recipients (Figure 2B) (EC50, 29.2±8.0 nM versus 25.5±13.3 nM). SNP-induced vasorelaxation was not affected by the recipient genotype (Figure 2C and D). We also analyzed VCAM-1 expression in the EC layer of the aortic root. VCAM-1 expression tended to increase in males but not females (Supplemental Figure IVA–C), but data did not reach significance. Aortic arch samples were not available for analysis.
Figure 2.
Response to vasodilating agents in femoral arteries of Ldlr−/− (n=4 per sex) and Abcg1−/−Ldlr−/− (n=4 per sex) mice transplanted with wild-type BM (12 weeks WTD). Response to acetylcholine (Ach) (A–B) and sodium nitroprusside (SNP) (C–D).
Discussion
The role of ABCG1 in atherogenesis is complex, but important in view of its potential role in response to HDL raising therapies. Although Abcg1−/− mice show prominent foam cell accumulation in tissues,2 most, but not all, reports have found unchanged or reduced atherosclerosis in susceptible mice transplanted with Abcg1−/− BM.8,9,10 Studies in Abcg1−/− mice have also led to conflicting reports.11,12 In Abcg1−/−Apoe−/− mice fed WTD, atherosclerosis was decreased,12 whereas in Abcg1−/− mice with small foam cell lesions fed the Paigen diet atherosclerosis was increased.11 The present study provides the first demonstration of an athero-protective role for vascular ABCG1. Because ABCG1 is highly expressed in ECs and undetectable in vascular SMCs,1,2 our results are most likely caused by endothelial ABCG1 expression, and are consistent with earlier studies indicating an important role of ABCG1 in preserving endothelial functions.1,3
Interestingly, the increase in atherosclerosis in Abcg1−/−Ldlr−/− recipient mice after 23 weeks of diet was most pronounced in the aortic arch. Increased atherosclerosis in Enos−/−Apoe−/− mice is also most prominent in this location.7 The aortic arch is exposed to altered blood flow, reducing eNOS activity and NO bioavailability.13 We found decreased endothelium-dependent vasorelaxation reflecting decreased eNOS activity and NO production in Abcg1−/−Ldlr−/− male but not female recipients. Although males clearly had increased atherosclerosis in the arch, our samples size was too small to test for gender-specific differences. Sex differences in eNOS activity have been attributed to eNOS activity-inducing estrogens in females.14 NO has several anti-atherogenic properties, including attenuation of EC-leukocyte interactions.15 Abcg1−/− ECs also show increased expression of VCAM-1 and E-selectin.3 A variety of mechanisms are likely responsible for the atheroprotective functions of vascular ABCG1. Evaluations of VCAM-1 expression in the present study were limited, and further investigations are warranted.
Supplementary Material
Acknowledgments
Sources of funding This work has been funded by NIH grant HL102395 (to A.T.), the American Heart Association (Postdoctoral Fellowship 09POST2110109 to M.W.), and the Netherlands Organization for Scientific Research (NWO Rubicon grant 825.07.018 to M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Whetzel AM, Sturek JM, Nagelin MH, Bolick DT, Gebre AK, Parks JS, Bruce AC, Skaflen MD, Hedrick CC. ABCG1 deficiency in mice promotes endothelial activation and monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2010;30:809–817. doi: 10.1161/ATVBAHA.109.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR. Cholesterol efflux potential and anti-inflammatory properties of HDL after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30:1430–1438. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forstermann U, Mugge A, Alheid U, Haverich A, Frolich JC. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988;62:185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Dansky HM, Barlow CB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol. 2001;21:1662–1667. doi: 10.1161/hq1001.096625. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 8.Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, Kuipers F, Van Berkel TJ, Van Eck M. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 9.Baldán A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA. Impaired development of atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 10.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 11.Out R, Hoekstra M, Meurs I, de Vos P, Kuiper J, Van Eck M, Van Berkel TJ. Total body ABCG1 expression protects against early atherosclerotic lesion development in mice. Arterioscler Thromb Vasc Biol. 2007;27:594–599. doi: 10.1161/01.ATV.0000257136.24308.0c. [DOI] [PubMed] [Google Scholar]
- 12.Tarling EJ, Bojanic DD, Tangirala RK, Wang X, Lovgren-Sandblom A, Lusis AJ, Bjorkhem I, Edwards PA. Impaired Development of Atherosclerosis in Abcg1−/−Apoe−/− Mice. Identification of Specific Oxysterols That Both Accumulate in Abcg1−/− Apoe−/− Tissues and Induce Apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:1174–1180. doi: 10.1161/ATVBAHA.110.205617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280:19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R, Libby P, Peng H-B, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.