Abstract
Multiple sclerosis (MS) is an autoimmune disorder characterised by clinical relapse and remission and pathological demyelination with varying inflammation. Because it is suggested that T-cells expressing natural killer cell receptors (NKR) play important roles in regulating human autoimmune diseases, we have quantified populations T-cells expressing the NKR CD56, CD161 and CD94 in the peripheral blood of MS patients, in healthy control subjects (HS) and in patients with other neurological diseases (OND). CD161+ T-cells and CD94+ T-cells were significantly decreased in MS patients with primary progressive disease and secondarily change progressive disease respectively whereas CD56+ T-cell numbers were unchanged. In contrast NKT-cells that express the invariant V α 24-J α 18+ T-cell receptor identified here by specific receptor antibody and CD1d-tetrameric PBS57-loaded complexes, were increased in MS patients compared with HS. Reductions in CD161+ T-cells and CD94+ T cells relative to HS were also observed in the OND group and this was particularly prominent in Parkinsonian patients. A striking functional finding was that while NKT-cells in unfractionated peripheral blood from healthy subjects expanded in number and produced IFN- γ upon stimulation with α-galactosylceramide, NKT-cells from MS patients did not. Thus we have identified alterations in a number of potentially important lymphocyte sub-populations warranting further investigation in the immune response in MS.
Keywords: Natural killer receptor+ T-cells, Natural killer T-cells, Multiple sclerosis, α-galactosylceramide
1. Introduction
Multiple sclerosis (MS) is an acute and chronic demyelinating disease affecting the central nervous system that is clinically characterised by periods of relapse and remission and usually by a progressive course [1–4]. While the immune response, and especially autoreactive T-cells, are generally believed to mediate the pathology, much remains to be understood about how this process is generated [5]. T-cells of the Th-1 (T helper-1) cytokine profile can be cloned from the peripheral blood of patients with MS. These cells secrete large amounts of interferon- (IFN- γ) upon recognition of components of myelin [6–9].
Several types of regulatory cell populations are believed to be involved in suppression of disease development or in prevention of MS, or indeed in promoting recovery in patients with relapsing-remitting disease. Regulatory roles for CD8+ T-cells [10], for B-cells [11] and for natural killer (NK−) cells [12] in suppressing the pathogenic Th1-cells in experimental autoimmune encephalitis, the animal model of MS, have previously been reported. However, the situation in human MS is less clear cut, and while several immune cell abnormalities have been described in MS [13] including reductions in the numbers of NK-cells [14], in natural killer T-cells (NKT-cells)[15,16], and in CD8+ T-regulatory cells [17], the relative contributions of individual regulatory cell subsets remains to be clearly defined.
In recent years an important role for T-cells which bear natural killer (NK) receptors has been recognised in regulating autoimmunity [18,19]. Included in this group are the invariant NKT-cells which express NK-cell surface receptors and a highly restricted T-cell receptor (TCR) repertoire, encoded by V α 24 and J α 18 genes in humans [20]. NKT-cells produce large amounts of interleukin- (IL-) 4 and IFN- γ , are activated by α-galactosylceramide ( α-GalCer) presented by the CD1d molecule [21] and display diverse effector mechanisms participating in tumour surveillance [22] and in regulation of autoimmune diseases [19]. Altered numbers and functions of NKT-cells have been previously reported in MS [15,16,23] and stimulation with α-GalCer can suppress Th1-mediated disease by inducing IL-4 secretion [24].
The low frequency of CD1d-restricted NKT-cells expressing the invariant V α 24J α 18+ TCR in peripheral blood of healthy human subjects [25] raises debate over the potential role of these cells to prevent autoimmune diseases. Nonetheless, other T-cell subsets which lack an invariant TCR but which express various NK receptors, including CD56, CD57, CD161, CD94 and killer Ig-like receptors, are more abundant in peripheral blood. These natural killer receptor-positive (NKR+) T-cell populations have been reported to share some functional similarities with murine NKT-cells [26–28]. CD56 is a marker which correlates with the cytotoxic function of CD8+ T-cells [29]. CD56+ T-cells display both NK-like and T-cell restricted cytotoxicity and are capable of secreting cytokines, including IFN- γ , TNF- α and IL-4 which promote Th1 and Th2 adaptive immune responses [26,30]. CD161 is a co-stimulatory molecule that regulates the activity of CD1d-dependent T-cells [31]. CD94 expression on T-cells is considered protective against viral infection and against the potential development of autoimmunity [32]. T-cells bearing NKRs together with innate lymphocyte populations like γ δ T-cells, act as front-line immune regulatory cells [33,34]. The mechanisms controlling the expression of NKRs on T-cells still remain to be elucidated, although cytokines may have significant roles in determining their expression [35].
Given that NKR+ T-cells which include NKT-cells represent functionally distinct populations it seems crucial to examine these individual populations in order to begin to clarify immune response regulation in MS. The primary aims of this study were firstly, to evaluate the expression of the NK receptors CD56, CD161 and CD94 on peripheral blood T-cells freshly isolated from MS patients compared with healthy subjects; and secondly, to compare the numbers of invariant NKT-cells in MS and controls and thirdly to investigate the response of NKT-cells to α-GalCer in these two groups. While reduced numbers of invariant NKT-cells have been previously reported in the peripheral blood of MS patients, particularly in clinical remission [15,23,36], these studies did not address the response to α-GalCer or the issue of whether NKR+ T-cells may participate in the local regulation of MS. Due to the central role of NKR+ T-cells in immunity, a study evaluating their numbers in MS, and especially encompassing the clinical spectrum of MS disease activity, was carried out.
2. Materials and methods
2.1 Subjects
All of the MS patients studied were diagnosed using standard clinical criteria including MRI scanning and cerebrospinal fluid examination [37]. Patients were classed as relapsing-remitting MS (RR-MS, n=23), secondarily progressive MS (SP-MS, n=10), and primary progressive MS (PP-MS, n=7). As controls, patients with other neurological diseases (OND, n=27), subdivided into non-inflammatory (NI-OND, n=22) and inflammatory (I-OND, n=5) subgroups, as well as healthy control subjects (n=38) were studied. The clinical diagnoses of subjects in the NI-OND and I-OND subgroups are listed in Table 1. The MS patients ranged from 22–64 years of age (female: male, 3.5), the OND patients from 34–81 (female: male, 2.7) and the healthy subjects from 21 to 30 years (female: male, 1.5). In all cases, informed consent was obtained from each individual. Ethical approval for this study was obtained from the University College Hospital Galway Ethics Committee and from the National University of Ireland, Galway Research Ethics Committee.
Table 1.
Clinical diagnoses of subjects with other neurological diseases (OND)
| Subgroup | Diagnosis (number of subjects) |
|---|---|
| Non-Inflammatory –OND | Epilepsy (n=5) |
| Headache (n=7) | |
| Paresthesias (n=2) | |
| Parkinson’s disease (n=6) | |
| Syncope (recurrent) (n=1) | |
| Syringomyelia (n=1) | |
| Inflammatory-OND | Chorea (undiagnosed), autoimmune cause suspected (n=1) |
| Chronic inflammatory demyelinating polyneuropathy/interstitial pneumonitis (n=1) | |
| Irritable bowel syndrome/migraine (n=1) | |
| Peripheral neuropathy, autoimmune cause suspected (n=1) | |
| Transverse myelitis (n=1) |
Our first study of the clinical phenotypes of circulating NKR+ T-cells surveyed 69 patients and controls employing a masked protocol in order to minimize clinical and technical bias. To determine whether treatment affected NKR+ T-cell numbers, the study was subsequently expanded to include 14 MS patients naïve to therapy. The study of invariant NKT-cell frequencies and the analysis of peripheral blood lymphocyte responses to α-GalCer examined MS subjects who were all naïve to immunomodulatory therapy with interferons or glatiramer.
2.2 Peripheral blood mononuclear cell (PBMC) isolation
PBMCs were isolated by conventional density gradient centrifugation. Briefly, peripheral blood was layered over Histopaque (5 ml; Sigma Chemical Co., St. Louis, MO) and centrifuged (300 x g, 25 min), the resulting buffy coat carefully removed, and washed twice with Hank’s Balanced Salts Solution (Gibco, Paisley,UK). Finally cells were re-suspended in complete RPMI 1640 medium (Gibco) containing 10% foetal calf serum, L-glutamine, and penicillin/streptomycin before counting.
2.3 Staining antibodies and flow cytometry
Fluorochrome-labelled monoclonal antibodies specific for human CD3 (Cy5), CD56 (fluorescein isothiocyanate-FITC), CD94 (FITC), CD161(FITC), and isotype-matched controls were obtained from Serotec (Oxford, UK). Monoclonal anti-V α 24 antibody labelled with phycoerythrin (PE) was obtained from Coulter Immunotech (Marseilles, France) and anti-V α 24J α 18-PE, specific for invariant NKT cells [38] from BD Pharmingen (Oxford, UK). Cell suspensions were derived from peripheral blood and cell counts adjusted in complete RPMI medium. Two- and three-colour flow cytometry was performed by staining 1 x 105 cells with 4 μl of the relevant monoclonal antibodies at 4°C. After washing with phosphate-buffered saline (0.1 M, pH 7.0) containing 1% bovine serum albumin (PBS-BSA), cells were fixed in 500 μl of Cellfix (BD Biosciences, Oxford, UK) prior to acquisition and analysis using FACsCalibur® and CellQuest® lysis software (Becton Dickinson, Oxford, UK).
To investigate the presence of CD1d-restricted (or invariant) NKT-cells amongst peripheral blood lymphocytes, PBMCs were stained using PBS57-loaded CD1d tetramers labelled with PE as fluorochrome (kindly provided by the National Institute of Health tetramer-facility at Emory University, Atlanta, Georgia). PBS57 is an analogue of α-GalCer which is a ligand for the invariant TCR on NKT-cells [21]. The activity of PBS57 is indistinguishable from α-GalCer but is more soluble [39]. For staining, 1 x 105 cells were incubated with a 1:100 dilution of the PBS57-loaded tetramers for 1 h at 4°C. As a negative control, cells were also stained with unloaded tetramer. After incubation, cells were washed in PBS-BSA, surface stained with fluorochrome-labelled anti-CD3 antibody, and following a 30 min incubation at 4°C and washing with PBS-BSA, cells were acquired and analysed by flow cytometry as described above.
2.4 NKT-cell response to α-GalCer
106 PBMC were cultured in 24-well tissue culture plates in the presence of 10 μg/ml α-GalCer (Alexis Biochemicals, San Diego, USA), or medium alone as control. After 7 days in culture, the numbers of invariant NKT-cells in stimulated and unstimulated cultures were compared using flow cytometry (see above).
For measurement of IFN- γ production, supernatants were collected from the above cultures at 72–120 hours. IFN- γ released by unstimulated (medium alone) and α-GalCer stimulated cells was measured using the capture ELISA system from R&D Systems, Oxon, UK.
2.5 Statistics
Differences between groups of non-parametric data were analysed using the Mann-Whitney U statistic using Graphpad Instat® software (GraphPad Software Inc); P < 0.05 was considered as significant.
3. Results
3.1 Representative patterns of natural killer receptor expression on peripheral blood T-cells
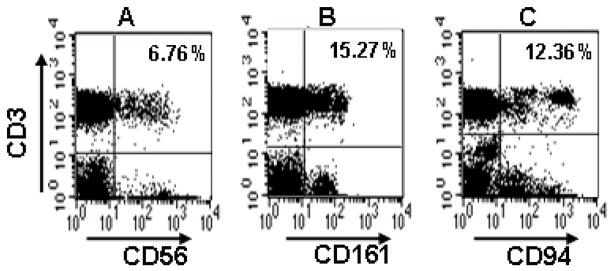
Using flow cytometry, we defined NKR+ T-cells as CD3+ T-cells expressing the NK-cell markers CD56, CD161 or CD94. The percentages of T-cells amongst peripheral blood mononuclear cells were determined firstly in healthy control subjects, of which representative dot plots are shown in Fig. 1. Overall in healthy subjects, the frequencies of CD161+ T-cells and CD94+ T-cells were similar, with median values of 12.5% and 12.1%, respectively. A smaller proportion (6.7%) of T-cells expressed the CD56 marker (Fig. 1).
Fig. 1.
Representative flow cytometric dot plots showing NKR+ T-cells in a healthy control subject. Lymphocytes were gated on the basis of cell size and granularity (FSC/SSC). The percentages of CD3+ T-cells expressing the natural killer receptors CD56, CD161 or CD94 are shown in the upper-right quadrants in A, B and C, respectively.
3.2 NKR+ T-cells in neurological disease
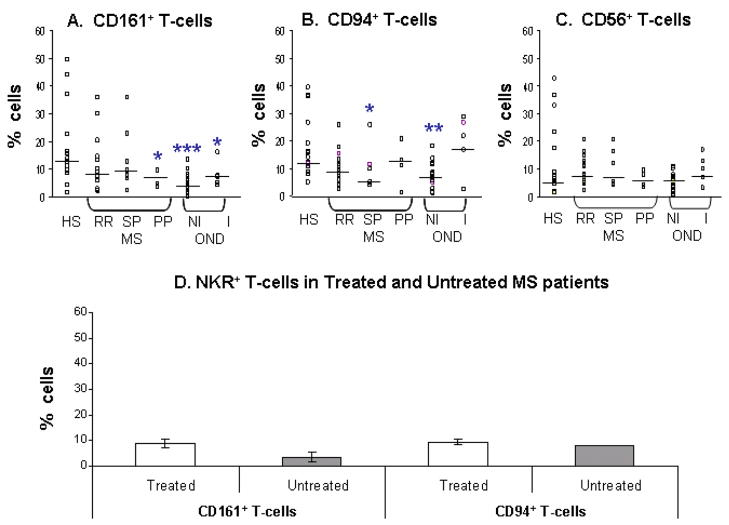
When NKR+ T-cell populations were examined in patients with neurological diseases, including all of the MS and OND patients, the proportion of T-cells expressing CD161 was significantly reduced compared with healthy subjects (median, 7.15% versus 12.5%; P = 0.0009). Likewise, a significant decrease (P = 0.01) was observed in the frequency of CD94+ T-cells in the neurological disease group compared with healthy subjects (medians, 8.3% versus 12.1%). CD56+ T-cells in the neurological disease group were less frequently detected (median, 6.4%) compared with either CD161+ T-cells or CD94+ T-cells, but there was no significant difference in the numbers of these cells compared with healthy subjects (median, 6.7%).
The composition of NKR+ T-cells was then examined in subgroups of MS patients (relapsing remitting (RR)-MS, primary progressive (PP)-MS and secondary progressive (SP)-MS) and in inflammatory and non-inflammatory other neurological disease control groups (I-OND and NI-OND respectively)(Fig. 2(a–c)). The proportion of CD161+ T-cells was significantly reduced in the PP-MS (median, 7.2%; P = 0.04), in the I-OND group (7.4%; P = 0.03) and in the NI-OND group (4.7%; P =0.0001), but not in the RR-MS or in the SP-MS patient groups compared with healthy subjects. It is noteworthy that when studied in three PP-MS patients, the extent of the reduction in CD161+ T-cells in relation to EDSS scores (range 5–7) was directly proportional to disability. The numbers of CD94+ T-cells were also significantly reduced in the SP-MS group (median, 4.9%; P = 0.04) and in the NI-OND group (6.7%; P = 0.001) but not in the RR-MS, PP-MS or in the I-OND patient groups compared with healthy subjects. For the six SP-MS patients studied, the EDSS disability scores (range 4–6.5) correlated with and were inversely proportional to the reduction in CD94+ T-cell numbers. There was no significant difference in the CD56+ T-cell numbers in the MS patient groups (median values ranging from 5.32% in SP-MS to 7.32% in RR-MS) and in the OND patient groups (median values for I-OND and NI-OND, 8.5% and 6.26%, respectively) compared with healthy subjects. Hence, significant changes in T-cells expressing CD161 or CD94, but not CD56, were found in subgroups of MS patients (i.e., PP-MS and SP-MS groups) and also in OND patients. The large reduction in CD161+ and CD94+ T-cells in the NI-OND group is noteworthy: this unanticipated observation was surprising, and may be related to the prominence of Parkinson’s disease in this group. Five of the 6 patients with this disease had reduced numbers of NKR+ T-cells compared with healthy subjects (see discussion).
Fig. 2.
NKR+ T-cells are reduced in multiple sclerosis. Percentages of NKR+ T-cells in the CD3+ T-cell populations of healthy subjects (HS), multiple sclerosis (MS) subgroups (RR-MS, relapsing remitting; SP-MS, secondary progressive; PP-MS, primary progressive), and other neurological diseases (OND) subgroups (NI-OND, non-inflammatory OND; I-OND, inflammatory OND). Percentages of (A) CD161+ T-cells, (B) CD94+ T-cells and (C) CD56+ T-cells for each of the groups are shown. Horizontal bars indicate median values. Significance values comparing HS to MS or OND subgroups are shown by *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) Comparison of NKR+ T-cells in the peripheral blood of untreated (naïve to therapy) and treated MS patients. Data shows median values and bars indicate standard error..
Immunomodulatory therapy had been prescribed in 17 of the 26 MS patients who were studied here and was equally distributed between the interferons (IFN-1 α , IFN-1β ) and glatiramer acetate. In the study design used in the initial NKR+ T-cell phenotyping experiments, a blinded protocol was used in order to minimize intrusion of observer or technical bias. Because of the possibility that the therapy might influence the circulating numbers of NKR+ T-cells, we compared the frequencies of CD161+ and CD94+ T-cell populations in a further 10 MS patients receiving no treatment. The results showed that there were no significant differences in the percentages of CD161+ T-cells and CD94+ in untreated compared with treated MS patients (Fig. 2(d)).
3.3 Increased numbers of NKT-cells in MS but lack of response to α-GalCer
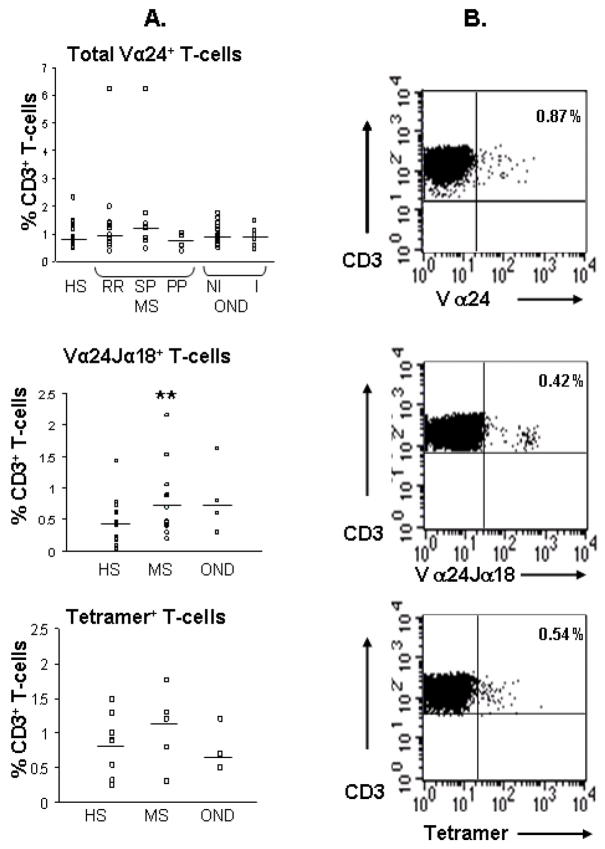
It has previously been shown that the expression of the V α 24J α 18+ TCR chain [20], or the use of CD1d-loaded tetramers [40] defines invariant CD1d-restricted NKT-cell populations. Therefore, flow cytometry was used firstly to quantify total numbers of V α 24+ T-cells amongst the CD3+ populations of lymphocytes and, secondly, to quantify the expression of the invariant V α 24J α 18+ T-cell receptor using specific antibodies and PBS57 loaded CD1d tetramers. As shown in Fig. 3, a small minority of circulating T-cells expressed the V α 24+ TCR, but there was no significant difference in the total numbers of V α 24+ T-cells in the MS (median ranging from of 0.77% in the PP-MS to 1.2% in the SP-MS group) or OND patient groups (I-OND, 0.89%; NI-OND, 0.93%) compared with healthy subjects (median, 0.87%). The proportion of T-cells expressing the invariant V α 24J α 18+ TCR was significantly increased in the MS patient group (medians, 0.71%; P = 0.02) compared with HS (median, 0.41 %) and in the OND group (median, 0.70 %) but not significantly so. When the MS subgroups were analysed, the significant increases in NKT-cell numbers were identified in the RR-MS group only (median, 0.7%) and not in the PP-MS group (median, 0.47%) or the SP-MS group (median, 0.43%). Likewise, when CD1d tetramers were used to identify the invariant T-cell population, the findings were similar to those using the invariant TCR chain antibody (Fig. 3). Thus, changes in the numbers of invariant NKT-cells were found in the MS compared with healthy subjects. All MS subjects studied in this group were not on any treatment at the time of analysis.
Fig. 3.
NKT-cells are increased in multiple sclerosis. (A) Percentages of total V α 24+ T-cells, V α 24J α 18+ T-cells and tetramer+ T-cells amongst CD3+ T-cell populations in healthy subjects (HS), multiple sclerosis (MS) subgroups (RR-MS, relapsing remitting; SP-MS, secondary progressive; PP-MS, primary progressive), and other neurological diseases (OND) subgroups (NI-OND, non-inflammatory OND; I-OND, inflammatory OND). Horizontal bars show median values. Significance values comparing HS to MS or OND subgroups are shown by *, P < 0.05 and **, P < 0.01. (B) Representative dot plots showing CD3+ T-cells that are V α 24+, V α 24J α 18+ or tetramer+ in their upper-right quadrants.
V α 24-J α 18+ NKT cells specifically recognize α-GalCer presented by CD1d [21]. We next examined reactivity to α-GalCer by culturing PBMC from MS subjects (who were untreated, and not receiving any immunomodulatory drugs) with α-GalCer for 7 days. After this time, the proportions of NKT cells were re-examined by flow cytometry. As shown in Fig. 4(a and b), in marked contrast to healthy controls where the numbers of invariant NKT cells were significantly expanded after stimulation with α-GalCer, NKT cells from the seven MS subjects failed to respond to α-GalCer. In each of these subjects there was no difference in the proportions of NKT cells in α-GalCer-stimulated cultures compared with unstimulated cells. When IFN- γ levels were measured in the culture supernatants of MS patients (Fig. 4(c)), there was no significant increase in production in response to α-GalCer stimulation (mean (SD), 123 (134) pg/ml) beyond the levels in unstimulated cultures (202 (254) pg/ml). This was in contrast to healthy subjects where α-GalCer significantly increased the levels of IFN- γ observed in the supernatants (324 (208) pg/ml versus 133 (134) pg/ml) in unstimulated cultures.
Fig. 4.
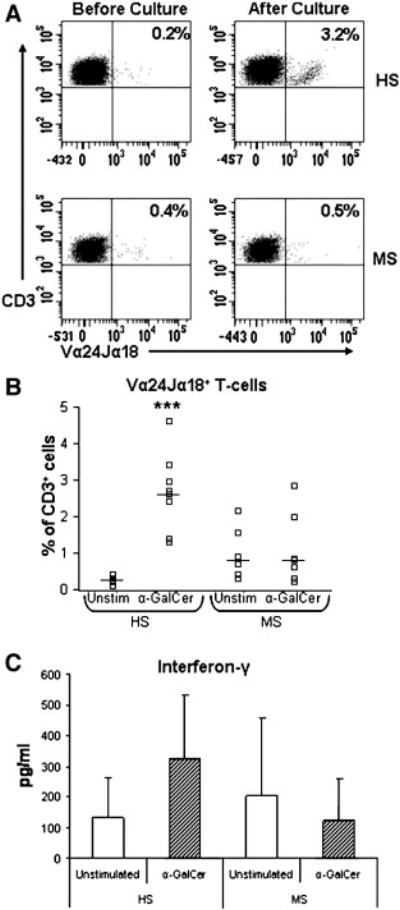
NKT- cell responses to α –GalCer are impaired in MS. (A). Representative flow cyometric profiles of V α 24-J α 18+ T-cells (NKT-cells) from one healthy subject (HS) and one MS patient. The frequency of NKT-cells before and after culture with α –GalCer is shown in the upper right hand quadrants in each plot. (B). Percentages of NKT cells in unstimulated (medium alone) and α –GalCer stimulated cultures in 8 healthy subjects and 7 MS patients. Horizontal bars indicate median levels. (C). IFN- γ production in unstimulated PBMC cultures or in α –GalCer stimulated cultures in HS and MS patients. Data shows mean levels and error bars show the standard deviation. Significance values comparing HS to MS patients are shown by *, P < 0.05; **, P < 0.01; ***, P < 0.001.
4. Discussion
The pathophysiology of MS is characterised by an exaggerated T-cell response that is believed to be directed at myelin-derived antigen, concomitant with inflammatory cytokine production and a polarized Th1-cell response; features of the characteristic central nervous system plaque lesion [4–9]. Many lymphocyte subsets have been implicated as the immune cells involved in mediating this, but the present study focused on NKR+ T-cells including NKT-cells in the peripheral blood of MS patients. The study design included sampling of patients at different stages and levels of disease activity and from the application not only of CD1d tetrameric complexes but also specific monoclonal antibodies to identify CD1d-restricted invariant NKT-cells. Overall, this study indicates that the numbers of NKR+ T-cells expressing CD161 and CD94 are decreased in MS patients while circulating levels of NKT-cells were significantly increased compared with controls. Importantly NKT-cells from MS patients failed to respond to stimulation with α-GalCer consistent with a state of anergy that may be induced by prior exposure to antigens eliciting innate immune responses and including lipids and glycolipids.
NKR+ T-cells constitute a heterogenous cell population which represents a significant proportion of the total T-cell population [18,19]. In this study, CD161+ T-cells and CD94+ T-cells accounted for approximately 12% of the total CD3+ T-cell population in healthy subjects, consistent with previous reports [41–43]. There were fewer CD56+ T-cells than either CD161+ T-cells or CD94+ T-cells; again consistent with previous findings [44]. In MS, decreased numbers of CD161+ T-cells were found, specifically in the PP-MS group compared with healthy subjects. The CD161 surface molecule is a memory cell marker which is co-stimulatory for CD1d restricted NKT-cells [31] and is involved in regulating lymphocyte transmigration [45]. Moreover, Poggi et al. [46] demonstrated an increased number of γ δ+T-cells expressing CD161 in peripheral blood from MS patients. This they propose confers upon γ δ+T-cells an increased ability to cross the vascular endothelium and contribute to demyelination. The potential role of CD161+ T-cells, including a regulatory role, in MS may be inferred from their capability to secrete IFN-γ upon stimulation [28]. Furthermore, the numbers of CD94+ T-cells were decreased in MS patients and specifically in the SP-MS group. CD94 recognises and binds HLA-E, and ligation can be stimulatory or inhibitory for T-cells and NK-cells depending upon the dimerisation status of this molecule [47,48]. Signals generated upon binding usually protect healthy cells against autoimmune lysis during infection or malignancy [49]. While changes in numbers were found, further studies, and especially functional ones, to delineate the exact role of this cell population are needed. We would emphasize that the reduction in the proportions of NKR+ T-cells in MS may be clinically relevant since reduced numbers could insufficiently activate populations required for controlling disease activity: this has been shown for the functional activities of NKR+ T-cells in tumour immunity [50]. Given the immunoregulatory role of NKR+ T-cells, a loss in the numbers of specific subsets of NKR+ T-cells could contribute to lack of control of Th1-cell activity. Importantly, the reductions in these NKR+ T cell numbers may reflect a decrease in immune inhibition with consequent progression of the neuro-degenerative phase of MS. We noted a trend to reduction of certain cell phenotypes in MS subsets with relative reduction of CD161+ T cells proportional to EDSS-scored disability for PP-MS and reduction of CD94+T cells for SP-MS, but the numbers of MS patients in these subgroups were insufficient for statistical corroboration.
Although the majority of NKT-cells in mice express the invariant V α 14J α 18+ TCR chain [51], only ≤ 1% of T-cells of individuals in this study expressed the homologous V α 24J α 18+ TCR. The results here are consistent with those showing that invariant NKT-cells in humans occur in low numbers in peripheral blood [25]. In MS, NKT cell numbers were significantly higher in frequency compared with healthy subjects which contrasts with previous reports of no difference [36] or reductions [15,16,23] in circulating levels of NKT cells in MS. The differences in findings between these studies may reflect the various methods used (single-strand conformation polymorphism [15], flow cytometry [23] and mRNA analysis with a J α 18 specific fluorescent probe [16] for identification of invariant NKT-cells, the study populations, and that any changes observed were small in a minor population of cells. Notably, our results obtained with two differing approaches, i.e. using α-GalCer-loaded CD1d tetrameric complexes and monoclonal antibodies to identify CD1d-restricted NKT-cells, strengthen the conclusion that there are significant differences in the numbers of CD1d-restricted NKT-cells in the MS compared to control patients. It is however more striking that while the proportions of invariant NKT-cells are elevated in freshly isolated peripheral blood of MS patients compared with healthy controls, the response to α-GalCer stimulation is also markedly different. The invariant NKT-cells of healthy controls significantly expanded in number following stimulation with α-GalCer while in MS the proportions of NKT cells remained the same before and after α-GalCer stimulation. Moreover, α-GalCer did not induce IFN- γ production in MS subjects indicating a hypo-responsiveness of NKT cells in vitro. The results suggest that some functions of NKT cells in MS are impaired in ways similar to patients with advanced cancers [52,53]. Administration of α-GalCer is a possible therapeutic agent in MS [24] in light of the effects upon murine EAE [54], and hence our findings carry significant implications for MS immunotherapy that would curb this enthusiasm somewhat.
The results of this study showed that reduced numbers of both CD161+ T-cells and CD94+ T-cells and increased numbers of NKT-cells were found in OND patients, in particular the non-inflammatory subgroup. These individuals were afflicted with Parkinson’s disease, headaches, epilepsy, burning leg discomfort, etc. The disease processes in these patients may have involved neuro-degeneration: Parkinson’s disease is a degenerative disease, and five of six such patients had severe reductions of NKR+ T-cells (Hogan et al., unpublished results). Alternatively, the pharmacological therapy for the OND patients may have influenced the peripheral blood subsets. Further evaluation of the functionality and antigenic specificities of the NKR+ T-cells are required to clarify their putative roles in relation to MS pathogenesis.
In conclusion, the results of the present study show that in the studied MS patients, the numbers of circulating NKR+ T-cells and NKT-cells are altered compared to healthy subjects and that peripheral blood NKT-cell reactivity against α-GalCer was markedly impaired in MS. Though it cannot be excluded that the differences observed between MS patient and healthy subject groups may in part reflect other clinical factors, these findings have identified a number of potentially important lymphocyte sub-populations that warrant further investigation in the immune response in MS pathogenesis.
Acknowledgments
The financial support of grants from the Millennium Fund (to JO’K), and the National Institute of Health (NS51666) and the National Multiple Sclerosis Society (NMSS RG3473) (to ELH and APM) are gratefully acknowledged. Also, CMG is the recipient of a Higher Education Award from Kildare County Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McAlpine D, Compston N. Some aspects of the natural history of disseminated sclerosis. Q J Med. 1952;21:135–167. [PubMed] [Google Scholar]
- 2.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. (I) Clinical course and disability Brain. 1989;112:133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343:938–95. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 4.Frohman EM, Racke MK, Raine CS. Multiple sclerosis – the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 5.Hellings N, Raus J, Stinissen P. Insights into the immunopathogenesis of multiple sclerosis. Immunol Res. 2002;25:27–51. doi: 10.1385/IR:25:1:27. [DOI] [PubMed] [Google Scholar]
- 6.Ota K, Matsui M, Milford EL, Mackin GA, et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 7.Pette M, Fujita K, Kitze B, Whitaker JN, et al. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;11:1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- 8.Tejada-Simon MV, Hong J, Rivera VM, Zhang JZ. Reactivity pattern and cytokine profile of T cells primed by myelin peptides in multiple sclerosis and healthy individuals. Eur J Immunol. 2001;31:907–17. doi: 10.1002/1521-4141(200103)31:3<907::aid-immu907>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi T, Yamamura T, Inobe J, et al. Analysis of proteolipid protein (PLP)-specific T-cells in multiple sclerosis: identification of PLP 95-116 as an HLA-DR2, w15-associated determinant. Int Immunol. 1995;11:1771–1778. doi: 10.1093/intimm/7.11.1771. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Nun A, Wekerle H, Cohen IR. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature. 1981;292:60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SD, Dittel BN, Hardardottir F, Janeway CA. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T, et al. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinaldi L, Gallo P, Calabrese M, Ranzato F, et al. Longitudinal analysis of immune cell phenotypes in early stage multiple sclerosis: distinctive patterns characterize MRI-active patients. Brain. 2006;129:1993–2007. doi: 10.1093/brain/awl179. [DOI] [PubMed] [Google Scholar]
- 14.Kastrukoff LF, Morgan NG, Zecchini D, et al. A role for natural killer cells in the immunopathogenesis of multiple sclerosis. J Neuroimmunol. 1998;86:123–133. doi: 10.1016/s0165-5728(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 15.Illes Z, Kondo T, Newcombe J, et al. Differential expression of NK T-cell Vα 24Jα Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol. 2000;164:4375–4381. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- 16.Demoulins T, Gachelin G, Bequet D, Dormont D. A biased Vα 24+ T-cell repertoire leads to circulating NKT-cell defects in a multiple sclerosis patient at the onset of his disease. Immunol Lett. 2003;90:223–228. doi: 10.1016/j.imlet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–34. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Van Kaer L. Natural killer T-cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82:315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 19.Linsen L, Somers V, Stinissen P. Immunoregulation of autoimmunity by natural killer T-cells. Hum Immunol. 2005;66:1193–1202. doi: 10.1016/j.humimm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T-cell antigen receptor (TCR) expression by human peripheral blood CD4−8− /T-cells demonstrates preferential use of several V genes and an invariant TCR chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα 14 NKT-cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 22.Seino K, Motohashi S, Fujisawa T, et al. Natural killer T-cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki M, Kondo T, Gumperz JE, et al. Th2 bias of CD4+ NKT-cells derived from multiple sclerosis in remission. Int Immunol. 2003;15:279–288. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- 24.Miyake S, Yamamura T. Therapeutic potential of glycolipid ligands for natural killer (NK) T-cells in the suppression of autoimmune diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:315–322. doi: 10.2174/1568008054863772. [DOI] [PubMed] [Google Scholar]
- 25.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T-cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T-cells, and CD3+CD56+ natural T-cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 27.Kenna T, Golden-Mason L, Porcelli SA, et al. NKT-cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT-cells. J Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 28.O'Keeffe J, Doherty DG, Kenna T, et al. Diverse populations of T-cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer. Eur J Immunol. 2004;34:2110–2119. doi: 10.1002/eji.200424958. [DOI] [PubMed] [Google Scholar]
- 29.Pittet MJ, Speiser DE, Valmori D, et al. Cutting edge: cytolytic effector function in human circulating CD8+ T-cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 30.Loza MJ, Metelitsa LS, Perussia B. NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol. 2002;32:3453–3462. doi: 10.1002/1521-4141(200212)32:12<3453::AID-IMMU3453>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T-cells expressing invariant Vα 24 Jα Q T-cell receptor alpha chains. J Exp Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8+ T-cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 34.Carding SR, Egan PJ. γ δ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 35.Mingari MC, Vitale C, Cambiaggi A, et al. Cytolytic T lymphocytes displaying natural killer (NK)-like activity: expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target T-cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 36.Gausling R, Trollmo C, Hafler DA. Decreases in interleukin-4 secretion by invariant CD4−CD8− Vα 24 Jα Q T-cells in peripheral blood of patients with relapsing-remitting multiple sclerosis. Clin Immunol. 2001;98:11–17. doi: 10.1006/clim.2000.4942. [DOI] [PubMed] [Google Scholar]
- 37.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 38.Prussin C, Foster B. TCR Vα 24 and Vβ 11- co-expression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862–5870. [PubMed] [Google Scholar]
- 39.Liu Y, Goff RD, Zhou D, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T-cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheu BC, Chiou SH, Lin HH, et al. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8+ T lymphocytes in human cervical carcinoma. Cancer Res. 2005;65:2921–2929. doi: 10.1158/0008-5472.CAN-04-2108. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T-cells with different functional activities. J Immunol. 2006;176:211–216. doi: 10.4049/jimmunol.176.1.211. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Hernandez Y, Pedraza-Sanchez S, Blandon-Vijil V, et al. Peripheral blood CD161+ T-cells from asthmatic patients are activated during asthma attack and predominantly produce IFN-gamma. Scand J Immunol. 2007;65:368–375. doi: 10.1111/j.1365-3083.2006.01885.x. [DOI] [PubMed] [Google Scholar]
- 44.Kelly-Rogers J, Madrigal-Estebas L, O'Connor T, Doherty DG. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Hum Immunol. 2006;67:863–868. doi: 10.1016/j.humimm.2006.08.292. [DOI] [PubMed] [Google Scholar]
- 45.Poggi A, Costa P, Zocchi MR, Moretta L. Phenotypic and functional analysis of CD4+ NKRP1A+ human T lymphocytes. Direct evidence that the NKRP1A molecule is involved in transendothelial migration. Eur J Immunol. 1997;27:2345–50. doi: 10.1002/eji.1830270932. [DOI] [PubMed] [Google Scholar]
- 46.Poggi A, Zocchi MR, Costa P, et al. IL-12-mediated NKRP1A up-regulation and consequent enhancement of endothelial transmigration of Vdelta2+ TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients. J Immunol. 1999;162:4349–54. [PubMed] [Google Scholar]
- 47.Lee N, Llano M, Carretero M, Ishitani A, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199–204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T-cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 49.Borrego F, Masilamani M, Marusina AI, Tang X, Coligan JE. The CD94/NKG2 family of receptors: from molecules and cells to clinical relevance. Immunol Res. 2006;35:263–278. doi: 10.1385/IR:35:3:263. [DOI] [PubMed] [Google Scholar]
- 50.Metelitsa LS, Naidenko OV, Kant A, et al. Human NKT-cells mediate antitumor cytotoxicity directly by recognizing targeted cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 51.Eberl G, Fehling HJ, von Boehmer H, MacDonald HR. Absolute requirement for the pre-T-cell receptor α chain during NK1.1+ TCRα β cell development. Eur J Immunol. 1999;29:1966–1971. doi: 10.1002/(SICI)1521-4141(199906)29:06<1966::AID-IMMU1966>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 52.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN- γ production by invariant NKT-cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 53.Yanagisawa K, Seino K, Ishikawa Y, et al. Impaired proliferative response of Valpha 24+ NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168:6494–9. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 54.Jahng AW, Maricic I, Pedersen B, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]