Abstract
Human posttraumatic epilepsy (PTE) is highly heterogeneous, ranging from mild remitting to progressive disabling forms. PTE results in simple partial, complex partial, and secondarily generalized seizures with a wide spectrum of durations and semiologies. PTE variability is thought to depend on the heterogeneity of head injury and patient's age, gender, and genetic background. To better understand the role of these factors, we investigated the seizures resulting from calibrated fluid percussion injury (FPI) to adolescent male Sprague–Dawley rats with video electrocorticography. We show that PTE incidence and the frequency and severity of chronic seizures depend on the location and severity of FPI. The frontal neocortex was more prone to epileptogenesis than the parietal and occipital, generating earlier, longer, and more frequent partial seizures. A prominent limbic focus developed in most animals, regardless of parameters of injury. Remarkably, even with carefully controlled injury parameters, including type, severity, and location, the duration of posttraumatic apnea and the age and gender of outbred rats, there was great subject-to-subject variability in frequency, duration, and rate of progression of seizures, indicating that other factors, likely the subjects' genetic background and physiological states, have critical roles in determining the characteristics of PTE.
Keywords: drug screening, electrocorticography, endophenotype, model, partial seizures, syndrome, trauma
Introduction
Human posttraumatic epilepsy (PTE) varies greatly in its progression, semiology and severity, but the reasons for this are unclear. Seizures may appear days to decades after injury (Caveness 1963; Jabbari et al. 1986; Annegers et al. 1998; Christensen et al. 2009), with variable frequency of occurrence and patterns of evolution (Caveness et al. 1979; Aarabi et al. 2000; Eftekhar et al. 2009). Some forms of PTE have low seizure frequency and remit spontaneously (20–26%; Aarabi et al. 2000; Eftekhar et al. 2009), some are stable (∼70%; Salazar et al. 1985), while others are progressive and lead to increasing disability (∼10%; Salazar et al. 1985). PTE may also have a late relapse, sometimes after 10 years or more (Walker 1962; Weiss and Caveness 1972). PTE patients may have a single or multiple epileptic foci, most often in the frontal or the temporal lobe (Marks et al. 1995; Wohlrab et al. 1997; Diaz-Arrastia et al. 2000; Hudak et al. 2004; Diaz-Arrastia et al. 2009) and may present with very localized or spreading focal seizures (Caveness 1963; Weiss and Caveness 1972; Walker and Blumer 1989). Ictal behaviors depend on the location of the epileptic focus, the pattern of seizures' spread and their duration and manifest as simple partial (Becker et al. 1979; Salazar et al. 1985, 1995; Jabbari et al. 1986; Pohlmann-Eden and Bruckmeir 1997), complex partial, or secondarily generalized tonic–clonic seizures (Caveness and Liss 1961; Becker et al. 1979; Salazar et al. 1985, 1995; Wohlrab et al. 1997). This heterogeneity in human PTE has been proposed to depend on variation in the type, location, severity, number of initiating injuries and of secondary insults, as well as on the different genetic backgrounds, ages, and genders of the victims (Caveness 1963; Caveness et al. 1979). In support of this hypothesis, subsequent work showed that the probability of developing PTE depends on the severity and type of the injury, particularly on the presence of penetrating injury, intraparenchymal hemorrhage, and acute seizures (Annegers et al. 1998; Herman 2002; Englander et al. 2003; Temkin 2003; Christensen et al. 2009). Other work has highlighted the influence of genetic background (Noebels 2003; Sutula 2004; Diaz-Arrastia and Baxter 2006), age (Annegers et al. 1980; Oztas et al. 1990; Frey 2003; Christensen et al. 2009; Jyoti et al. 2009; Kelly 2010), and gender (Brandt et al. 2003; Christensen et al. 2005) on epileptogenesis. However, the fundamental mechanisms causing the great subject-to-subject variability in PTE are still unclear. While several aspects of traumatic brain injury (TBI) are known to be epileptogenic (Willmore and Ueda 2009), including intraparenchymal hemorrhage (Lee et al. 1997; Seiffert et al. 2004; Maggio et al. 2008), ischemia (Epsztein et al. 2008), hypoxia (Kadam et al. 2010), excitotoxicity (Gupta and Gupta 2006), inflammation (Vezzani et al. 2008), and oxidative stress (Costello and Delanty 2004), human studies are typically limited by inadequate information on the mechanical details of the injuries and neither clinical nor experimental studies have determined which factor is primarily responsible for the observed variability in PTE. Therefore, animal models based on realistic etiologies that reproduce human head injury and PTE are needed to investigate the causes of this variability. The fluid percussion injury (FPI) mechanically reproduces human blunt contusive closed head injury and its pathological consequences (Thompson et al. 2005). The site of injury affects FPI sequelae, including cognitive effects, hippocampal damage, and distribution of reactive astrocytosis (Vink et al. 2001; Floyd et al. 2002). Consistent with this variability, FPI also appears to reproduce the variability of human PTE. Frontal parasagittal FPI resulted in a high incidence of epilepsy and a high frequency of nonconvulsive partial seizures emerging in the early weeks after injury, progressing in frequency and duration, and leading to dual pathology with frontal and limbic seizures (D'Ambrosio et al. 2004, 2005, 2009). Conversely, more severe parietal FPI in older rats was reported to induce lower epilepsy incidence and seizure frequency, with limbic seizures with tonic–clonic convulsions but no neocortical epilepsy (Kharatishvili et al. 2006). However, significant differences in FPI methodology and in the monitoring and evaluation of seizures prevent meaningful comparisons of these results from different laboratories.
The purpose of the current study is to better understand how head injury variants determine the variability of PTE and standardize the FPI model of PTE. We carefully controlled experimental conditions to systematically examine 1) whether FPI delivered at different locations or intensities results in different PTE syndromes and 2) which part of the cortex is more prone to epileptogenesis. We recorded the video-electrocorticography (ECoG) for up to 5 months postinjury and probed the epileptogenicity of a single event of FPI delivered at 2 different intensities to frontal, parietal, and occipital parasagittal cortices.
Materials and Methods
Animals
This work used 99 outbred male Sprague–Dawley rats (age 33–35 days; Charles Rivers) housed in a specific pathogen-free facility 2–3 per cage to allow socialization, under controlled conditions of temperature and humidity and a 12 h/12 h light/dark cycle. Water and food were available ad libitum. All procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
FPI
FPI was performed as previously described (D'Ambrosio et al. 2009) with care taken to ensure consistent procedure, size, shape, and location of craniotomy, absence of damage to the dura and that no air bubbles were present in the cannula or Luer-Lok connection of the pressure cylinder that could dampen the pressure wave shock and diminish the injury severity. Six different experimental groups were used with 3 different locations of FPI at 2 different mechanical severities. Rats were anesthetized in a plexiglass chamber with 4% halothane, intubated, thereafter kept anesthetized with halothane (1–1.5% halothane, 30% oxygen, and air) and mounted on a stereotaxic frame. A heating pad kept rectal temperature constant at 37 °C. A 3-mm burr hole (Fig. 1A) was drilled 3-mm right of midline at bregma −2 mm (rostral parasagittal; rpFPI), bregma −4 mm (medial parasagittal; mpFPI), or bregma −6 mm (caudal parasagittal; cpFPI). Temporal FPI could not be studied because temporal implantation of the cannula would require the resection of chewing muscles, which the animals would not survive. Immediately before injury, animals were removed from the stereotactic frame, disconnected from the ventilator, and a 8-ms pressure pulse of either 2.0 atm or 3.4 atm peak pressure was delivered with the FPI device (Scientific Instruments). The pressure pulse was monitored on an oscilloscope at high temporal resolution with an analog pressure transducer (Entran EPN-D33-100P-IX, Measurement Specialties Inc.) and digitally stored to assess trial-to-trial variability. As in our previous studies, the pendulum and the piston were carefully lubricated and accurately positioned to ensure reproducibility of the pressure wave shock. The acute posttraumatic period of apnea was set at 10 s to standardize the effects of posttraumatic hypoxia, after which the animals were reconnected to the ventilator and ventilated with an oxygen/air mixture until spontaneous breathing resumed. Sham-injured animals underwent the exact same procedure except that the FPI device stopcock was closed when the pressure pulse was generated. Animals were then extubated, the wound site closed, and righting reflexes monitored.
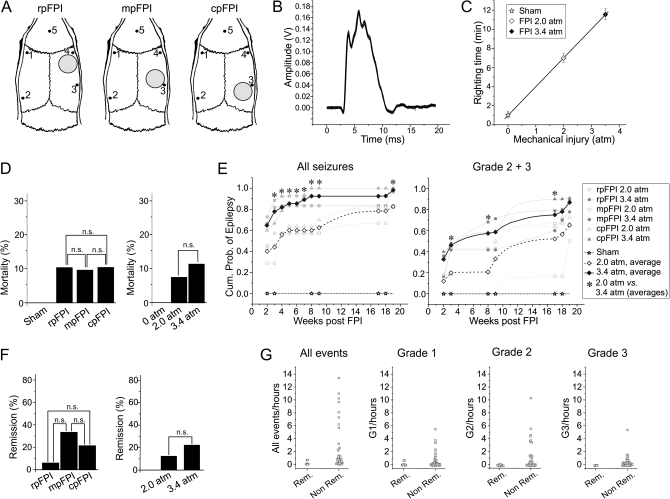
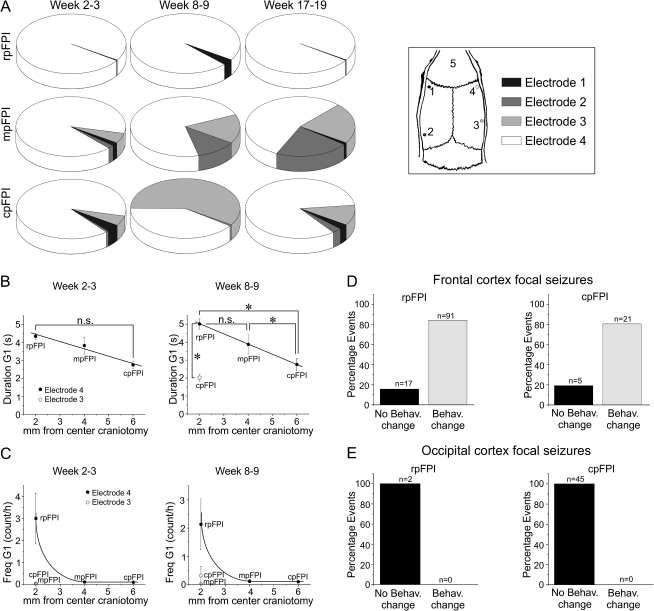
Figure 1.
Basic properties of frontal, parietal, and occipital FPI, its mechanical reproducibility, acute mortality, and ensuing epilepsy. (A) Location of craniotomy and recording (#1–4) and reference (#5) electrode positions for rpFPI, mpFPI, and cpFPI. (B) Graph shows 23 overlapped digitized pressure traces resulting from FPI at 3.4 atm. Amplitude of pressure transducer output is measured in volts. Traces demonstrate the high reproducibility of the mechanical injury. (C) The plot shows mean righting times after 0 atm (sham), 2.0 atm, and 3.4 atm FPI (all locations combined). Righting time increases linearly with severity of injury. (D) Histograms show the acute (≤1 week) mortality for rats injured at different locations (left panel) and for rats receiving moderate and severe trauma (right panel). n.s. indicates no statistical significance. Note that FPI at all locations results in comparable acute mortality when animals are mechanically ventilated after injury. (E) The incidence of PTE increases over time after injury in all experimental groups and depends on the severity of injury (2.0 vs. 3.4 atm) but not on the location of FPI. On the left, the cumulative probability of detecting seizures (G1, G2, and G3) in FPI rats and in sham-injured rats is plotted versus time after injury. On the right, the cumulative probability of detecting unprovoked spreading seizures (G2 and G3) in FPI and sham-injured rats is plotted versus time after injury. Asterisks indicate significant differences in the cumulative incidence of seizures between rats injured at 2.0 atm and rats injured at 3.4 atm. (F) Histograms show percentage of remitters after injury at different locations (left panel) and after injury at 2.0 and 3.4 atm (right panel). n.s. indicates no significance. (G) Remitters (Rem) had low preremission seizure frequency, had few G1 seizures, and virtually no G2 and G3 seizures. Nonremitters (Non Rem) presented with varying and often high frequency of seizures of all types over the period of study.
Electrode Implantation and Video-ECoG
One week after injury, animals were intubated and anesthetized with halothane (1–1.5%) while guiding craniotomies ∼Ø = 0.75 mm were drilled and 5 epidural electrodes (stainless-steel screws of Ø = 1 mm) implanted for chronic video-ECoG. One midline electrode in the frontal bone was used as reference, and 2 electrodes were placed at level of frontal (bregma 0 mm) and parietal/occipital cortices (bregma −5 mm for the ipsilateral side and −7 mm for the contralateral side), 4.5–5 mm from the midline (Fig. 1A). Each electrode was connected with insulated wire to a gold-plated pin in a plastic pedestal, and the entire assembly cemented onto the skull with dental acrylic. Rats were housed separately after implantation to preserve headsets. Lost headsets were reimplanted once, if possible, otherwise animals were included in the study only until the last day of recording. Electrical brain activity was amplified (×5000) and filtered (0.3 Hz high-pass, 100 Hz low-pass) using a Neurodata 12 or a M15 amplifier (Grass Instruments), acquired at 512 Hz per channel with DT3010 acquisition boards (DataTranslation Inc.), stored, and analyzed on computers equipped with SciWorks and Experimenter V3 software (Datawave Technologies Inc.). All ECoG recordings lasted at least 24 h, to fully sample the circadian cycle, with each animal monitored for the same amount of time weekly from 2 to 19 weeks postinjury. Video was recorded with VCRs using one digital camera for each pair of cages (1 animal per cage) to detect subtle changes in behavior.
Quality Control of Surgical Procedures
As in previous work, we took precautions to ensure that epileptiform ECoG events were induced by FPI and not by cortical damage for heating or compression due to substandard drilling or epidural electrode implantation. The skull and drill bit were cooled during drilling with room temperature sterile saline and care taken to never deform the skull or the dura with the drill bit. The depth of epidural electrodes was carefully adjusted to avoid brain compression. As previously described (D'Ambrosio et al. 2005, 2009) glial fibrillary acidic protein (GFAP) immunostaining was performed in most FPI and sham-injured animals after the completion of recordings. Cases of neocortical damage due to electrode implantation, as indicated by ∼0.5- to 1.5-mm diameter focal GFAP+ astroglial reactivity under the electrodes (D'Ambrosio et al. 2009 for illustration), were excluded from analysis.
Identification of Seizures and Definitions
Several well-trained raters manually analyzed video-ECoG data, acquired at 3 time points (2–3, 8–9, 17–19 weeks after injury) from the 6 different experimental groups. Seizures were characterized by epileptiform ECoG patterns with trains of 150- to 250-ms long spikes with amplitudes at least twice the previous 2-s baseline and were never seen in sham-injured animals. Seizures occurring within 5 s of each other were defined as one seizure event. Consistent with our previous demonstration of short clinical seizures in both rat and humans (D'Ambrosio et al. 2009), seizure durations ranged 1–144 s. Events with stereotyped behavioral changes coinciding with epileptiform ECoG activity were clinical seizures, but some seizures had no overt behavioral change (subclinical seizures). Behavioral changes without a simultaneous epileptiform ECoG were not considered seizures. As all FPI animals were at risk of developing PTE, we diagnosed epilepsy upon their first proven ictal event (Beghi et al. 2005; Fisher et al. 2005; Fisher and Leppik 2008). Animals that did not show seizures were counted as not epileptic only through the last day of recording. Cases of remission from PTE were defined as animals that had no seizures in 3 or more 24 h recordings from a certain period of time onward. While no amount of recordings and electrodes can ever prove an animal to be truly seizure free, 72 h recordings by 5-electrode ECoG was deemed adequate for this study. All identified cases of remission were included in the group analysis of seizure frequency, progression of PTE, and apparent focus location, unless specified otherwise. ECoG seizures were categorized as: 1) grade 1 (G1), if limited to a neocortical focus; 2) grade 2 (G2), if appearing first in a limited neocortical area and then spread to other cortical areas; 3) grade 3 (G3), if appearing bilateral at their neocortical onset. Based on a previous study performed using acute paired epidural and depth-electrode recordings (D'Ambrosio et al. 2005), G1 and G2 seizures are likely neocortical, while G3 seizures are of limbic origin. In accord with previous work (D'Ambrosio et al. 2004, 2005), age-dependent idiopathic seizures, typically 1- to 10-s long, bilateral in onset, with a sharp-wave pattern that was larger in amplitude in the parietal–occipital cortex, were not seen in this study because that seizure type typically appears no earlier that ∼5–6 months of age.
Behavioral Analysis
The semiology of the behavior associated with electrical seizures was classified with the behavioral scale previously developed for rpFPI (D'Ambrosio et al. 2005, 2009): 0 = no detected behavioral change (subclinical); 1 = freeze-like motion arrest of the whole body with no alteration in body posture, and with the head and neck maintaining their posture; 2 = freeze-like pause in behavior as in class 1 but accompanied by facial automatisms (twitching of vibrissae, sniffing, eye blinking, jaw automatisms); 3 = head nodding without change in body posture; 4 = body myoclonus without change in body posture; 5 = interruption of normal behavior with loss of posture consisting of the rat crouching down with its whole body, typically with head propped on forelimbs, and losing posture of the neck and head, or rare cases of falling atonia; 6 = behavioral arrest as in class 5 but followed by motor manifestations (facial automatisms, contralateral limb dystonia, or myoclonus); 7 = tonic–clonic convulsion; and 8 = partial status epilepticus, defined as cluster of seizures lasting at least 30 min. For neocortical seizures (G1 and G2), the electrographic onset was simultaneous with the behavioral onset of the seizure. For limbic seizures (G3), the behavioral onset preceded by 1–20 s the electrographic onset of the seizure, as expected for origination from a subcortical focus.
GFAP Immunoreactivity
Animals were deeply anesthetized and perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed, postfixed, and cryoprotected in sucrose in PBS. Free floating coronal sections (30 μm) were treated to block nonspecific staining and incubated with rabbit GFAP antibody (1:4000 dilution; Dako). Secondary antibody treatment included overnight incubation in a 1:300 solution of biotinylated goat rabbit immunoglobulin (IgG). Biotin/avidin/horseradish peroxidase complexes were formed by incubation in a 1:500 Elite ABC kit (Vector Labs). Sections were then developed in 3,3-diaminobenzidine with H2O2, mounted on glass slides, air dried, dehydrated through alcohols, cleared in xylene, and cover slipped. At least 6 coronal sections that included electrode coordinates were examined per animal.
Morphological Analysis
Hippocampal and temporal cortex atrophy were assessed by evaluating the ipsilateral versus contralateral asymmetry of the maximal axes of the hippocampus and thickness of the cortex, in GFAP-stained sections. Size and shape of the rhinal fissure were used to ensure slices were cut perfectly coronally. Sections that did not satisfy this requirement were discarded from analysis. The scale previously reported (D'Ambrosio et al. 2005) was used to define asymmetry levels: “negligible,” “mild,” “moderate,” and “pronounced.”
Statistical Analysis
All data are presented as mean ± standard error of the mean, and n indicates number of rats unless otherwise specified. To evaluate the effects of different trauma severities, different FPI pressures were compared at the same FPI location. To evaluate the effects of different FPI locations, different FPI locations were compared with the same FPI pressure. Sham-injured rats receiving craniotomy in the 3 different locations were pooled together as one control group for all experimental cases.
On some occasions, seizure frequency data were logarithmically transformed to facilitate graphical presentation. Since the logarithm of zero is −∞ and observing no seizures in a finite period of time only suggests a seizure frequency of less than one event per observation period, observations of zero seizures were assigned a floor value of 1 per observation duration (in hours). Statistical tests (t-test for unpaired data, Mann–Whitney U test, linear regression, bivariate correlation, Fisher's Exact test) were performed with SPSS 8.0 (SPSS Inc.). A P < 0.05 was considered significant.
Results
For analysis of the effect of site and severity of injury, 67 animals were prepared: rpFPI (n = 7 injured at 2.0 atm and n = 10 at 3.4 atm), mpFPI (n = 7 at 2.0 atm and n = 12 at 3.4 atm), cpFPI (n = 11 at 2.0 atm and n = 15 at 3.4 atm), and sham-injury animals (n = 5). We also included an independent batch of animals (5 shams and 18 rpFPI injured at 3.4 atm) from a previous analysis of the temporal evolution of rpFPI-induced PTE (D'Ambrosio et al. 2005). In all, 89 FPI and 10 sham-injured animals were examined. Nine FPI rats died acutely after injury and are included only for calculating of mortality rate. A total of 7963 h of ECoG recordings were obtained from the surviving animals from 2 to 19 weeks after FPI.
FPI Reproducibility, Righting Time, and Acute Mortality
FPI was delivered to frontal, parietal, and occipital cortices (Fig. 1A) with a pressure pulse that was highly reproducible in amplitude, duration, and area. A pilot study demonstrated that 3.4 atm FPIs (n = 23) were delivered with a peak pressure amplitude of 3.40 ± 0.01 atm, a duration of 8.03 ± 0.02 ms, and an integrated area of 14.53 ± 0.06 atm·ms (Fig. 1B). Thus, the peak pressure changed from trial to trial less than 6%, and the integrated pressure over the duration of the pulse changed less than 7%. As determined by the pressure wave shock profile, experimental injuries in the present study were delivered with peak pressure amplitudes of either 2.00 ± 0.02 atm (n = 25) or 3.35 ± 0.01 atm (n = 55). In humans, duration of loss of consciousness is used as a functional measure of the severity of trauma. In rodents, an analogous measure is the righting time after injury. Sham-injured rats (n = 10) righted in 1.0 ± 0.3 min, while rats injured at 2.0 (n = 25) and 3.4 atm (n = 55) righted in 7.0 ± 0.4 min and 11.6 ± 0.6 min, respectively (Fig. 1C), indicating that the functional severity of trauma is proportional to the mechanical intensity of the insult, with the small variance in righting time within a group a good indication of FPI reproducibility. There was no acute (<1 week) mortality following technically successful sham injuries (n = 10) and mortality ranged 9.5 to 10.3% following rpFPI (n = 39), mpFPI (n = 21), and cpFPI (n = 29; Fig. 1D, left panel). Acute mortality was 7.4% and 11.3% after trauma at 2.0 atm (n = 27) and at 3.4 atm (n = 62), respectively, but this difference was not significant (P = 0.7, Fisher's Exact Test; Fig. 1D, right panel).
Incidence of PTE
The cumulative probability of developing PTE increased over time for all experimental groups (Fig. 1E, left panel) but did not differ significantly among rpFPI (n = 28), mpFPI (n = 12), and cpFPI (n = 15) rats injured at the same pressure (all P > 0.05, Fisher's Exact Test). No sham-injured animals developed epilepsy within 18 weeks of observation (n = 10). More severe trauma resulted in a higher probability of PTE (Fig. 1E, left panel). When data obtained from different locations of injury were combined (n = 25 at 2.0 atm, n = 55 at 3.4 atm), the cumulative probabilities of epilepsy after 2.0 atm injury was significantly lower than after 3.4 atm injury at 3, 9, and 19 weeks postinjury (P < 0.005, Fisher's Exact Test). Latencies for development of seizure after injury was variable, ranging ≤2–18 weeks for rpFPI, ≤2–19 weeks for mpFPI, and ≤2–17 weeks for cpFPI. The cumulative incidence of G2 and G3 seizures, which spread to wider areas of the brain, increased with time postinjury for all experimental groups (Fig. 1E, right panel). Early after injury, it was significantly higher for rats receiving more severe injury (n = 52) than for those receiving milder trauma (n = 25; P = 0.043, Fisher's Exact Test) and remained nominally higher at week 9 (P = 0.077) and at week 19 (P = 0.46, Fisher's Exact Test). Thus, the incidence of spreading seizures is higher after severe injury, particularly in the first 8 weeks.
Probability of Remission from PTE
Cases of remission occurred after rpFPI (1/17; 5.9%), mpFPI (4/12; 33.3%), and cpFPI (3/14; 21.4%; Fig. 1F). Following FPI at 2.0 and 3.4 atm, remission was observed in 12.5% (2/16) and 25.9% (7/27) of injured rats, respectively. While remission was less frequent after 3.4 atm rpFPI (1/12) than after 3.4 atm mpFPI and cpFPI (5/15, combined), there were no significant group differences (all P > 0.13, Fisher's Exact Test). All remitters were characterized by low preremission seizure frequency (0.2 ± 0.1 events/h; range 0.04–0.7/h), while seizure frequency for nonremitters tended to be much higher (2.8 ± 0.6 events/h; range 3/week–14/h; Fig. 1G). Remitters also presented with only one type of seizure, and no animal with more than one seizure type went into remission. Specifically, 6 of 8 remitting FPI rats had only G1 seizures (range: 1–17 events; median: 3 events) and the 2 remaining remitters had just 1 G3 seizure, each.
Temporal Progression of Seizure Frequency
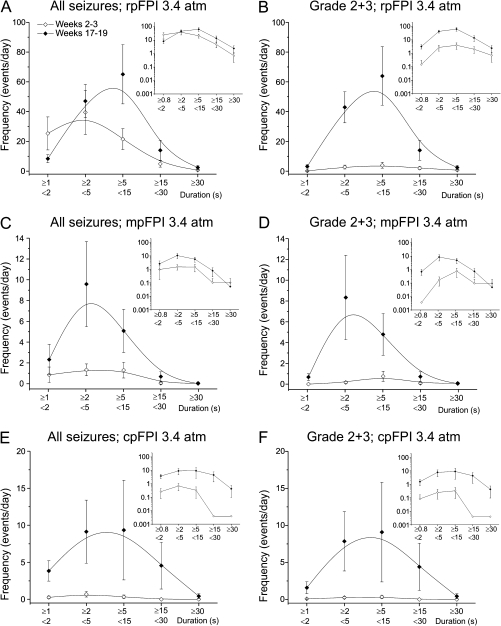
The duration of clinical seizures in FPI rats ranged widely (Table 1; Fig. 2; D'Ambrosio et al. 2005). Comparison of seizure duration at 2–3 and 17–19 weeks postinjury demonstrates clear progression regardless of location of injury (Fig. 2A,C, and E), with a similar distribution when only G2 and G3 spreading seizures are considered (Fig. 2B,D, and F). Only rostral parasagittal injury resulted in significant numbers of chronic seizures in the early weeks postinjury, suggesting that the frontal neocortex experiences more rapid epileptogenesis.
Table 1.
Range of duration of chronic recurrent seizures induced by FPI
| Group | Weeks 2–3 |
Weeks 8–9 |
Weeks 17–19 |
|||
| Seconds | Count | Seconds | Counts | Seconds | Counts | |
| rpFPI 2.0 atm | 1–36 | 69 | 1–40 | 140 | 1–24 | 552 |
| rpFPI 3.4 atm | 1–61 | 1269 | 1–99 | 856 | 1–71 | 1263 |
| mpFPI 2.0 atm | 3–8 | 2 | 1–10 | 4 | 1–26 | 106 |
| mpFPI 3.4 atm | 1–35 | 73 | 1–27 | 107 | 1–30 | 370 |
| cpFPI 2.0 atm | 1–20 | 49 | 1–24 | 60 | 1–144 | 691 |
| cpFPI 3.4 atm | 1–27 | 112 | 1–41 | 107 | 1–37 | 350 |
Note: rp, rostral parasagittal; mp, medial parasagittal; cp, caudal parasagittal.
Figure 2.
Duration of clinical seizures induced by rpFPI, mpFPI, and cpFPI. Overall frequency of all seizure types (A, C, E) or spreading G2 and G3 seizures (B, D, F) early (weeks 2–3; hollow symbols) and late (17–19 weeks; filled symbols) after 3.4 atm rpFPI (A, B), mpFPI (C, D), and cpFPI (E, F). Insets show the same data on a logarithmic scale. Note that seizures are frequent 2–3 weeks after rpFPI (A) but much rarer after mpFPI (C) and cpFPI (E), indicating more rapid epileptogenesis in the frontal neocortex. However, a wide range of seizure durations is a reliable feature of the PTE syndrome regardless of injury location.
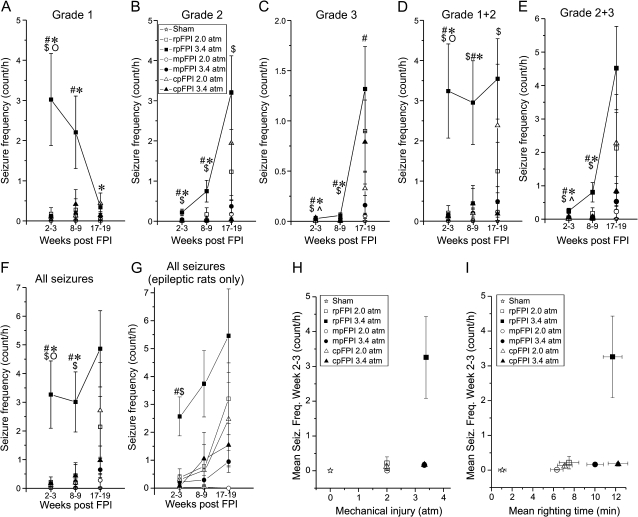
Further analyses of seizure frequency progression were performed by combining all seizure durations for different seizure types (G1, G2, G3; Fig. 3). Following 3.4 atm rpFPI, the frequency (events/hour) of G1 seizures decreased from 3.0 ± 1.1 (n = 26) at 2–3 weeks to 0.4 ± 0.1 (n = 15) at 17–19 weeks postinjury; the frequency of G2 events increased from 0.2 ± 0.1 at weeks 2–3 post-FPI to 3.2 ± 0.9 at weeks 17–19, respectively (Fig. 3A,B). The frequency of focal neocortical seizures (grades 1 and 2 combined) thus remained stable over the entire 19 weeks of observation (2.9–3.6 events/h), suggesting that the epileptic neocortical focus was fully developed just 2–3 weeks after 3.4 atm rpFPI (Fig. 3D). The mean frequency of neocortical seizures (G1 and G2) after rpFPI was much higher than after mpFPI (weeks 2–3: P < 0.0001; weeks 8–9: P = 0.014; weeks 17–19: P = 0.1; unpaired t-test after log transform; Fig. 3D) or cpFPI (weeks 2–3: P < 0.0001; weeks 8–9: P = 0.021; weeks 17–19: P = 0.025; unpaired t-test after log transform; Fig. 3D). These data suggest that the neocortical focus develops faster after injuries in the frontal cortex than in medial parasagittal and caudal parasagittal cortices. Regardless of injury location, limbic seizures (G3) contributed little to the epileptic syndrome up to ∼2 months post-FPI, accounting for at most 0.06 ± 0.03 events/h at 8–9 weeks (Fig. 3C). Nonetheless, the frequency of G3 seizures was higher in rpFPI rats than in mpFPI and cpFPI animals during this time period (all P < 0.024, unpaired t-test after log transform). At 17–19 weeks postinjury, following a period of limbic epileptogenesis, the frequency of G3 seizures increased to 1.3 ± 0.4, 0.16 ± 0.10, and 0.79 ± 0.42 events/h after rpFPI, mpFPI, and cpFPI, respectively. At this time, the frequency of limbic seizure after rpFPI significantly exceeded that in mpFPI (P = 0.028, unpaired t-test after log transform) but not cpFPI rats. Thus, limbic epileptogenesis is delayed compared with that in neocortex. The overall frequency of seizures was lower after milder (2.0 atm) than more severe (3.4 atm) trauma (Fig. 3F). After rpFPI at 2.0 atm, seizures initially occurred at much lower frequency than after rpFPI at 3.4 atm: 0.22 ± 0.18 events/h (P = 0.004) at 2–3 weeks postinjury, 0.45 ± 0.45 events/h (P = 0.014) at 8–9 weeks postinjury, and 2.14 ± 1.06 events/h (P = 0.6, unpaired t-test after log transform) at 17–19 weeks postinjury. These data suggest that seizure frequency is higher after more severe trauma and that PTE induced by moderate rpFPI progresses over time, although at a slower pace than PTE from severe rpFPI. To better appreciate the overall progression of PTE after injury in different locations, we plotted the frequency of all seizures (neocortical and limbic) at different times after injury. Only rats that were epileptic at week 2–3 and were recorded at all time points were included in this analysis (Fig. 3G). The frequency of seizure at 2–3 weeks after 3.4 atm FPI was higher after rpFPI (n = 10) than after mpFPI (n = 5; P = 0.008, unpaired t-test after log transform) or cpFPI (n = 4, P = 0.001, unpaired t-test after log transform), indicating more rapid early progression after rostral injury.
Figure 3.
Temporal progression of seizure frequencies and their dependence on location and severity of FPI. (A–G) Frequency of distinct seizure types determined 2–3, 8–9, and 17–19 weeks after rpFPI, mpFPI, and cpFPI at 2.0 and 3.4 atm. RpFPI at 3.4 atm induces a higher frequency of neocortical seizures at weeks 2–3 and 8–9 post-FPI, compared with all other groups. Statistically significant differences (2-tailed unpaired t-test after logarithmic transformation) are indicated by # (rpFPI 3.4 atm vs. mpFPI 3.4 atm), $ (rpFPI 3.4 atm vs. cpFPI 3.4 atm), * (rpFPI 2.0 atm vs. 3.4 atm), o (mpFPI 2.0 vs. 3.4 atm), (cpFPI 2.0 atm vs. 3.4 atm). (H–I) For each experimental group, mean seizure frequency at 2–3 weeks postinjury is plotted against the intensity of mechanical (H) and functional (righting time; I) injury. RpFPI rats injured at 3.4 atm present higher seizure frequency than mpFPI and cpFPI rats despite comparable mechanical injury and righting time.
We found that 1) rpFPI generated higher seizure frequency than mpFPI and cpFPI, 2) 3.4 atm injury generated higher seizure frequencies than 2.0 atm injury. These studies were carried out over many months. To control for an effect of time, we also compared the frequencies of neocortical seizures in this study with those observed in a recent study that was based on different rpFPI 3.4 atm animals (n = 51; Eastman et al. 2010). We confirmed that the observed differences remained statistically significant when this alternative data set was used: seizure frequency of rpFPI 3.4 atm versus mpFPI 3.4 atm (P = 0.005), rpFPI 3.4 atm versus cpFPI 3.4 atm (P = 0.0015), and rpFPI 3.4 atm versus rpFPI 2.0 atm (P = 0.015). Thus, the findings are independent of time.
Dependence of Seizure Frequency on Mechanical Injury and Righting Time
To assess the relationships between neocortical epileptogenesis and the mechanical and functional severity of injury, the 2–3 week postinjury seizure frequencies for the 6 experimental groups were plotted against the average peak pressures from the FPI pressure transducer (Fig. 3H) and also against mean righting times (Fig. 3I). Moderate (2 atm) injuries producing comparable postinjury righting times induced a low frequency of seizure regardless of location, while 3.4 atm injury resulted in higher seizure frequencies than the comparable 2 atm injuries. However, a much higher frequency of seizure was obtained after 3.4 atm rpFPI than after mpFPI or cpFPI, despite comparable mechanical and functional injuries at all 3 locations. This suggests that the frontal cortex may be particularly prone to epileptogenesis after severe injury.
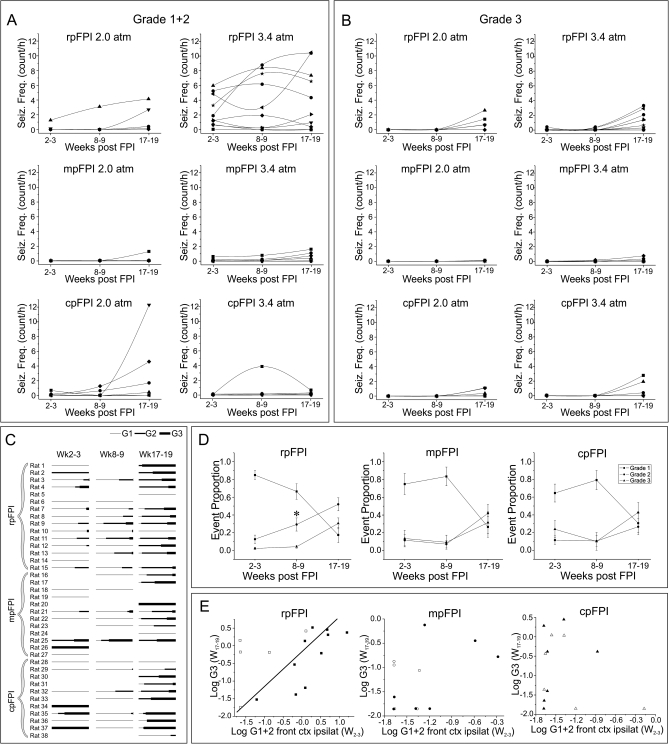
Rat-to-Rat Variability of the Temporal Progression of Seizures
To determine whether variability in FPI mimics variability in human PTE, we examined the animal-to-animal variability in progression of FPI-induced PTE. Plots of individuals' frequencies of neocortical (Fig. 4A) and limbic seizures (Fig. 4B) revealed a highly variable epilepsy progression in animals injured with same location and severity of FPI. We observed rats with low neocortical seizure frequency 2–3 weeks postinjury with rapid worsening to high seizure frequency by weeks 17–19; rats with high seizure frequency at weeks 2–3 that did not progress; and rats that displayed a decreasing frequency of seizure that, in some cases, qualified as epilepsy remission. RpFPI rats injured at 3.4 atm had neocortical seizure frequencies that ranged widely. Conversely, neocortical seizure frequencies at weeks 2–3 were low for rpFPI rats injured at 2.0 atm and for mpFPI and cpFPI rats injured at 2.0 and 3.4 atm (Fig. 4A). Rat-to-rat variability in progression of neocortical epilepsy was observed in each group suggesting that the frequency of chronic seizures in the early weeks and months postinjury is a poor predictor of the progression of neocortical epilepsy. The frequency of limbic seizures was comparably low in all groups through 8–9 weeks post-FPI (Fig. 4B). A subsequent rise in the frequency of limbic seizures at 17–19 weeks post-FPI varied both between groups and among identically treated individuals.
Figure 4.
Rat-to-rat variability in progression of posttraumatic epilepsy after FPI. The frequency of neocortical seizures (A) and of limbic seizures (B) is plotted for rpFPI, mpFPI, and cpFPI rats injured at 2.0 atm and 3.4 atm. Note the variable progression of epilepsy in different subjects and the overall lower propensity to epileptogenesis after mpFPI. (C) Rat-to-rat variability in temporal progression of seizure types. Proportions of G1, G2, and G3 seizures are represented with thin, intermediate, and thick line, respectively, at all time points. No line indicates no seizures detected. Note the overall tendency to worsening of seizure type with the time postinjury but with significant rat-to-rat variability. (D) Temporal progression in the proportion of G1, G2, and G3 seizures for rpFPI, mpFPI, and cpFPI animals (2.0 and 3.4 atm combined). Note the faster increase in the frequency of G2 seizures after rpFPI. (E) Relationship between log-transformed frequencies of neocortical seizures at weeks 2–3 postinjury and limbic seizures at weeks 17–19, after rpFPI, mpFPI, and cpFPI at 2.0 atm (hollow symbols) and 3.4 atm (filled symbols). A significant correlation (Pearson; P = 0.03) between the frequencies of early neocortical and later limbic seizures was found only for the rpFPI group. Line represents linear fitting of the data.
The rat-to-rat variability in the temporal progression of seizure type severity was further examined in epileptic rats that were recorded at each time point (Fig. 4C). A vector plot was produced in which seizure type was represented by the thickness of the vector and the relative proportion of each seizure type by its length. Vectors were displayed at each time point postinjury. Similar to the human, we observed animals with remitting, stable, or progressive PTE.
Temporal Evolution of Seizure Types
To address whether injury location affects the evolution of epilepsy, we examined the course of development of electrical seizure types in mpFPI and cpFPI (2.0 and 3.4 atm combined) and compared it with rpFPI rats. In all cases, G1 events accounted for a large proportion (65–90%) of seizures in the first 2 months after injury but just 15–30% by 17–19 weeks postinjury (Fig. 4D). G2 seizures were a low and stable proportion (∼10–20%) of seizures 2–3 weeks after mpFPI and cpFPI but increased to ∼30–40% by 17–19 weeks. In contrast, following rpFPI, the proportion of G2 seizures increased linearly over the entire observation period (∼10% at weeks 2–3, ∼30% at weeks 8–9, and ∼50% at weeks 17–19). There was a significant difference between the proportion of G2 seizures observed after rpFPI and that in pooled data from mpFPI and cpFPI groups at weeks 8–9 (n = 14 rpFPI and n = 18 mpFPI + cpFPI; P = 0.037, Mann–Whitney U test). In all groups, limbic seizures accounted for a small proportion of seizures in the first 2 months after injury and increased later.
Because seizure-induced kindling is hypothesized to have a role in the progression of human epilepsy, we investigated whether a higher frequency of neocortical seizures (G1 and G2 combined) 2–3 weeks after FPI in the frontal cortex ipsilateral to the injury was associated with an increased frequency of G3 seizures 4.5 months after trauma (Fig. 4E). There was a significant positive correlation between the frequencies of neocortical seizures at weeks 2–3 and that of G3 seizures at weeks 17–19 after rpFPI (r = 0.49, n = 15, P = 0.03) but not after mpFPI (r = 0.46, n = 12, P = 0.07) and cpFPI (r = −0.22, n = 13, P = 0.23; all Pearson correlations after log transform, 1-tailed), even though cpFPI had a frequency of G3 seizures comparable with that rpFPI. These data suggest that the neocortical focus induced by rpFPI may kindle the subcortical focus driving G3 seizures. However, G3 seizures can also be induced by more caudal injuries (mpFPI, cpFPI) without evidence of frontal lobe mediated kindling.
Localization of Partial Seizure Onset
Because human PTE is often multifocal, we investigated the onset sites of cortical seizures induced by FPI (Fig. 5A). After rpFPI, we observed a frontal dominance of neocortical seizures persisting over time. At 2–3 weeks postinjury, more than 90% of focal nonspreading seizures originated from the frontal cortex even with parietal or occipital trauma. At a later time, significantly more parietal/occipital and contralateral seizures were seen after mpFPI and cpFPI. MpFPI and cpFPI rats had greater proportion of contralateral seizures compared with rpFPI animals. FPI rats may only have neocortical focal seizures (∼41%), only limbic seizures (6%), or a combination of the 2 (∼53%; Table 2).
Figure 5.
Seizure frequency, duration, and behavioral changes associated with neocortical seizures detected at different neocortical sites. (A) Pie charts showing time-dependent changes in the proportion of G1 events detected by each of the 4 active epidural electrodes (1–4 in the inset) after rpFPI, mpFPI, and cpFPI (2.0 and 3.4 atm combined) at 2–3, 8–9, and 17–19 weeks posttrauma. At 2–3 weeks post-FPI, G1 seizures mostly originate from frontal cortex even when trauma is applied to caudal cortex. For rpFPI rats, the frontal lobe dominance of focal seizures does not change over time after injury. Following mpFPI and cpFPI, substantial fractions of G1 seizures are detected by contralateral or nonperilesional electrodes, indicating multifocal neocortical epilepsy. Electrodes' positions in respect to the skull are shown at right. (B) Inverse correlation between the duration of focal seizures and the distance between the site of onset and the injury site. Frontal cortex G1 seizures detected by electrode 4 (filled symbols) are longer when induced by rpFPI 3.4 atm than when induced by mpFPI or cpFPI at same severity of injury, suggesting that mechanisms of seizure maintenance and termination are recruited to a greater extent when injury is delivered closer to the frontal epileptic focus. Perilesional G1 seizures induced by cpFPI at 3.4 atm (hollow symbol; detected by electrode 3) are shorter than those detected by electrode 4 after rpFPI at same severity, indicating that the frontal cortex sustains seizures better than the occipital cortex. Asterisks indicate P < 0.05 (Mann–Whitney U test); n.s. indicates no significant difference. (C) Frequency of frontal cortex G1 seizures detected by electrode 4 (filled symbols) at weeks 2–3 and 8–9 is higher after 3.4 atm rpFPI than after mpFPI and cpFPI at the same severity. The frequency of these seizures decreases rapidly with the distance from the injury site. Perilesional G1 seizures induced by mpFPI and cpFPI at 3.4 atm (hollow symbol; detected by electrode 3) are much rarer than those induced by rpFPI, indicating that the frontal cortex is more prone to epileptogenesis after injury. (D–E) G1 seizures detected in the frontal cortex by electrode 4 after rostral or caudal injury are most commonly coincident with behavioral changes (D), typically motor arrest with or without facial automatisms, while G1 seizures originating from the occipital cortex (detected by electrode 3) were never associated with changes in behavior (E), regardless of the location of injury. n indicates number of events.
Table 2.
ECoG-based location of seizure onset
| rpFPI | rpFPI | mpFPI | mpFPI | cpFPI | cpFPI | All groups | |
| 2.0 atm | 3.4 atm | 2.0 atm | 3.4 atm | 2.0 atm | 3.4 atm | ||
| FC | 20% | 41.6% | 40% | 45.4% | 22.2% | 33.3% | 36.4% |
| OC | 0% | 4.2% | 0% | 9.1% | 0% | 0% | 3% |
| FC + OC | 0% | 0% | 0% | 0% | 11.1% | 0% | 1.6% |
| L | 0% | 0% | 20% | 9.1% | 11.1% | 8.3% | 6% |
| FC + L | 20% | 37.5% | 40% | 9.1% | 22.2% | 33.4% | 28.8% |
| OC + L | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| FC + OC + L | 60% | 16.7% | 0% | 27.3% | 33.4% | 25% | 24.2% |
Note: FC, frontal cortex; L, limbic system; OC, occipital cortex; rp, rostral parasagittal; mp, medial parasagittal; cp, caudal parasagittal; atm, atmospheres. Data are percentages of animals in each experimental group. All times postinjury are combined.
At 2–3 weeks postinjury, depending on location of FPI, 69–77% of epileptic animals had only one neocortical focus (located 97% of the time in the frontal cortex regardless the injury location) and 23–30% had 2 foci (usually in frontal ipsi- and contra-lateral cortices or in ipsilateral frontal and occipital cortices) and none presented more than 2 foci. By weeks 17–19, 20–46% of rats presented with a single focus, while the remainder had 2–4 foci in roughly equal proportions.
Properties of Neocortical Onset Partial Seizures
To examine the properties of the neocortex generating the focus without confounding effects of other areas involved in the spread of seizures, we examined the frequency and duration of frontal G1 seizures induced by different injury locations. Frontal cortex G1 seizures induced at 3.4 atm by rpFPI lasted longer (5.0 ± 0.3 s at weeks 8–9; n = 592 events) than when induced by mpFPI (3.9 ± 0.5 s at weeks 8–9; n = 56) or cpFPI (2.8 ± 0.4 s at weeks 8–9; n = 30). G1 seizure durations decreased linearly with the distance between the frontal cortex where the electrographic discharges began, and the center of the craniotomy where the trauma was applied (weeks 2–3: β = −0.55, r2 = 0.004, P = 0.025; weeks 8–9: β = −0.73, r2 = 0.005, P = 0.043, linear regression; Fig. 5B). With caudal trauma, the G1 seizures arising in perilesional occipital cortex were shorter than the perilesional seizures with frontal injury (Fig. 5B right panel, hollow vs. filled pentagon at 2 mm from center of craniotomy). The frequency of frontal G1 events (Fig. 5C) at weeks 2–3 and 8–9 after severe trauma was high in rats injured rostrally but decreased rapidly with distance from the site of contusive injury. Together these results suggest that frontal cortex is able to generate and maintain seizures with greater frequency than parietal and occipital cortices.
Behavioral Seizures
The semiology of human partial seizures depends on the location of the focus and the extent and regions of spread. Thus, frontal lobe seizures have different behaviors than parietal or occipital seizures. We investigated whether similar focus-dependent behavioral changes occur in the rat after FPI. Behavioral changes were seen with 80% of nonspreading frontal G1 seizures, usually consisting of arrest or loss of posture, sometimes followed by facial automatisms and body myoclonus (Fig. 5D). Behavioral changes were never detected with focal non-spreading events originating from the caudal parasagittal cortex, consistent with the lack of motor function of the occipital cortex (n = 47 events from rpFPI and cpFPI rats; Fig. 5E). Humans with occipital seizures often experience visual symptoms, which cannot be verified in rats. The previously published scale of behavioral correlates of partial seizures induced by rpFPI (D'Ambrosio et al. 2005, 2009) applies equally well to mpFPI and cpFPI seizures, as no new ictal behaviors were observed after more caudal injuries.
Regardless of injury location, G1 (Fig. 6A) and G2 (Fig. 6B) seizures arising from the frontal cortex were most commonly associated with behavioral arrest with (behavioral class 2) or without (behavioral class 1) facial automatisms, while some G2 and most G3 seizures several months postinjury were most commonly manifested as stereotyped loss of posture with the animal crouching down on the cage floor, remaining motionless until the electrographic discharge ends (behavioral class 5; Fig. 6C). The clinical manifestations of G3 seizures preceded the electrographic discharge by 1–20 s, consistent with origination in a subcortical focus with propagation to neocortex. In the vast majority of cases, active behavior resumed immediately at the end of the cortical electrographic discharge (Fig. 6C), with rare postictal transition into sleep.
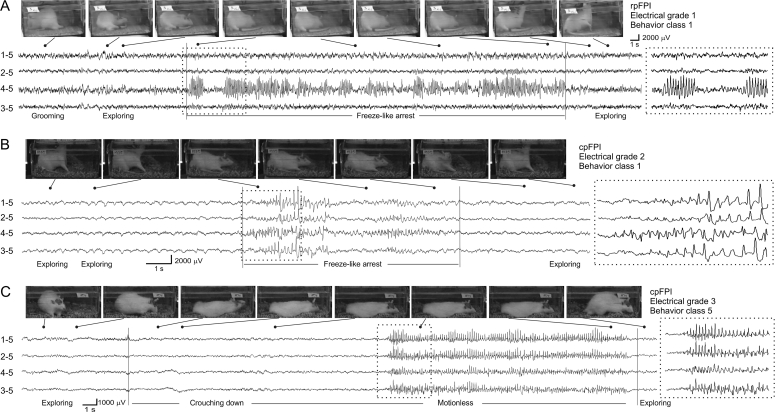
Figure 6.
ECoG traces and video frames of chronic seizures induced by FPI. (A) A ∼42s-long G1 seizure with behavioral arrest following 3.4 atm rpFPI. The rat was engaged in grooming and exploratory behavior before the onset of the seizure, exhibited a freeze-like arrest concomitantly with the ECoG event and then resumed its exploratory behavior immediately upon termination of the epileptiform discharge. (B) A cpFPI rat injured at 2.0 atm having a 8.5 s-long cortical spreading seizure (G2) associated with behavioral arrest without loss of posture. (C) A cpFPI rat injured at 3.4 atm having a ∼32s-long G3 limbic seizure associated with loss of posture. The clinical manifestation of this event begins with the rat crouching down to the floor of the cage prior to detection of the neocortical discharge. The rat then remained motionless until electrographic discharge ended. In all panels, dotted rectangles highlight ECoG traces expanded in insets at right. Numbers left of all ECoG traces represent the electrode and its reference.
Structural Changes in FPI-Injured Brains
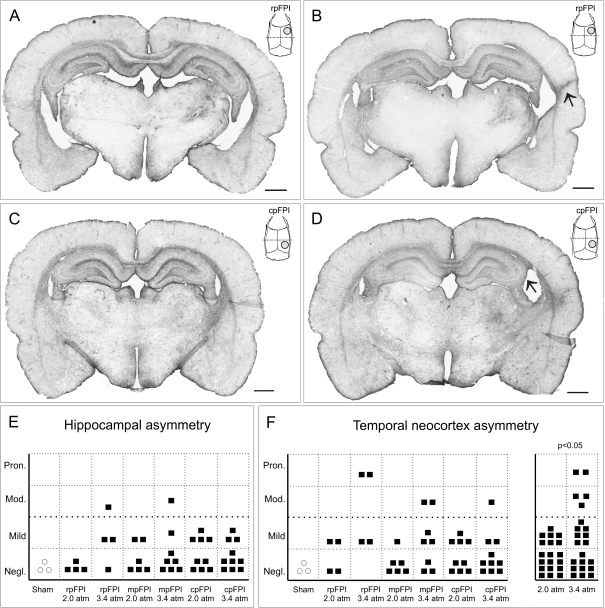
Because reactive astrogliosis is a prominent feature of human PTE and a sensitive indicator of damage, we examined GFAP immunostaining as previously described (D'Ambrosio et al. 2004, 2005). Brain structural changes were examined in the animals used for the study of PTE after 2.0 or 3.4 atm FPI inflicted rostrally (n = 4 and 4), medially (n = 6 and 8), and caudally (n = 9 and 10), as well as in sham-injured rats (n = 3). After 3.4 atm, rpFPI ipsilateral hippocampi and temporal cortices had varying degrees of sclerosis, even among identically injured animals (Fig. 7A,D). In the current study, we found that the structural asymmetry in the hippocampus and temporal cortex ranged from negligible to pronounced (Fig. 7E,F). While the trauma location did not significantly affect the severity of this hippocampal and temporal neocortical asymmetry, higher mechanical pressure caused a significantly higher temporal cortex asymmetry (P < 0.05; Fisher's Exact test; Fig. 7F, right panel). Regardless of injury location, all FPI animals also had a consistent, yet variable increase in GFAP+ immunoreactivity in the ipsilateral thalamus and corpus callosum.
Figure 7.
Hippocampal and temporal neocortex atrophy in posttraumatic epileptic rats after frontal, parietal, and occipital FPI. GFAP-stained coronal sections at Bregma −3/−4 mm obtained from rpFPI rats (A, B) and cpFPI rats (C, D) injured at 3.4 atm. Examples illustrate negligible hippocampal and temporal neocortical asymmetry (A and C), pronounced shrinkage of the ipsilateral temporal neocortex (B, arrow), and moderate shrinkage of the hippocampus (D, arrow). Insets in (A–D) represent the position of the coronal section (dotted line) with respect to the FPI craniotomy site (gray circle) and the rat skull. Scale bar is 1 mm in all panels. Hippocampal (E) and temporal neocortex (F) asymmetry in rpFPI, mpFPI, and cpFPI rats injured at 2.0 atm and 3.4 atm. Varying degrees of asymmetry are evident in each experimental group. The temporal cortex asymmetry increases significantly with the severity of FPI (F, right panel). Pron, pronounced; Mod, moderate; Negl, negligible.
Discussion
We examined the effect of location and severity of contusive closed head injury in the rat to understand factors that may contribute to the heterogeneity of human PTE. The main findings are 1) the location and severity of FPI affects the incidence and severity of PTE, 2) the frontal cortex is more susceptible to posttraumatic epileptogenesis than parietal and occipital cortices, 3) limbic epileptogenesis proceeds slower than neocortical but is a prominent component of the PTE syndrome regardless of injury location, and 4) there is wide variation among animals in the severity and course of PTE that is independent from location and severity of injury.
Elucidating the role of factors hypothesized to influence human PTE and its variability (Caveness 1963; Caveness et al. 1979) requires examining them separately under controlled experimental conditions with clearly defined criteria for comparison of human and rat PTE.
Controlling Variables Affecting Posttraumatic Epileptogenesis
In this study, animals' age, gender, and strain were controlled and a carefully calibrated contusive closed head injury was delivered at 3 different locations with 2 different mechanical severities. Posttraumatic hypoxia was also controlled because it profoundly affects outcome and mortality in animals (Ishige et al. 1987a; Ishige et al. 1987b; Shima and Marmarou 1991) and humans (Chesnut et al. 1993; The Brain Trauma Foundation 2000; Chi et al. 2006) and is epileptogenic (Kadam et al. 2010). This was done by intubating the animals, administering a volatile anesthetic, and controlling posttraumatic apnea. This strategy is known to decrease mortality after FPI (Levasseur et al. 1989) because artificial ventilation rescues the animals from the respiratory arrest that occurs more often with caudal FPI, when the pressure shock reaches the pons (Floyd et al. 2002). The use of respiratory support permits a controlled comparison of FPI at different locations, an overall lower mortality, as well as greater clinical relevance since humans with the most severe head injuries are rescued by intensive care to enter the pool of patients suffering from PTE.
Criteria and Limitations of Comparing Human Versus Rodent Posttraumatic Epilepsy Variants
A meaningful comparison of humans and rats PTE also requires the understanding of its potential pitfalls. First, there are species differences in the behavioral manifestations of seizures. The larger association cortices in humans means that very localized seizures are less likely to have somatosensory or motor manifestations, while seizures with purely subjective or cognitive effects in humans would be judged subclinical in a rat. However, a comparison of PTE based only on easily recognized motor events, such as tonic–clonic seizures, would miss nonconvulsive complex partial seizures (CPSs), which are a significant PTE component (Caveness et al. 1962; Walker 1965; Becker et al. 1979; Salazar et al. 1985, 1995; Langendorf and Pedley 1997; D'Ambrosio and Miller 2010). Second, PTE evolves over time, but postinjury latencies cannot easily be compared across these 2 species with different maturation rates and lifespan. Most ECoG/electroencephalography (EEG) data on human PTE are obtained 17–18 years after epilepsy diagnosis (Salanova et al. 2002; Benbadis et al. 2003) but a rat lifespan is about 3 years. Third, all epidemiological head injury data include a wide range of ages, both genders, cases with different numbers, severities and types of head injuries, and widely varying sequelae. Even when broken down by functional severity, very diverse types of injuries (e.g., closed vs. penetrating injury; single blunt injury vs. acceleration/deceleration motor vehicle accidents), with different sequelae (e.g., ischemia/hypoxia or recurrent blood brain barrier opening) and different risk factors for PTE (e.g., intracerebral or subdural hemorrhage or acute seizures) are all grouped together. Fourth, it is difficult to accurately determine the frequency of different seizure types in patients (Langendorf and Pedley 1997). PTE may not be identified in humans if the seizures are subtle or subclinical (Geier et al. 1977; Williamson et al. 1985a, 1985b; Williamson and Spencer 1986; Williamson et al. 1992; Kerling et al. 2006; D'Ambrosio et al. 2009; Dichter 2009), and clinical studies of PTE are typically based on self-reports which can miss many seizures (Kerling et al. 2006). Human CPSs can manifest with a wide variety of convulsive or nonconvulsive behaviors and subjective experiences or sometimes as just a lapse of response to the environment that can easily be misdiagnosed (Penfield and Jasper 1954; Swartz 1992; Leppik 1997) or confused with psychiatric conditions (Williamson et al. 1985a, 1985b; Williamson and Spencer 1986). In addition, simple partial seizures (SPSs), despite being part of the PTE syndrome (Becker et al. 1979; Salazar et al. 1985, 1995; Jabbari et al. 1986), are often unreported or misdiagnosed, if they have only subjective symptoms without motor activity (Devinsky et al. 1988, 1989). Also, seizures with limited electrical spread are often not detected by standard 20-electrode scalp EEG but are evident in invasive ECoG recordings (Williamson et al. 1985b; Williamson and Spencer 1986; Devinsky et al. 1989; Cukiert et al. 2001; Binnie and Stefan 2003; Worrell et al. 2008), but much of the literature is based on either behavioral assessment alone (Caveness 1963; Walker 1965) or scalp EEG based on only 4–16 electrodes (Caveness 1954; Devinsky et al. 1988).
In contrast, the present animal study uses calibrated contusive closed head injury with a controlled hypoxic period, inflicted on a homogeneous population of male juvenile rats with prospective evaluation by invasive video-ECoG in all animals. Unlike clinical studies, this study is optimized to detect all seizures making up the PTE syndrome, as needed to investigate factors affecting PTE. Thus, the incidence and frequency of seizures observed in the rat are likely to exceed those reported in epidemiological literature, although the model's pathophysiology is still appropriate to investigate mechanisms and variability of PTE.
Location and Severity of FPI Affect Incidence of PTE, Seizure Frequency, and Speed of Epileptogenesis but Do Not Fully Account for PTE Variability
Greater mechanical injury resulted in greater acute functional impairment (Fig. 1C) and chronic neocortical atrophy (Fig. 7F). It also caused a higher probability of PTE (Fig. 1E) just as in humans (Annegers et al. 1998; Aarabi et al. 2000; Herman 2002). This suggests that FPI shares important mechanisms of epileptogenesis with human PTE. The weakness of the correlation between injury site and PTE incidence could be due to the known diffuse injury component of FPI (Thompson et al. 2005). Comparative data do not exist for human PTE. In FPI rats, seizure frequency ranged from 10/h to less than 1 a month (Fig. 3) and seizure duration ranged from 1 s to 2.5 min to status epilepticus (Table 1; Fig. 2; D'Ambrosio et al. 2005, 2009), in agreement with the variability of human frontal lobe seizures determined by invasive ECoG (Williamson et al. 1985a; Williamson and Spencer 1986; So 1998; Jobst et al. 2000). This large variability in seizure duration did not depend on injury location or severity (Fig. 2) but rather appeared to be an intrinsic property of the epileptic foci (D'Ambrosio et al. 2009).
FPI severity does affect the early rate of epileptogenesis since animals injured at 3.4 atm had a higher epilepsy incidence (Fig. 1E) with a higher seizure frequency (Fig. 3) than at 2 atm in the early weeks after injury. This difference was not just due to a higher proportion of nonepileptic animals after milder injury but also to an overall lower seizure frequency and progression rate in epileptic animals (Fig. 3G). Even after controlling for injury location and severity, animals presented quite different latencies to PTE, ranging from ≤2 weeks to 5 months (Fig. 4), indicating that other important factors are at play. Notably, rpFPI at 3.4 atm resulted in neocortical focus that generates full seizure frequency within 3 weeks, while at 2 atm, comparable seizure frequencies did not occur even after 4.5 months (Fig. 3D). The dependence of PTE progression on injury severity could be due to early seizures or hemorrhage, both risk factors for human PTE, and may partially explain the great variability in the time to diagnosis of human PTE. FPI in the rat produced remitting, stable, and progressive PTE (Fig. 4C). There was a trend for remission to be more likely with caudal FPI locations (Fig. 1F). Higher seizure frequency and the occurrence of more severe spreading G2 and G3 seizures or of multiple seizure types predicted failure to remit (Fig. 1G). This is consistent with clinical data. Cases of remitting, stable, and progressive PTE are also seen in humans (Caveness 1963; Salazar et al. 1985, 1995). PTE may remit whether or not the patients take antiepileptic medications (Jennett and Lewin 1960; Walker 1962; Caveness 1963; Weiss and Caveness 1972; Caveness et al. 1979; Collaborative Group for the Study of Epilepsy 1992; Pohlmann-Eden and Bruckmeir 1997; Berg et al. 2009), and a remission is more likely in patients with low seizure frequency, a single seizure type, and SPSs rather than CPSs (Caveness 1963; Salazar et al. 1985).
Although many features of FPI-induced PTE depend on the location and severity of injury, a remarkable rat-to-rat variability was still observed in latency to seizure onset, seizure frequency, and epilepsy evolution over time despite the standardization of the posttraumatic hypoxia, the uniform age, and gender of the animals, and the small intertrial variation in the FPI pressure peak (Fig. 1B). Thus, factors other than the location and severity of the injury are also likely to contribute to the heterogeneity of human PTE. Human genetic background is thought to be a key factor in PTE variability (Caveness 1976; Caveness et al. 1979), and our studies employed genetically diverse outbred rats. The genetic background of animals has been shown to affect TBI outcome (Tan et al. 2009) and susceptibility to both acquired (Winawer et al. 2007; Müller et al. 2009) and idiopathic epilepsy (Inoue et al. 1990; Noebels 2003). In humans, recent studies show that genetic polymorphisms, such as apolipoprotein E epsilon-4 allele (Chiang et al. 2003; Diaz-Arrastia et al. 2003; Lendon et al. 2003; Nathoo et al. 2003), haptoglobin Hp2-2 allele (Panter et al. 1985; Sadrzadeh et al. 2004), or IL-1β (511) allele 2 (Kanemoto et al. 2000; Virta et al. 2002), may partially determine neurologic outcome after TBI and posttraumatic epileptogenesis (Sorbi et al. 1995; Hadjigeorgiou et al. 2005; Diaz-Arrastia and Baxter 2006). The physiological state of the animal at injury may also affect epileptogenesis. TBI paired with peripheral bone fracture—a common occurrence in human head injury—resulted in more severe brain inflammation (Maegele et al. 2005, 2007) that may affect epileptogenesis (Vezzani et al. 2008; Maroso et al. 2010). Further work is needed to determine which genes and physiological states are the most important determinants of posttraumatic epileptogenesis.
The Frontal Cortex Is More Prone to Epileptogenesis than Parietal and Occipital Neocortices
Head trauma is a common cause of frontal lobe epilepsy in humans (Rasmussen 1983; Williamson 1992), and PTE patients most often develop frontal and temporal lobe seizures (Walker 1965; Hudak et al. 2004; Diaz-Arrastia et al. 2009). Human frontal and temporal cortices may be especially susceptible to contusion (Pohlmann-Eden and Bruckmeir 1997; Hartzfeld et al. 2008) due to the shape of the bony vault (Gurdjian et al. 1966), which may explain why posttraumatic contusion is greater in frontal than in occipital cortex, even with caudal injuries (Goggio 1941; Sano et al. 1967; Adams et al. 1980; Bešenski et al. 1996). However, another factor at play may be the increased propensity to posttraumatic epileptogenesis of the frontal cortex. In the FPI rat, we found that frontal neocortex generated higher seizure frequency and faster epileptogenesis (Fig. 3), longer seizure durations (Fig. 5B), and frontal lobe seizures were the most common neocortical seizures in the early weeks postinjury regardless of injury location (Fig. 5A). When considering only perilesional G1 seizures—to eliminate effects attributable to the bony vault and isolate the effects of the focal component of FPI from its diffuse effects—both mpFPI and cpFPI induced many fewer (Fig. 5C) and shorter (Fig. 5B) perilesional seizures in the parietal/occipital cortex (electrode 3) than rpFPI does in the frontal cortex (electrode 4). The mechanisms responsible for the predilection of the frontal lobe to epileptogenesis are unknown but could range from an intrinsically higher susceptibility to tissue damage due to the applied pressure, to the known tendency of prefrontal neurons to burst discharges and hypersynchronization (Shinomoto et al. 2009). Interestingly, we observed that the duration of frontal G1 seizures decreased linearly when the center of the craniotomy—where trauma was applied—was more distant from the frontal lobe (Fig 5B). This indicates that FPI also recruits mechanisms of seizure maintenance and termination. However, seizure frequency decreased nonlinearly with distance from the center of craniotomy (Fig. 5C), suggesting cellular and network mechanisms of ictogenesis differ from those responsible for seizure maintenance and termination.
Variable Semiology of FPI-Induced PTE
Human PTE presents with a wide range of semiologies (Caveness 1963; Weiss and Caveness 1972), and our data indicate this variability is likely to partially depend on the location and severity of injury. Clinical manifestations of partial seizures depend on the ictal sites and the areas of seizure spread (Commission on Classification and terminology of ILAE 1989; Engel 2006), and the most common forms of human PTE are frontal and temporal lobe epilepsies, with or without dual pathology or secondarily generalization, while parietal or occipital epilepsies are uncommon (Jennett 1961; Ludwig et al. 1975; Williamson 1992; Marks et al. 1995; Diaz-Arrastia et al. 2000, 2009; Hudak et al. 2004). Similarly, our data show that FPI-induced PTE can be multifocal, with mpFPI and cpFPI more prone to induce multiple foci (Fig. 5; Table 2) and seizures with different behavioral correlates depending on the location of the epileptic focus (Fig. 5D,E) and of different duration depending on the site of FPI (Fig. 5B). Most animals had frontal neocortical or limbic epilepsy, regardless of FPI location, and parietal/occipital seizures were rare (Table 2). Ictal behavioral changes associated with neocortical G1 and G2 seizures began simultaneously with the ECoG discharge (Fig. 6A,B), while those associated with G3 seizures, which are of limbic origin (D'Ambrosio et al. 2005), most commonly began several seconds before the cortical discharge (Fig. 6C). Frontal lobe seizures in FPI animals were electrically and behaviorally consistent with those seen in humans, being most commonly associated with behavioral arrest, with or without mild automatisms (Geier et al. 1977; Williamson et al. 1985a; Williamson and Spencer 1986; Bancaud and Talairach 1992; Lüders et al. 1992, 1995; Swartz 1992; Salanova et al. 1995; So 1998; Ikeda et al. 2009). The rare parieto–occipital neocortical seizures induced by rpFPI were never associated with behavioral arrest (Fig. 5E, left panel). Accordingly, ictal behavioral arrests after cpFPI were seen with frontal lobe seizures (Fig. 5D, right panel) but not with perilesional parieto–occipital seizures (Fig. 5E, right panel). The latter appeared subclinical but might be associated with subjective visual phenomena (Williamson et al. 1992) that cannot be assessed in animals. Frontal and limbic seizures with loss of body posture became progressively more common in rats over time (D'Ambrosio et al. 2005, 2009) and had semiology reminiscent of human CPSs. Many of these seizures consist of stereotyped crouching down (Fig. 6C) sometimes with hindlimb dystonia, facial automatisms, or body myoclonus.
Evidence for 2 Different Mechanisms in Posttraumatic Limbic Epileptogenesis
An unresolved question is why frontal neocortical foci develop fully within 1 month post-FPI, while it takes several months for limbic foci to appear (D'Ambrosio et al. 2005). One possibility is that frontal neocortical seizures may kindle the hippocampus since about 56% of frontal neocortical seizures spread to the hippocampus (D'Ambrosio et al. 2005). Alternatively, limbic epileptogenesis could occur because of direct hippocampal injury but developing more slowly because deeper structures sustain a milder mechanical injury. Our data suggest that both mechanisms might have a role in limbic epileptogenesis, depending on the injury site. With rpFPI, the early rate of occurrence of neocortical frontal lobe seizures predicted the frequency of limbic seizures 4.5 months postinjury (Fig. 4E), suggesting that kindling may take place. After cpFPI, the frequency of frontal seizures was approximately one tenth of that seen after rpFPI and did not correlate with the later development of the limbic focus, indicating that this limbic focus is likely due to direct injury. Consistent with this, rpFPI produces less hippocampal cell loss than cpFPI (Floyd et al. 2002). These hypothetical mechanisms of epileptogenesis need to be directly tested with experiments to determine the effects of shutting down the neocortical focus on development of subcortical foci.
Comparison of FPI-Induced PTE across Laboratories
Strikingly different PTE syndromes have now been described after FPI in 2 different laboratories. We (D'Ambrosio et al. 2004, 2005, 2009; Eastman et al. 2010) have observed frequent neocortical partial seizures that appear weeks after rpFPI, and gradually worsen in frequency and duration. A subcortical focus appeared months later and progressed to drive most seizures 7–8 months after injury. The semiology of most seizures was analogous to human convulsive SPSs and CPSs, and tonic-clonic convulsions were not observed. The Racine scale (Racine 1972) did not adequately characterize all observed seizures, and a new behavioral scale was developed. Kharatishvili et al. (2006), in contrast, reported similarly progressing PTE syndrome after mpFPI but with lower incidence, longer latency, lower seizure frequency, and semiology with tonic–clonic seizures of limbic origin, and no neocortical seizures. Our data indicate that this lower seizure frequency is partially explained by mpFPI, which results in 10-fold lower seizure frequency than rpFPI under identical experimental conditions. However, the absence of neocortical seizures and the presence of tonic–clonic convulsions cannot be explained with variations in location and severity of injury. Our controlled comparisons show that neocortical epilepsy is always induced, to different extent, regardless of the location of FPI, and we observed no tonic–clonic convulsions in any experimental group.
Thus, other methodological differences (Table 3) could be a factor. Chronic tonic–clonic seizures have been observed after experimental neonatal hypoxia (Kadam et al. 2010; Klein et al. 2009). It is thus likely that severe posttraumatic hypoxia, which occur in the absence of acute postinjury respiratory support (Ishige et al. 1987a; Ishige et al. 1987b), contributed to the development of tonic–clonic seizures in the FPI model of Kharatishvili et al. (2006), in which posttraumatic apnea was not controlled. Also, that model uses older animals, which are more sensitive to head injury and epileptogenesis (Kelly 2010), and a much more severe and diffuse injury than ours (larger craniotomy and longer FPI pulses), which is likely to cause greater damage to the brainstem, which is involved in generating tonic–clonic convulsions (Browning 1987; Gale and Browning 1988). Differences in the detection and scoring of ictal activity may also contribute to the apparent absence of neocortical seizures and overall lower seizure frequency. We report all seizures with behavioral changes and epileptiform ECoG detected with 5 epidural electrodes (D'Ambrosio et al. 2004, 2009), sometimes with 1–2 additional depth electrodes (D'Ambrosio et al. 2005). Kharatishvili et al. (2006), in contrast, used only 2 epidural and 1 depth electrodes and only report Racine scale seizures lasting longer than 29 s. This approach may miss relevant events since many verifiable human seizures have a shorter duration (Devinsky et al. 1988; Leppik 1997) and since neocortical seizures are better detected by epidural electrodes. Another factor that may contribute to the different findings by Kharatishvili et al. (2006) is the very chronic depth electrode implantation that leads to inflammation (Holguin et al. 2007). Prolonged implantation of depth electrodes has been shown to facilitate kindling and worsen evoked seizures (Blackwood et al. 1982; Löscher et al. 1995), so the year-long depth electrode implantation employed by these authors during a time of rapid skull growth may cause additional brain injury. Consistent with this possibility, Kharatishvili et al. (2007) did not see chronic seizures when depth electrodes were implanted for less than a month, and Hunt et al. (2009), who did not employ depth electrodes, did not observe Racine scale 4 or 5 seizures chronically after cortical impact injury in the mouse.
Table 3.
Comparison of methods to induce PTE by FPI and main results in different laboratories
| Study | D'Ambrosio et al., 2004, 2005, 2009 and present study | Kharatishvili et al., 2006 |
| Location of craniotomy | Rostrala or medialb parasagittal at bregma −2 mma or −4 mmb, 3-mm lateral. Right convexity | Medialc parasagittal at bregma −4 mm, 3-mm lateral. Left convexity |
| Diameter craniotomy | 3 mm | 5 mm |
| Percussion pressure | 3.3–3.5 atm | 2.6–3.3 atm |
| Percussion duration | 8.03 ± 0.02 ms | 21–23 ms |
| FPI device design/build | University of Washington | Medical College of Virginia |
| Species | Sprague–Dawley rat (outbred) | Sprague–Dawley rat (outbred) |
| Rat age at injury | 33–35 d.o. | Not reported |
| Rat weight at injury | 100–125 g | 305–390 g |
| Anesthetic | Volatile. Halothane 1–1.5% | Injected. Sodium pentobarbital, 58 mg/kg; chloral hydrate, 60 mg/kg; magnesium sulfate, 127.2 mg/kg; propylene glycol, 42.8%; absolute ethanol, 11.6% |
| Intubation | Yes | No |
| Posttraumatic apnea | 10 s (set experimentally) | 11 s–1.5 min (spontaneous) |
| Acute mortality (<1 week) | 11%a, 8%b | 31–33% |
| Righting time | ∼12 mina, ∼10 minb | Not reported |
| Body temperature during surgery | Rectal (37 °C) | Not reported |
| Electrodes type and location | FC, OC ipsilateral (epidural) | PC ipsi-/contralateral (epidural); H ipsilateral (1 depth el.) |
| FC, OC contralateral (epidural); H ipsilateral (1-2 depth el.) | ||
| Monitoring | Up to 7 monthsa or up to 4.5 monthsb postinjury by noninvasive epidural el. Up to 2 weeks by depth el. | Up to 12 months postinjury by depth electrode |
| Management of lost headsets | One noninvasive reimplant at most | One invasive reimplant at most |
| Management of chronic electrode damage | Observed by GFAP+ immunoreactivity | Not reported |
| Seizure definition | Epileptiform ECoG lasting ≥1 s with ictal behavior as per D'Ambrosio et al. (2004, 2005, 2009) | Epileptiform ECoG lasting ≥5 s with ictal behavior as per Racine scale. Only events ≥29 s reported. |
| Diagnosis of epilepsy | At least one chronic seizure after head injury | At least 2 chronic seizures |
| Frequency of recordings | 24 h weekly | 24 h every 4 or 7 weeks |
| Time first recording | Week 2 post-FPI | Weeks 7–9 post-FPI |
| Latency of epileptogenesis | 2–19 weeks | 7–52 weeks |
| Total hours of recording per time point | 48–72 | 24 |
| Distinguishing age-dependent idiopathic epilepsy in rat strain | Waveform identified and seizures quantified reported separately from PTE | Not reported |
| Incidence PTE | 90%a or 80b (week 8 post-FPI) 100% (week 19 post-FPI) | 6% (week 8 post-FPI) 11–23% (week 19 post-FPI) |
| Average seizure frequency | ∼3 sz/ha, ∼0.2 sz/hb (weeks 8–9 post-FPI), ∼5 sz/ha, ∼0.6 sz/hb (weeks 17–19 post-FPI) | ∼0.3 to 0.86 sz/day (weeks 8–49 post-FPI) |
| Observed seizure duration range | 1–99 sa; 1–35 sb | 29–210 s |
Note: d.o., day old; FC, frontal cortex; H, hippocampus; OC, occipital cortex; PC, parietal cortex; el., electrode.
Rostral parasagittal FPI in D'Ambrosio et al. (2004, 2005, 2009) and present study.
Medial parasagittal FPI as in present study.
Medial parasagittal FPI in Kharatishvili et al. (2006).
Conclusions
The frontal lobe and limbic system of the rat have major roles in posttraumatic epileptogenesis. The location and severity of injury determine some of the heterogeneity of PTE in the rat, and these factors likely have comparable roles in human PTE. However, much variability in seizure frequency and temporal evolution of the epileptic syndrome remains when these factors are controlled. Genetic background and physiological state at time of injury are likely contributors to this residual variability. Our data demonstrate numerous similarities between FPI-induced and human PTE and indicate that FPI is a relevant model of human PTE capable of reproducing important features, such as its heterogeneity. They also show that FPI delivered to the frontal lobe is particularly well suited for investigation of mechanisms and treatment of neocortical epileptogenesis.
Funding
National Institutes of Health (NS053928 to R.D.).
Acknowledgments
We thank Drs Clifford Eastman and Nancy Temkin for helpful discussion. Conflict of Interest: None declared.
References
- Aarabi B, Taghipour M, Haghnegahdar A, Farokhi M, Mobley L. Prognostic factors in the occurrence of posttraumatic epilepsy after penetrating head injury suffered during military service. Neurosurg Focus. 2000;8(1):1–6. doi: 10.3171/foc.2000.8.1.155. [DOI] [PubMed] [Google Scholar]
- Adams JH, Scott G, Parker LS, Graham DI, Doyle D. The contusion index: a quantitative approach to cerebral contusions in head injury. Neuropathol Appl Neurobiol. 1980;6:319–324. doi: 10.1111/j.1365-2990.1980.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Groover RV, Laws ER, Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser A, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Talairach J. Clinical semiology of frontal lobe seizures. Adv Neurol. 1992;57:3–58. [PubMed] [Google Scholar]
- Becker DP, Grossman RG, McLaurin RL, Caveness WF. Head injuries—panel 3. Arch Neurol. 1979;36:750–758. doi: 10.1001/archneur.1979.00500480024005. [DOI] [PubMed] [Google Scholar]
- Beghi E, Berg A, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Shinnar S, Temkin N, Thurman D, Tomson T. Comment on epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(10):1698–1699. doi: 10.1111/j.1528-1167.2005.00273_1.x. [DOI] [PubMed] [Google Scholar]
- Benbadis SR, Heriaud L, Tatum WO, Vale FL. Epilepsy surgery, delays and referral patterns—are all your epilepsy patients controlled? Seizure. 2003;12:167–170. doi: 10.1016/s1059-1311(02)00320-5. [DOI] [PubMed] [Google Scholar]
- Berg AT, Levy SR, Testa FM, D'Souza R. Remission of epilepsy after two drug failures in children: a prospective study. Ann Neurol. 2009;65:510–519. doi: 10.1002/ana.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bešenski N, Broz R, Jadro-Šantel D, Pavić D, Mikulić D. The course of the traumatizing force in acceleration head injury: CT evidence. Neuroradiology. 1996;38:S36–S41. doi: 10.1007/BF02278116. [DOI] [PubMed] [Google Scholar]
- Binnie CD, Stefan H. The EEG in epilepsy. In: Binnie C, Cooper R, Mauguière F, Osselton J, Prior P, Tedman B, editors. Clinical neurophysiology. Vol 2: EEG, paediatric neurophysiology, special techniques and applications. Amsterdam (NL): Elsevier; 2003. pp. 268–303. [Google Scholar]
- Blackwood DH, Martin MJ, McQueen JK. Enhanced rate of kindling after prolonged electrode implantation into the amygdala of rats. J Neurosci Methods. 1982;5(4):343–348. doi: 10.1016/0165-0270(82)90004-8. [DOI] [PubMed] [Google Scholar]
- Brandt C, Glien M, Potschka H, Volk H, Löscher W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdale in rats. Epilepsy Res. 2003;55:83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Browning RA. Effect of lesion on seizures in experimental animals. In: Fromm GH, Faingold CL, Browning RA, Burnham WM, editors. Epilepsy and the reticular formation: the role of the reticular core in convulsive seizures. New York: Alan R. Liss; 1987. pp. 137–162. [Google Scholar]
- Caveness WF. Electroencephalography in head injury. Clin Neurosurg. 1954;2:116–134. [PubMed] [Google Scholar]
- Caveness WF. Onset and cessation of fits following craniocerebral trauma. J Neurosurg. 1963;20:570–583. doi: 10.3171/jns.1963.20.7.0570. [DOI] [PubMed] [Google Scholar]
- Caveness WF. Epilepsy, a product of trauma in our time. Epilepsia. 1976;17(2):207–215. doi: 10.1111/j.1528-1157.1976.tb03398.x. [DOI] [PubMed] [Google Scholar]
- Caveness WF, Liss HR. Incidence of post-traumatic epilepsy. Epilepsia. 1961;2:123–129. [PubMed] [Google Scholar]
- Caveness WF, Meirowsky AM, Rish BL, Mohr JP, Kistler JP, Dillon JD, Weiss GH. The nature of post-traumatic epilepsy. J Neurosurg. 1979;50(5):545–553. doi: 10.3171/jns.1979.50.5.0545. [DOI] [PubMed] [Google Scholar]
- Caveness WF, Walker AE, Ascroft PB. Incidence of posttraumatic epilepsy in Korean veterans as compared with those from World War I and World War II. J Neurosurg. 1962;19:122–129. doi: 10.3171/jns.1962.19.2.0122. [DOI] [PubMed] [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Chi JH, Knudson MM, Vassar MJ, McCarthy MC, Shapiro MB, Mallet S, Holcroft JJ, Moncrief H, Noble J, Wisner D, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61(5):1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- Chiang MF, Chang JG, Hu CJ. Association between apolipoprotein E genotype and outcome of traumatic brain injury. Acta Neurochir. 2003;145(8):649–653. doi: 10.1007/s00701-003-0069-3. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46(6):956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- Collaborative Group for the Study of Epilepsy. Prognosis of epilepsy in newly referred patients: a multicenter prospective study of the effects of monotherapy on the long-term course of epilepsy. Epilepsia. 1992;33(1):45–51. doi: 10.1111/j.1528-1157.1992.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Commission on classification and terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsy and epileptic syndrome. Epilepsia. 1989;30(4):389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Costello DJ, Delanty N. Oxidative injury in epilepsy: potential for antioxidant therapy? Expert Rev Neurother. 2004;4(3):541–553. doi: 10.1586/14737175.4.3.541. [DOI] [PubMed] [Google Scholar]
- Cukiert A, Buratini JA, Machado E, Sousa A, Vieira O, Argentoni M, Foster C, Baldauf C. Results of surgery in patients with refractory extratemporal epilepsy with normal or localizing magnetic resonance findings investigated with subdural grids. Epilepsia. 2001;42(7):889–894. doi: 10.1046/j.1528-1157.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, Sheerin AH, Gupta P, Diaz-Arrastia R, Ojemann J, et al. Functional definition of seizures provides new insights into posttraumatic epileptogenesis. Brain. 2009;132:2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Miller JW. What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epilepsy Curr. 2010;10(3):61–66. doi: 10.1111/j.1535-7511.2010.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Kelley K, Porter RJ, Thodore WH. Clinical and electrographic features of simple partial seizures. Neurology. 1988;38(9):1347–1352. doi: 10.1212/wnl.38.9.1347. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Sato S, Kufta CV, Ito B, Rose DF, Theodore WH, Porter RJ. Electroencephalographic studies of simple partial seizures with subdural electrode recordings. Neurology. 1989;39:527–533. doi: 10.1212/wnl.39.4.527. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Agostini MA, Frol AB, Mickey B, Fleckenstein J, Bigio E, Van Ness PC. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch Neurol. 2000;57:1611–1616. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Agostini MA, Madden CJ, Van Ness PC. Post-traumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia. 2009;50(Suppl 2):14–20. doi: 10.1111/j.1528-1167.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Baxter VK. Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J Head Trauma Rehabil. 2006;21(4):361–374. doi: 10.1097/00001199-200607000-00007. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Gong Y, Fair S, Scott KD, Gargia MC, Carlie MC, Agostini MA, Van Ness PC. Increased risk of late posttraumatic seizures associated with inheritance of APOE epsilon4 allele. Arch Neurol. 2003;60(6):818–822. doi: 10.1001/archneur.60.6.818. [DOI] [PubMed] [Google Scholar]
- Dichter MA. Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia. 2009;50(Suppl 2):41–45. doi: 10.1111/j.1528-1167.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Temkin NR, D'Ambrosio R. ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. Exp Neurol. 2010;224(2):369–388. doi: 10.1016/j.expneurol.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhar B, Sahraian MA, Nouralishahi B, Khaji A, Vahabi Z, Ghodsi M, Araghizadeh H, Soroush MR, Esmaeili SK, Masoumi M. Prognostic factors in the persistence of posttraumatic epilepsy after penetrating head injuries sustained in war. J Neurosurg. 2009;110:319–326. doi: 10.3171/2008.4.17519. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr Report of the ILAE classification core group. Epilepsia. 2006;47(9):1558–1568. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, Hughes R, Bergman W. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84:365–373. doi: 10.1053/apmr.2003.50022. [DOI] [PubMed] [Google Scholar]
- Epsztein J, Ben-Ari Y, Represa A, Crepel V. Late-onset epileptogenesis and seizures genesis: lessons from models of cerebral ischemia. Neuroscientist. 2008;14(1):78–90. doi: 10.1177/1073858407301681. [DOI] [PubMed] [Google Scholar]
- Fisher R, Leppik I. Debate: when does a seizure imply epilepsy? Epilepsia. 2008;49(Suppl 9):7–12. doi: 10.1111/j.1528-1167.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Golden KM, Black RT, Hamm RJ, Lyeth BG. Craniectomy position affects Morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma. 2002;19(3):303–316. doi: 10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Gale K, Browning RA. Anatomical and neurochemical substrates of clonic and tonic seizures. In: Dichter MA, editor. Mechanisms of epileptogenesis. New York: Plenum Press; 1988. p. 111. [Google Scholar]
- Geier S, Bancaud J, Talairach J, Bonis A, Szikla G, Enjelvin M. The seizures of frontal lobe epilepsy. A study of clinical manifestations. Neurology. 1977;27:951–958. doi: 10.1212/wnl.27.10.951. [DOI] [PubMed] [Google Scholar]
- Goggio AF. The mechanism of counter-coup injury. J Neurol Psychiatry. 1941;4:11–22. doi: 10.1136/jnnp.4.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta YK, Gupta M. Post traumatic epilepsy: a review of scientific evidence. Indian J Physiol Pharmacol. 2006;50(1):7–16. [PubMed] [Google Scholar]
- Gurdjian ES, Lissner HR, Hodgson VR, Patrick LM. Mechanisms of head injury. Clin Neurosurg. 1966;12:112–128. doi: 10.1093/neurosurgery/12.cn_suppl_1.112. [DOI] [PubMed] [Google Scholar]
- Hadjigeorgiou GM, Paterakis K, Dardiotis E, Dardioti M, Aggelakis K, Tasiou A, Xiromerisiou G, Komnos A, Zintzaras E, Scarmeas N, et al. IL-1RN and IL-1B gene polymorphisms and cerebral hemorrhagic events after traumatic brain injury. Neurology. 2005;65(7):1077–1082. doi: 10.1212/01.wnl.0000178890.93795.0e. [DOI] [PubMed] [Google Scholar]
- Hartzfeld P, Elisevich K, Pace M, Smith B, Gutierrez JA. Characteristics and surgical outcomes for medial temporal post-traumatic epilepsy. Br J Neurosurg. 2008;22(2):224–230. doi: 10.1080/02688690701818901. [DOI] [PubMed] [Google Scholar]
- Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Holguin A, Frank MG, Biedenkapp JC, Nelson K, Watkins LR, Rudy JW, Maier SF. Characterization of the temporo-spatial effects of chronic bilateral intrahippocampal cannulae on interleukin-1beta. J Neurosci Methods. 2007;161(2):265–272. doi: 10.1016/j.jneumeth.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak AM, Trivedi K, Harper CR, Booker K, Caesar RR, Agostini M, Van Ness PC, Diaz-Arrastia R. Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J Head Trauma Rehabil. 2004;19(4):290–295. doi: 10.1097/00001199-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol. 2009;215(2):243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Hirasawa K, Kinoshita M, Hitomi T, Matsumoto R, Mitsueda T, Taki J, Inouch M, Mikuni N, Hori T, et al. Negative motor seizure arising from the negative motor area: is it ictal apraxia? Epilepsia. 2009;50(9):2072–2084. doi: 10.1111/j.1528-1167.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- Inoue M, Peeters BW, van luijtelaar EL, Vossen JM, Coenen AM. Spontaneous occurrence of spike-wave discharges in five inbred strains of rats. Physiol Behav. 1990;48(1):199–201. doi: 10.1016/0031-9384(90)90285-c. [DOI] [PubMed] [Google Scholar]
- Ishige N, Pitts LH, Berry I, Carlson SG, Nishimura MC, Moseley ME, Weinstein PR. The effect of hypoxia on traumatic head injury in rats: alterations in neurologic function, brain edema, and cerebral blood flow. J Cereb Blood Flow Metab. 1987a;7(6):759–767. doi: 10.1038/jcbfm.1987.131. [DOI] [PubMed] [Google Scholar]
- Ishige N, Pitts LH, Hashimoto T, Nishimura MC, Bartkowski HM. Effect of hypoxia on traumatic brain injury in rats: part 1. Changes in neurological function, electroencephalograms, and histopathology. Neurosurgery. 1987b;20(6):848–853. doi: 10.1227/00006123-198706000-00005. [DOI] [PubMed] [Google Scholar]
- Jabbari B, Vengrow MI, Salazar AM, Harper MG, Smutok MA, Amin D. Clinical and radiological correlates of EEG in the late phase of head injury: a study of 515 Vietnam veterans. Electroencephalogr Clin Neurophysiol. 1986;64:285–293. doi: 10.1016/0013-4694(86)90151-3. [DOI] [PubMed] [Google Scholar]
- Jennett WB. Late epilepsy after blunt head injuries: a clinical study based on 282 cases of traumatic epilepsy. Ann R Coll Surg Engl. 1961;29:370–384. [PMC free article] [PubMed] [Google Scholar]
- Jennett WB, Lewin W. Traumatic epilepsy after closed head injuries. J Neurol Neurosurg Psychiatry. 1960;23:295–301. doi: 10.1136/jnnp.23.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst BC, Siegel AM, Thadani VM, Roberts DW, Rhodes HC, Williamson PD. Intractable seizures of frontal lobe origin: clinical characteristics, localizing signs, and results of surgery. Epilepsia. 2000;41(9):1139–1152. doi: 10.1111/j.1528-1157.2000.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Jyoti A, Sethi P, Sharma D. Aging accelerates the progression and manifestation of seizures in post-traumatic model of epilepsy. Neurosci Lett. 2009;453:86–91. doi: 10.1016/j.neulet.2009.01.082. [DOI] [PubMed] [Google Scholar]
- Kadam SD, White AM, Staley KJ, Dudek FE. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci. 2010;30(1):404–415. doi: 10.1523/JNEUROSCI.4093-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47(5):571–574. [PubMed] [Google Scholar]
- Kelly KM. Aging models of acute seizures and epilepsy. Epilepsy Curr. 2010;10(1):15–20. doi: 10.1111/j.1535-7511.2009.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerling F, Mueller S, Pauli E, Stefan H. When do patients forget their seizures? An electroclinical study. Epilepsy Behav. 2006;9(2):281–285. doi: 10.1016/j.yebeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Gröhn O, Pitkanen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain. 2007;130:3155–3168. doi: 10.1093/brain/awm268. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of post-traumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Klein PM, Rakhade S, Jensen FE. Hippocampal depth recordings from long evans rats subjected to neonatal seizures reveal development of spontaneous limbic seizures. Proceedings of the American Epilepsy Society Meeting. 2009 Dec 4–8; Boston (MA): Epilepsia. 2009;50(Suppl 11):385. [abstract] [Google Scholar]
- Langendorf F, Pedley TA. Post-traumatic seizures. In: Engel J, Pedley TA, editors. Epilepsy a comprehensive textbook. Philadelphia: Lippincott-Raven; pp. 2469–2474. [Google Scholar]
- Lee KR, Drudy I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. J Neurosurg. 1997;87:73–78. doi: 10.3171/jns.1997.87.1.0073. [DOI] [PubMed] [Google Scholar]
- Lendon CL, Harris JM, Pritchard AL, Nicoll JA, Teasdale GM, Murray G. Genetic variation of the APOE promoter and outcome after head injury. Neurology. 2003;61(5):683–685. doi: 10.1212/01.wnl.0000078033.81925.80. [DOI] [PubMed] [Google Scholar]
- Leppik IE. The classification of seizures. In: Leppik IE, editor. Contemporary diagnosis and management of the patient with epilepsy. Newtown (PA): Handbooks In Health Care; 1997. pp. 8–15. [Google Scholar]
- Levasseur JE, Patterson JL, Jr, Ghatak NR, Kontos HA, Choi SC. Combined effect of respirator-induced ventilation and superoxide dismutase in experimental brain injury. J Neurosurg. 1989;71(4):573–577. doi: 10.3171/jns.1989.71.4.0573. [DOI] [PubMed] [Google Scholar]
- Löscher W, Wahnschaffe U, Hönack D, Rundfeldt C. Does prolonged implantation of depth electrodes predispose the brain to kindling? Brain Res. 1995;697:197–204. doi: 10.1016/0006-8993(95)00843-f. [DOI] [PubMed] [Google Scholar]
- Lüders HO, Dinner DS, Morris HH, Wyllie E, Comair YG. Cortical electrical stimulation in humans. The negative motor areas. Adv Neurol. 1995;67:115–129. [PubMed] [Google Scholar]
- Lüders HO, Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J, Hahn JH. A negative motor response elicited by electrical stimulation of the human frontal cortex. Adv Neurol. 1992;57:149–157. [PubMed] [Google Scholar]
- Ludwig B, Marsan CA, Buren JV. Cerebral seizures of probable orbitofrontal origin. Epilepsia. 1975;16:141–158. doi: 10.1111/j.1528-1157.1975.tb04731.x. [DOI] [PubMed] [Google Scholar]
- Maegele M, Riess P, Sauerland S, Bouillon B, Hess S, McIntosh TK, Mautes A, Brockmann M, Koebke J, Knifka J, et al. Characterization of a new rat model of experimental combined neurotrauma. Shock. 2005;23(5):476–481. doi: 10.1097/01.shk.0000159929.87737.5c. [DOI] [PubMed] [Google Scholar]
- Maegele M, Sauerland S, Bouillon B, Schäfer U, Trübel H, Riess P, Neugebauer EA. Differential immunoresponses following experimental traumatic brain injury, bone fracture and “two-hit”-combined neurotrauma. Inflamm Res. 2007;56(8):318–323. doi: 10.1007/s00011-007-6141-3. [DOI] [PubMed] [Google Scholar]
- Maggio N, Shavit E, Chapman J, Segal M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: toward understanding the functional consequences of cerebrovascular insults. J Neurosci. 2008;28(3):732–736. doi: 10.1523/JNEUROSCI.3665-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DA, Kim J, Spencer DD, Spender SS. Seizure localization and pathology following head injury in patients with uncontrolled epilepsy. Neurology. 1995;45:2051–2057. doi: 10.1212/wnl.45.11.2051. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Hoffmann K, Schughart K, Löscher W. Differences in sensitivity to the convulsant pilocarpine in substrains and sublines of C57BL/6 mice. Genes Brain Behav. 2009;8(5):481–492. doi: 10.1111/j.1601-183X.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- Nathoo N, Chetty R, van Dellen JR, Barnett GH. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol. 2003;56:132–136. doi: 10.1136/mp.56.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Oztas B, Kaya M, Camurcu S. Age related changes in the effect of electroconvulsive shock on the blood brain barrier permeability in rats. Mech Ageing Dev. 1990;51:149–155. doi: 10.1016/0047-6374(90)90097-y. [DOI] [PubMed] [Google Scholar]
- Panter SS, Sadrzadeh SM, Hallaway PE, Haines JL, Anderson VE, Eaton JW. Hypohaptoglobinemia associated with familial epilepsy. J Exp Med. 1985;161:748–754. doi: 10.1084/jem.161.4.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Jasper HH. Epilepsy and the functional anatomy of the human brain. Boston (MA): Little, Brown; 1954. [Google Scholar]
- Pohlmann-Eden B, Bruckmeir J. Predictors and dynamics of posttraumatic epilepsy. Acta Neurol Scand. 1997;95(5):257–262. doi: 10.1111/j.1600-0404.1997.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen T. Characteristics of a pure culture of frontal lobe epilepsy. Epilepsia. 1983;24:482–493. doi: 10.1111/j.1528-1157.1983.tb04919.x. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh SM, Safari Y, Bozorgmehr J. Haptoglobin phenotypes in epilepsy. Clin Chem. 2004;50(6):1095–1097. doi: 10.1373/clinchem.2003.028001. [DOI] [PubMed] [Google Scholar]
- Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43:170–174. doi: 10.1046/j.1528-1157.2002.33800.x. [DOI] [PubMed] [Google Scholar]
- Salanova V, Morris HH, Van Ness P, Kotagal P, Wyllie E, Lüders H. Frontal lobe seizures: electroclinical syndromes. Epilepsia. 1995;36(1):16–24. doi: 10.1111/j.1528-1157.1995.tb01659.x. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam head injury study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Schwab K, Grafman JH. Penetrating injuries in the Vietnam war: traumatic unconsciousness, epilepsy, and psychosocial outcome. Neurosurg Clin N Am. 1995;6(4):715–726. [PubMed] [Google Scholar]
- Sano K, Nakamura N, Hirakawa K, Masuzawa H, Hashizume K, Hayashi T, Fujii S. Mechanism and dynamics of closed head injuries (preliminary report) Neurol Med Chir. 1967;9:21–33. doi: 10.2176/nmc.9.21. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Marmarou A. Evaluation of brain-stem dysfunction following severe fluid-percussion head injury to the cat. J Neurosurg. 1991;74:270–277. doi: 10.3171/jns.1991.74.2.0270. [DOI] [PubMed] [Google Scholar]
- Shinomoto S, Kim H, Shimokawa T, Matsuno N, Funahashi S, Shima K, Fujita I, Tamura H, Doi T, Kawano K, et al. Relating neuronal firing patterns to functional differentiation of cerebral cortex. PLoS Comput Biol. 2009;5(7):e1000433. doi: 10.1371/journal.pcbi.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So NK. Mesial frontal epilepsy. Epilepsia. 1998;39(Suppl 4):S49–S61. doi: 10.1111/j.1528-1157.1998.tb05125.x. [DOI] [PubMed] [Google Scholar]
- Sorbi S, Nacmias B, Piacentini S, Repice A, Latorraca S, Forleo P, Amaducci L. ApoE as a prognostic factor for post-traumatic coma. Nat Med. 1995;1(9):852. doi: 10.1038/nm0995-852. [DOI] [PubMed] [Google Scholar]
- Sutula TP. Mechanisms of epilepsy progression: current theories and perspectives from neuroplasticity in adulthood and development. Epilepsy Res. 2004;60:161–171. doi: 10.1016/j.eplepsyres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Swartz BE. Pseudo-absence seizures. A frontal lobe phenome. J Epilepsy. 1992;5:80–93. [Google Scholar]
- Tan AA, Quigley A, Smith DC, Hoane MR. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J Neurotrauma. 2009;26(4):539–548. doi: 10.1089/neu.2008.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44(Suppl 10):18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The joint section on neurotrauma and critical care. Resuscitation of blood pressure and oxygenation. J Neurotrauma. 2000;17(6–7):471–478. doi: 10.1089/neu.2000.17.471. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22(1):42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vink R, Mullins PGM, Temple MD, Bao W, Faden AI. Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. J Neurotrauma. 2001;18(8):839–847. doi: 10.1089/089771501316919201. [DOI] [PubMed] [Google Scholar]
- Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26(3):192–195. doi: 10.1016/s0887-8994(01)00380-0. [DOI] [PubMed] [Google Scholar]
- Walker AE. Post-traumatic epilepsy. World Neurol. 1962;3:185–194. [PubMed] [Google Scholar]
- Walker AE. Posttraumatic epilepsy. J Med Assoc of State of Ala. 1965;35(2):93–100. [PubMed] [Google Scholar]
- Walker AE, Blumer D. The fate of World War II veterans with posttraumatic seizures. Arch Neurol. 1989;46:23–26. doi: 10.1001/archneur.1989.00520370025013. [DOI] [PubMed] [Google Scholar]
- Weiss GH, Caveness WF. Prognostic factors in the persistence of posttraumatic epilepsy. J Neurosurg. 1972;37:164–169. doi: 10.3171/jns.1972.37.2.0164. [DOI] [PubMed] [Google Scholar]
- Williamson PD. Frontal lobe seizures. Problems of diagnosis and classification. Adv Neurol. 1992;57:289–309. [PubMed] [Google Scholar]
- Williamson PD, Spencer DD, Spencer SS, Novelly RA, Mattson RH. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985a;18:497–504. doi: 10.1002/ana.410180413. [DOI] [PubMed] [Google Scholar]
- Williamson PD, Spencer DD, Spencer SS, Novelly RA, Mattson RH. Complex partial status epilepticus: a depth-electrode study. Ann Neurol. 1985b;18:647–654. doi: 10.1002/ana.410180604. [DOI] [PubMed] [Google Scholar]
- Williamson PD, Spencer SS. Clinical and EEG features of complex partial seizures of extratemporal origin. Epilepsia. 1986;27(Suppl 2):S46–S63. doi: 10.1111/j.1528-1157.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- Williamson PD, Thadani VM, Darcey TM, Spencer DD, Spencer SS, Mattson RH. Occipital lobe epilepsy: clinical characteristics, seizure spread patterns, and results of surgery. Ann Neurol. 1992;31:3–13. doi: 10.1002/ana.410310103. [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Ueda Y. Posttraumatic epilepsy: hemorrhage, free radicals and the molecular regulation of glutamate. Neurochem Res. 2009;34:688–697. doi: 10.1007/s11064-008-9841-3. [DOI] [PubMed] [Google Scholar]
- Winawer MR, Kuperman R, Niethammer M, Sherman S, Rabinowitz D, Guell IP, Pondor CA, Palmer AA. Use of chromosome substitution strains to identify seizure susceptibility loci in mice. Mamm Genome. 2007;18(1):23–31. doi: 10.1007/s00335-006-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlrab G, Schmitt B, Boltshauser E. Benign focal epileptiform discharges in children after severe head trauma: prognostic value and clinical course. Epilepsia. 1997;38(3):275–278. doi: 10.1111/j.1528-1157.1997.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131(4):928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]