Abstract
Anxiety is linked to compromised interactions between the amygdala and the dorsal and ventral medial prefrontal cortex (mPFC). While numerous task-based neuroimaging studies show that anxiety levels predict amygdala–mPFC connectivity and response magnitude, here we tested the hypothesis that anxiety would predict functional connectivity between these brain regions even during rest. Resting-state functional magnetic resonance imaging scans and self-reported measures of anxiety were acquired from healthy subjects. At rest, individuals with high anxiety were characterized by negatively correlated amygdala–ventral mPFC functional connectivity, while low anxious subjects showed positively correlated activity. Further, high anxious subjects showed amygdala–dorsal mPFC activity that was uncorrelated, while low anxious subjects showed negatively correlated activity. These data show that amygdala–mPFC connectivity at rest indexes normal individual differences in anxiety.
Keywords: amygdala, anxiety, functional connectivity, medial prefrontal cortex, resting-state fMRI
Introduction
Recent work has demonstrated the usefulness of conceptualizing anxiety as a sustained psychological state, in contrast to fear that is usefully compared with anxiety as a transient reaction to a specific environmental event (Lang et al. 2000; Walker et al. 2003; Hasler et al. 2007; Somerville et al., forthcoming). Based on this characteristic feature of anxiety, we sought to determine if differences in functional brain connectivity would vary with individual differences in reported anxiety even during rest. We focused our study on the amygdala and medial prefrontal cortex (mPFC), given the numerous task-based functional neuroimaging investigations showing that anxiety levels predict the magnitude of responses to presented stimuli in both the amygdala (Bishop, Duncan and Lawrence 2004; Etkin et al. 2004; Somerville et al. 2004; Dickie and Armony 2008; Carlson et al. 2010) and the mPFC (Simpson et al. 2001; Bishop, Duncan, Brett et al. 2004; Bishop 2007; Hare et al. 2008; Simmons et al. 2008; Straube et al. 2009). These studies have generally adopted a framework that assumes the mPFC functions to actively regulate the amygdala (Hariri et al. 2003; Kim et al. 2003; Pezawas et al. 2005; Bishop 2007; Hare et al. 2008). This framework is supported by functional connectivity data showing that amygdala–mPFC coupling is inversely correlated with self-reported measures of anxiety or anxious temperament (i.e., greater connectivity predicts lower anxiety; e.g., Pezawas et al. 2005).
Findings from task-based studies of anxiety can be roughly divided into 2 subregions of the mPFC, relative to the genu of the corpus callosum—dorsal medial prefrontal cortex (dmPFC) and ventral medial prefrontal cortex (vmPFC). Here, we broadly define the dmPFC as including the supragenual anterior cingulate and the medial frontal gyrus, whereas the vmPFC includes the subgenual anterior cingulate and parts of the medial orbitofrontal cortex. Though results can differ depending on the task used (for review, see Bishop 2007), a consistent finding across these studies is that higher levels of anxiety are associated with both attenuated vmPFC activity and exaggerated dmPFC activity (Simmons et al. 2008; Straube et al. 2009).
What remains to be determined is how amygdala–prefrontal interactions relate to anxiety in the absence of presented stimuli—that is, at rest. Task-independent brain activity can be investigated using functional magnetic resonance imaging (fMRI) by measuring spontaneous, slow (<0.1 Hz) fluctuations in the brain that occur across time at rest (Biswal et al. 1995; see Fox and Raichle 2007 for review). Over the past few years, resting-state fMRI studies have offered important findings such as the delineation of resting-state functional networks (Fox et al. 2006; Vincent et al. 2008; Damoiseaux and Greicius 2009; Smith et al. 2009) that have proven to be reliable over time (Shehzad et al. 2009). One useful strategy is to use the resting activity of one brain region of interest (ROI) to identify other brain regions that are functionally connected (i.e., seed-based approach). For example, investigators have mapped highly detailed resting-state functional networks of the anterior cingulate (Margulies et al. 2007) and the striatum (Di Martino et al. 2008). Recent studies have demonstrated the utility for using resting state fMRI data to predict behavioral outcomes (Kelly et al. 2008; Wig et al. 2009).
In a recent investigation, Roy et al. (2009) used a seed-based approach to show that, at rest, fluctuations in amygdala activity are positively coupled with the vmPFC but negatively coupled with the dmPFC. Given the free-floating nature of anxiety discussed above, here we aimed to determine whether individual differences in normal anxiety levels are related to the “resting” functional connectivity between the amygdala and the mPFC—specifically, whether higher levels of anxiety compromise resting connectivity. Such data would inform the very baseline upon which task-based investigations of normal and pathological anxiety are conducted.
Materials and Methods
Subjects
Twenty-nine healthy volunteers (21 women; 19.6 ± 0.9 years of age; 28 right-handed) were recruited for the current study from 2 separate fMRI experiments (10 subjects from one and 19 subjects from the other). Of note, the latter 19 subjects’ resting-state fMRI scans were acquired after a task-based fMRI session, which followed the experimental procedure detailed in our previous study (Kim et al. 2010). All subjects were screened for current or past psychiatric illness using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV (First et al. 1997). No subject had taken psychotropic medications. Handedness was determined with the Edinburgh Handedness Inventory (Oldfield 1971). After the fMRI scanning sessions, each subject's anxiety level was assessed with the State Trait Anxiety Inventory Form Y (STAI-S, STAI-T; Spielberger et al. 1988) self-report questionnaires. In addition, the subjects completed the Beck Depression Inventory (BDI; Beck et al. 1961). The study protocol was approved by the Committee for the Protection of Human Subjects at Dartmouth College. Prior to the experiment, written, informed consent was obtained from all the subjects.
Image Acquisition
All subjects were scanned using a 3.0-T Philips Intera Achieva Scanner (Philips Medical Systems) equipped with a SENSE birdcage head coil. Anatomical T1-weighted images were collected using a high-resolution 3D magnetization-prepared rapid gradient echo sequence, with 160 contiguous 1-mm thick sagittal slices (echo time [TE] = 4.6 ms, repetition time [TR] = 9.8 ms, field of view [FOV] = 240 mm, flip angle = 8°, voxel size = 1 × 0.94 × 0.94 mm). Functional images were acquired using echo-planar -weighted imaging sequence. Each volume consisted of 36 interleaved 3-mm thick slices with 0.5-mm interslice gap (TE = 35 ms, TR = 2000 ms, FOV = 240 mm, flip angle = 90°, voxel size = 3 × 3 × 3.5 mm). During this 8-min long functional scan, subjects were asked to keep their eyes open and not to fall asleep while looking at the word “relax” on a back-projected screen.
fMRI Data Preprocessing
Following the procedure described by Roy et al. (2009), all fMRI data were preprocessed using the Analysis of Functional NeuroImages (AFNI; Cox 1996) and the FMRIB Software Library (FSL; Smith et al. 2004) tools. Using AFNI, raw functional images were first corrected for slice timing and then for head movement. None of the subjects included in the analysis had head movement exceeding 1.5 mm in any direction. Extreme outliers in the time series data were removed from the analysis. The resulting images were preprocessed with FSL, starting with spatial smoothing using a Gaussian kernel of 6 mm full-width at half-maximum. Images were further preprocessed using mean-based intensity normalization by the same factor and then applying temporal high-pass filter (Gaussian-weighted least squares straight line fitting with sigma = 100 s) and low-pass filter (Gaussian filter with half width at half maximum =2.8 s) to the data. These images were prewhitened to correct for time series autocorrelation. Functional and anatomical images were normalized into standard space using the Montreal Neurological Institute (MNI)-152 template. Anatomical images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) in order to use them as masks to extract time series data for each subject.
Seed-Based Functional Connectivity Analysis
An anatomical ROI of the amygdala was derived using the Harvard–Oxford probabilistic atlas that was included with FSL. We defined our ROI to only include voxels that had 50% or higher probability of being labeled as the amygdala (left: 1816 mm3, right: 2224 mm3; Fig. 1). All subsequent analyses were performed separately for the left and right amygdala. For each subject, mean time series data extracted from the amygdala ROI were used as a predictor (i.e., “seed”) in a general linear model to identify voxels that were significantly correlated with amygdala activity across time. In addition, time series data for 9 covariates of no interest (global signal, white matter, CSF, and 6 motion parameters for head movement) were computed and entered in the general linear model. To ensure that the results were due to variance unique to the seed regions, mean amygdala time series data were orthogonalized with respect to the 9 covariates of no interest. This analysis yielded subject-level statistical maps showing voxels that were positively or negatively correlated with mean amygdala time series. These maps were then entered into a group-level mixed-effects model, controlling for the effects of age, gender, and study group (i.e., when the resting-state fMRI scans were acquired relative to the task-based fMRI scans). This analysis generated group-level functional connectivity maps illustrating brain areas whose activity was positively or negatively correlated with amygdala activity during rest. Finally, in order to generate group-level statistical maps showing how anxiety modulates functional connectivity between the amygdala and other brain regions, a general linear model was constructed with anxiety scores as the predictor, and age, gender, and study group as covariates of no interest. Analyses for state and trait anxiety were carried out separately. For all statistical analyses, cluster-based correction for multiple comparisons implemented in FSL was used to determine the significance threshold (Z > 2.3, P < 0.05, corrected for multiple comparisons across the whole brain).
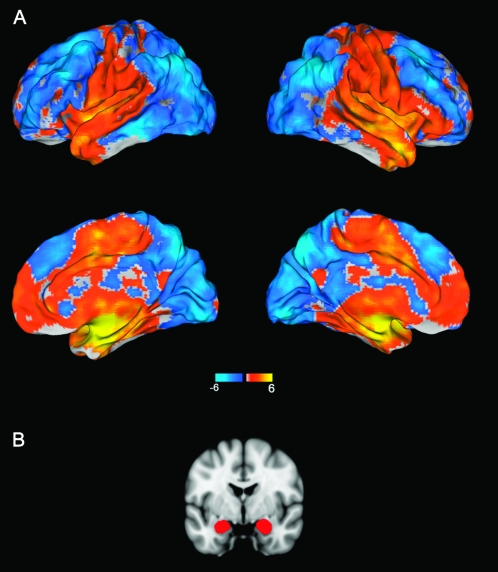
Figure 1.
(A) Resting-state functional connectivity of the amygdala with the rest of the brain across all 29 subjects. Of note, amygdala activity was positively correlated with vmPFC and negatively correlated with dmPFC. (B) Coronal slice of the brain depicting the amygdala regions that were used as seeds in the functional connectivity analyses.
Results
Subject Characteristics
All subjects had self-report scores for anxiety and depression within the normal range (mean ± SD: STAI-S = 32.97 ± 7.84; STAI-T = 36.28 ± 8.05; BDI = 3.83 ± 3.84). There were no significant differences in any of the self-report measures between males and females. Also, no significant differences in age, gender, or any of the self-reported measures were observed between the subjects who had their resting-state fMRI scans after task-based fMRI scans (n = 19) versus those who had them before a task (n = 10).
Amygdala Connectivity during Rest
Consistent with the findings of Roy et al. (2009), amygdala activity at rest was positively correlated with activity in ventral mPFC regions, including the medial frontal gyrus and the anterior cingulate cortex. Conversely, amygdala activity was negatively correlated with activity in dorsal mPFC regions including the supragenual anterior cingulate and the middle frontal gyrus (Fig. 1). Here, we focus on the mPFC, but other regions whose activity correlated with resting amygdala activity are presented in Supplementary Tables 1 and 2. There were no significant differences in the amygdala functional connectivity map between the subjects who had their resting-state fMRI scans after the task-based fMRI scans versus those who had not.
Amygdala Connectivity Predicted by Anxiety
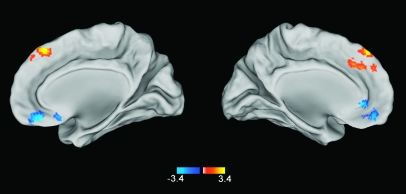
Across the whole brain, anxiety scores predicted resting-state functional connectivity between the amygdala and only 2 regions—the dmPFC and vmPFC. Resting-state functional connectivity between the right amygdala and the dmPFC was positively correlated with state anxiety (MNI 0, 32, 36; Z = 4.6, P < 0.05 corrected, cluster size = 5464 mm3; Fig. 2 and Supplementary Fig. 1). Put another way, the negative correlation between amygdala and dmPFC activity observed in all subjects was preserved in subjects with lower levels of state anxiety, but this pattern of connectivity broke down in subjects with higher levels of state anxiety. Conversely, resting-state functional connectivity between the right amygdala and the vmPFC (MNI 10, 40, −18; Z = 4.11, P < 0.05 corrected, cluster size = 5,368 mm3) was negatively correlated with state anxiety (Fig. 2 and Supplementary Fig. 2). That is, the positive relationship between activity in the amygdala and vmPFC observed in all subjects was preserved in subjects with lower levels of state anxiety but was compromised in subjects with higher levels of state anxiety. We note that this vmPFC cluster also extended into lateral portions of the ventral PFC (MNI 24, 54, −12).
Figure 2.
Statistical map showing how the strength of the amygdala functional connectivity is correlated with state anxiety across all 29 subjects. Across the whole brain, the strength of amygdala–vmPFC functional connectivity was negatively correlated with state anxiety. Conversely, the strength of amygdala–dmPFC functional connectivity was positively correlated with state anxiety.
This dissociation was also observed when trait anxiety was entered into the model as a predictor of functional connectivity between the right amygdala and dmPFC and vmPFC, albeit at a more liberal threshold (Z > 2.3, uncorrected). When the left amygdala was used as a seed, only the positive correlation between amygdala–dmPFC functional connectivity and anxiety measures (both state and trait) was observed, also at a liberal threshold (Z > 2.3, uncorrected).
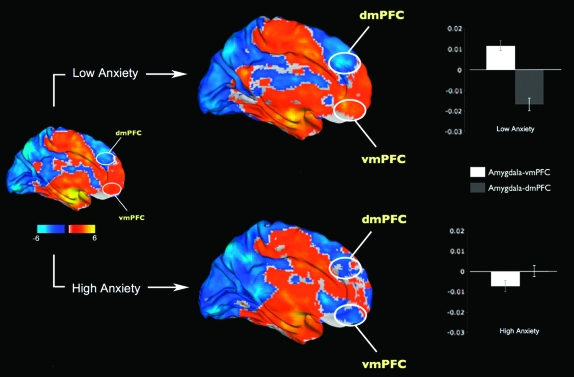
Based on these findings, we evaluated the directionality (i.e., positive or negative nature) of the functional connectivity values by dividing the subjects into high and low state anxiety groups using a median split (median STAI-S = 33). There were no significant differences in age or gender between the low and high anxiety groups. Results showed that, in the voxel clusters identified in the previous analysis (Fig. 2), the low anxiety group showed the expected negative functional connectivity between the amygdala and dmPFC (t(13) = −5.51, P < 0.001), whereas the high anxiety group did not show this relationship (t(14) = 0.15, P = 0.88; Fig. 3). Furthermore, whereas the low anxiety group showed the expected significant positive connectivity between activity in the amygdala and vmPFC (t(13) = 4.89, P < 0.001), the high anxiety group showed the opposite relationship where activity in the amygdala and vmPFC was negatively correlated (t(14) = −2.62, P = 0.02; Fig. 3).
Figure 3.
Resting-state functional connectivity of the amygdala with the rest of the brain, divided into high (N = 15) and low (N = 14) anxiety groups. The low anxiety group is characterized by a strong positive amygdala–vmPFC connectivity and negative amygdala–dmPFC connectivity. In contrast, the high anxiety group showed negative amygdala–vmPFC connectivity and disrupted amygdala–dmPFC connectivity. The white ovals depict approximate locations of the voxel clusters shown in Figure 2. Error bars indicate the standard error of the mean.
Discussion
In the current study, we demonstrated that the resting functional connectivity between the amygdala and mPFC varies as a function of self-reported anxiety. Across the whole brain, anxiety levels exclusively predicted functional connectivity between the amygdala and mPFC. Specifically, we observed a dissociation in functional connectivity between the amygdala and dorsal and ventral mPFC with respect to anxiety—that is, the negative connectivity normally seen between the amygdala and the dmPFC at rest was attenuated in high anxious subjects, whereas the positive connectivity normally observed between the amygdala and vmPFC at rest, manifested as negative connectivity in high anxious subjects. Importantly, this relationship was observed in the absence of external stimulus presentations.
Previous task-based neuroimaging studies have demonstrated that individual differences in anxiety levels predict amygdala responsivity (Bishop, Duncan and Lawrence 2004; Etkin et al. 2004; Somerville et al. 2004; Pezawas et al. 2005; Dickie and Armony 2008). For example, exaggerated amygdala responses to fearful faces have been associated with increased state (Bishop, Duncan and Lawrence 2004) as well as trait (Etkin et al. 2004) anxiety. A positron emission tomography (PET) study showed increased amygdala activity to cues that provoke anxiety by predicting threat (e.g., electric shocks; Hasler et al. 2007). Interestingly, this relationship was observed even when the stimuli used to evoke amygdala activity were unrelated to threat. For instance, Somerville et al. (2004) reported that higher levels of state anxiety were associated with increased amygdala activity to neutral faces. These data suggest that a relationship between amygdala activity and anxiety need not be examined in a negative experimental context (e.g., threat-related stimuli) but can be generalized to other, nonthreatening, experimental contexts. The present data extend this logic to show that a systematic relationship between amygdala activity and anxiety can also be observed at rest.
Task-based neuroimaging studies have also demonstrated that anxiety levels predict mPFC responses to different types of stimuli, with slightly mixed results (Simpson et al. 2001; Bishop, Duncan, Brett et al. 2004; Simmons et al. 2008; Straube et al. 2009). For example, higher levels of state anxiety were associated with “decreased” mPFC activity in response to unattended fearful faces in one study (Bishop, Duncan, Brett, and Lawrence 2004), whereas higher levels of anxiety were accompanied by “increased” activity in a different subregion of the mPFC to cues predicting electric shock in another study (Simpson et al. 2001). Recent investigations have shown that different subregions of the mPFC may be differentially related to anxiety—for example, during the anticipation of threat (e.g., electric shocks), activity in the dmPFC was positively correlated with anxiety whereas vmPFC activity was negatively correlated with anxiety (Straube et al. 2009). Another study reported that highly anxious subjects showed increased dmPFC activity and decreased vmPFC activity during a task that involved the viewing of angry and happy faces (Simmons et al. 2008). Collectively, these findings suggest that the dorsal and ventral regions of the mPFC may play different, if not opposing roles in anxiety and prompt further investigation into the relationship between anxiety and interactions between these brain regions and the amygdala.
There are extensive anatomical connections between the amygdala and the dmPFC/vmPFC (Aggleton et al. 1980; Pandya et al. 1981; Amaral et al. 1992; Ghashghaei et al. 2007), supporting their purported reciprocal interactions during emotional processing (Ochsner et al. 2002; Kim et al. 2004; van Reekum et al. 2007; Bishop 2008; Wager et al. 2008; Urry et al. 2009) and fear conditioning and extinction (LeDoux 2000; Davis and Whalen 2001; Milad and Quirk 2002; Phelps et al. 2004). When interpreting task-based mPFC responses to presented stimuli and their relation to anxiety, it would be important to consider the present data showing that the amygdala is differentially coupled with dmPFC and vmPFC circuitry as a function of anxiety at rest. For instance, in a study where one wanted to test the hypothesis that amygdala–vmPFC functional connectivity is strengthened during an emotion regulation task compared with a passive viewing task, individuals showing stronger positive amygdala–vmPFC functional connectivity at rest would be more sensitive to revealing a task-based change.
We observed that the typical positive correlation between amygdala–vmPFC functional connectivity at rest (Roy et al. 2009) is compromised in high anxious, psychiatrically healthy subjects. This result complements the findings of Pezawas et al. (2005), who demonstrated that stronger amygdala–vmPFC connectivity during the viewing of angry and fearful faces was associated with lower levels of anxious temperament. In our previous study using DTI, we observed that a stronger structural integrity in a pathway linking the amygdala and vmPFC also predicted lower anxiety levels (Kim and Whalen 2009). Combined with animal studies highlighting the importance of this circuitry in fear extinction (Milad and Quirk 2002), these findings suggest that a more coherent amygdala–vmPFC connectivity predicts a healthier behavioral outcome (i.e., lower anxiety). Findings from the current study further extend this notion by demonstrating that spontaneous fluctuations in the activity of the amygdala at rest positively correlate with fluctuations in the vmPFC in individuals with lower levels of anxiety. Perhaps positive functional connectivity between the amygdala–vmPFC circuitry (and concomitant negative connectivity with dmPFC) during rest represents an efficient amygdala–mPFC cross talk, which may mitigate the generation of anxious states. This amygdala–mPFC relationship is disrupted in individuals with high levels of anxiety who show a negative relationship between amygdala and vmPFC activity (and no relationship between amygdala and dmPFC activity).
Our data also demonstrated a positive correlation between amygdala–dmPFC functional connectivity during rest and anxiety. A prior resting-state fMRI study (Seeley et al. 2007) used an independent component analysis approach to define a “salience network” of the brain, which largely consisted of limbic regions, including parts of the amygdala, dmPFC, and the insula. Importantly, this study reported a strong positive correlation between anxiety levels and the strength of the functional connectivity between the dmPFC and the salience network. Our data show a similar positive correlation between dmPFC–amygdala functional connectivity and anxiety, related to the specific amygdala seed-based approach we employed. Moreover, our data suggest that resting negative functional connectivity between the amygdala and the dmPFC may be a healthy, canonical pattern and that individuals with higher levels of anxiety fail to engage the amygdala–dmPFC circuitry during rest. This interpretation of these task-independent data is consistent with a previous suggestion based on task-based studies showing that inhibitory feedback from the dmPFC serves to suppress the anxiogenic effects of amygdala activity (Pezawas et al. 2005; Hariri and Holmes 2006).
These data in healthy, nonpathological subjects may have implications for the study of psychiatric disorders. Anxiety disorders, particularly posttraumatic stress disorder (PTSD), have been characterized by a hyperactive amygdala, hypoactive vmPFC, and hyperactive dmPFC (see Shin and Handwerger 2009 for review). Specifically, individual differences in the degree to which the vmPFC is recruited and, in turn, how reactive the amygdala remains predicts symptom severity in PTSD (Shin et al. 2005; Rauch et al. 2006). Also, a PET investigation has shown that exaggerated dmPFC activity at rest predicts a greater likelihood of developing PTSD after being exposed to psychological trauma (Shin et al. 2009). Further, recent reports have documented abnormalities in other resting-state brain circuitries in generalized anxiety disorder (Etkin et al. 2009) and obsessive–compulsive disorder (Harrison et al. 2009). Although our data link resting-state amygdala–mPFC functional connectivity with self-reported anxiety levels within the normal range, they might inform investigations of amygdala–mPFC resting-state data in the anxiety disorders. Perhaps, they could specifically aid in interpreting amygdala–prefrontal connectivity in a control group that is to be compared with a patient group. Specifically, the resting baseline connectivity in healthy and pathological groups could be measured in addition to their responses during specific tasks, and the resting data could be used to explain a portion of the variability observed within the task-based responses.
As documented by the current literature on resting-state functional connectivity, the nature of negative functional connectivity remains unclear. While some research has suggested that negative correlations are artifacts of global signal regression (Murphy et al. 2009), recent work indicates that negative correlations have a biological, instead of artifactual basis (Fox et al. 2009). That said, since we chose to regress out the effect of global signal from our data to remove the effects of physiological noise, we are cautious in making strong interpretations of the observed negative functional connectivity between the amygdala and mPFC regions.
In the present study, the dissociation between amygdala–vmPFC and dmPFC connectivity was observed most robustly in relation to state anxiety. The fact that our brain data were more prominently associated with state anxiety relative to trait anxiety raises the possibility that the observed results may be due to the uniqueness of the scanning environment. That is, perhaps the experimental environment itself (i.e., being inside a loud MRI scanner in the dark) may be anxiogenic, affecting state anxiety levels, rather than trait anxiety. Importantly, there is empirical evidence showing that scanning without the presentation of any external stimuli could evoke a different amount of anxiety in each subject (Heinz et al. 2007).
However, we point out that both state and trait anxiety measures were highly correlated from our study sample (r = 0.81, P < 0.0001) and using trait anxiety also produced a similar dissociation between amygdala–vmPFC and dmPFC connectivity at a lower threshold. Thus, we are careful not to make strong claims that our data solely reflects state anxiety as opposed to trait anxiety. Further, a previous study from our laboratory (Kim and Whalen 2009) documented a correlation between trait anxiety and the structural integrity of a white matter pathway between the amygdala and prefrontal cortex (with state anxiety showing the trend toward significance). It is interesting to speculate that perhaps the nonspecific “resting” nature of the present study lent itself to be more relevant to state anxiety, while our previous study assessing more static structural measurements was more readily correlated with trait anxiety. Given that numerous previous functional and structural neuroimaging studies have differed in finding relationships with state (present report; Bishop, Duncan, and Lawrence 2004, 2007) or trait (Etkin et al. 2004; Dickie and Armony 2008; Kim and Whalen 2009; Carlson et al. 2010) anxiety, future studies will be needed to more carefully assess these highly correlated constructs.
One limitation of the current study is that our subjects were mostly women. To address this issue, we have verified that there were no statistically significant differences in terms of self-reported and functional connectivity measures between the 2 genders. We attempted to further control for these effects by removing variances that could be explained by gender from all of our analyses using general linear models. Even so, in order to truly test for potential between-gender differences in amygdala–mPFC functional connectivity measures, future studies should be designed to include equal number of men and women. Given that there are reports showing that the interaction between gender and anxiety is represented in the brain as differential patterns of amygdala activity (Dickie and Armony 2008), it would be interesting to investigate the potential effects of gender on the relationship between anxiety and amygdala–mPFC functional connectivity during rest.
Taken together, the findings from the current study show that individual differences in anxiety are reflected in the strength of amygdala–mPFC functional connectivity during rest. A closer investigation revealed a dissociation between vmPFC and dmPFC resting functional connectivity with respect to anxiety—individuals with high anxiety showed fluctuations in amygdala activity that were negatively correlated with vmPFC activity and unrelated to dmPFC activity. This altered pattern was observed in the absence of presented anxiety-inducing stimuli, but we note the potential importance of the imaging environment itself. Given that such an environment constitutes the baseline for neuroimaging studies of healthy and pathological anxiety, the present resting-state data strategy could be used as an adjunct to task-based studies to explain response variability in response to presented stimuli.
Supplementary Material
Supplementary Tables 1 and 2 and Figures 1 and 2 can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institute of Mental Health (R01 MH080716 to P.J.W. and F31 MH090672 to M.J.K.).
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss, Inc; 1992. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Ann N Y Acad Sci. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq017. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: interaction between sex and trait anxiety. Psychiatry Res. 2008;162:51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview (SCID) for DSM-IV axis 1 disorders. Washington (DC): American Psychiatric Association; 1997. [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, Mann K, Schumann G, Buchel C, Hariri AR, et al. Serotonin transporter genotype (5-HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry. 2007;61:1011–1014. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Neta M, Davis FC, Oler JA, Mazzulla EC, Whalen PJ. Behind the mask: the influence of mask-type on amygdala response to fearful faces. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq014. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress. 2009 doi: 10.1002/jts.20442. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Feinstein JS, Hitchcock C, Paulus MP, Stein MB. Anxiety vulnerability is associated with altered anterior cingulate response to an affective appraisal task. Neuroreport. 2008;19:1033–1037. doi: 10.1097/WNR.0b013e328305b722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Jr., Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM 2010. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI-Manual for the State Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1988. [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Schaefer HS, Alexander AL, Davidson RJ. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. J Cogn Neurosci. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.