Abstract
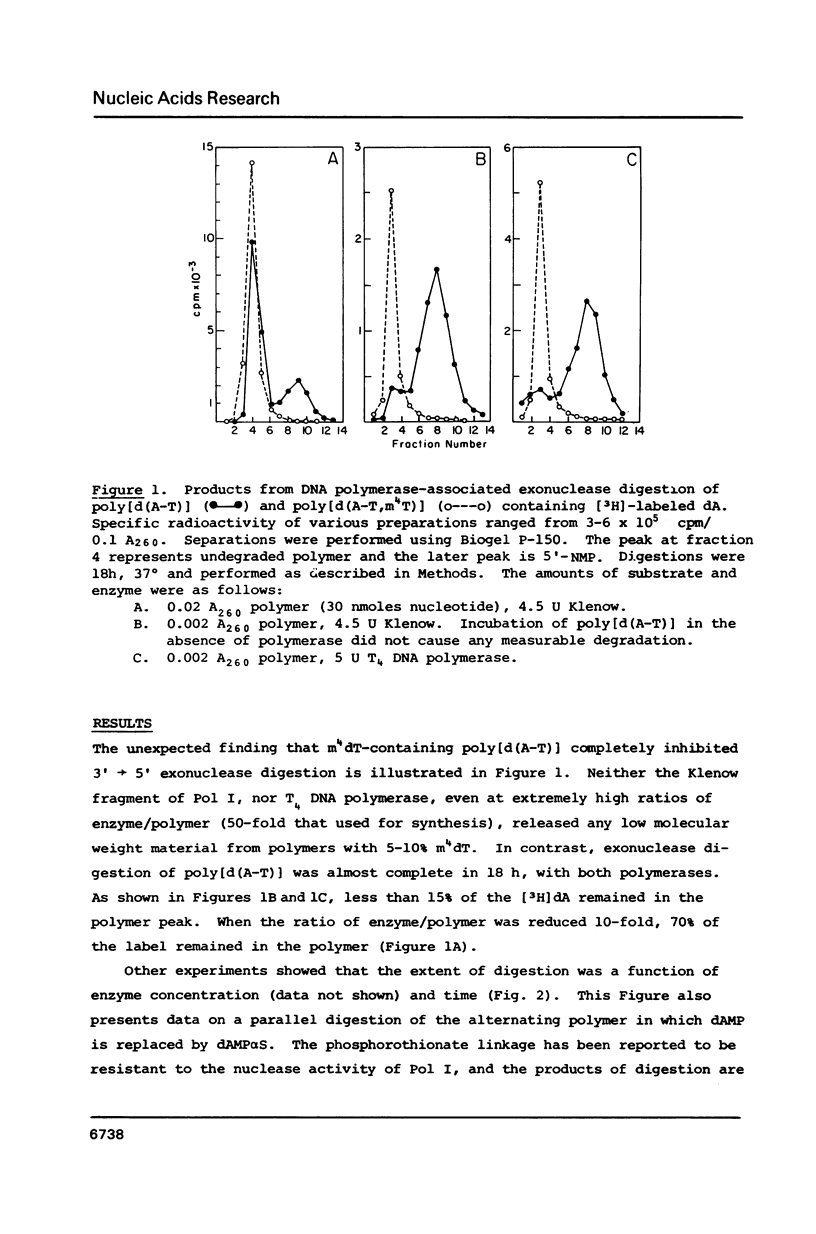
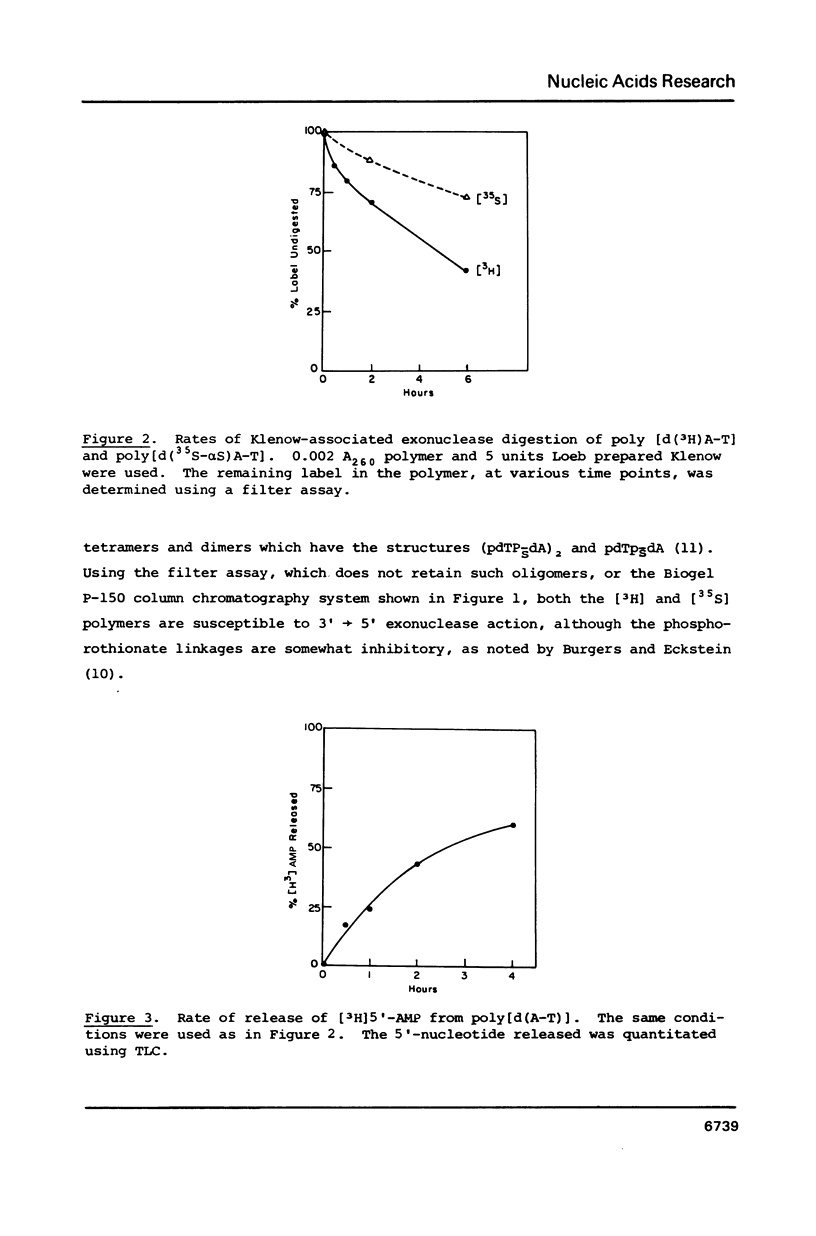
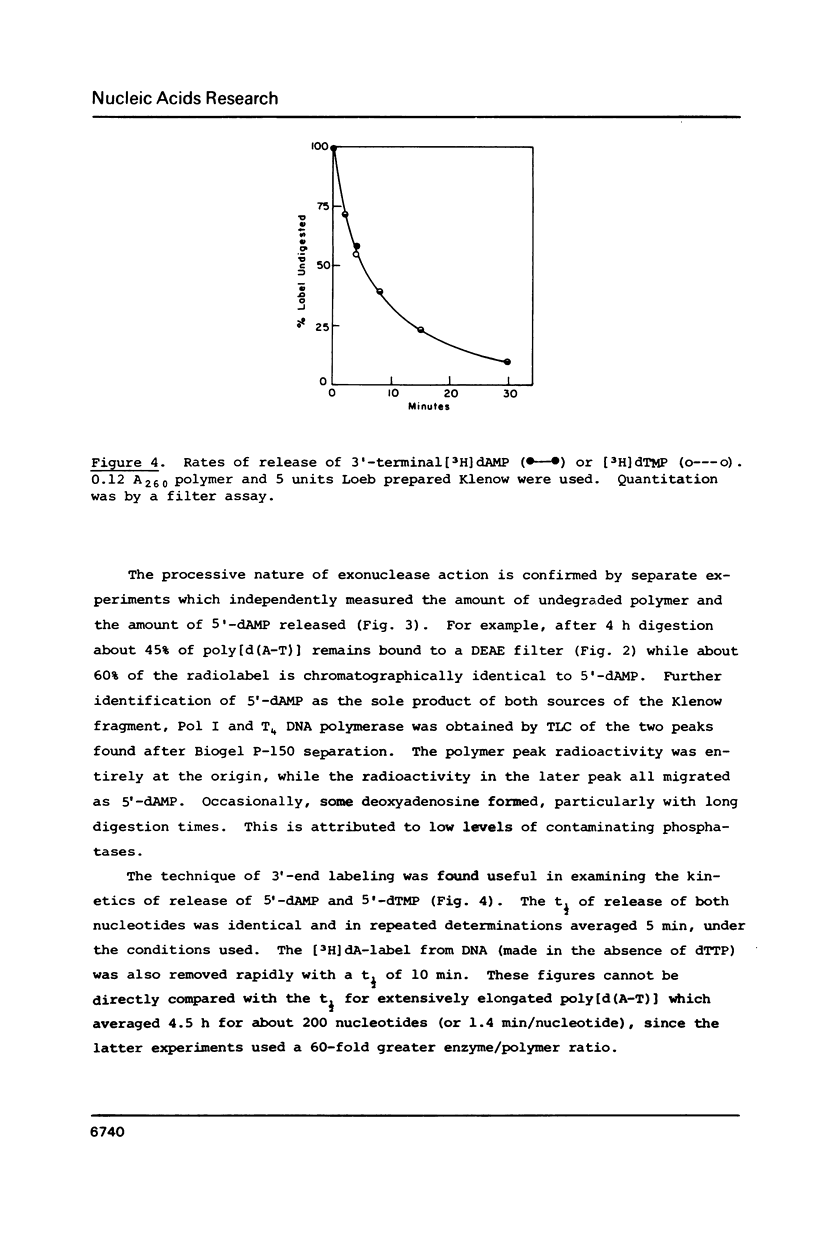
We previously reported that O4-alkyl dTTPs could replace, for short times, dTTP in polymer synthesis [Singer et al., PNAS 83, 26-32, 1986]. The reasons for such early termination of synthesis could be either proofreading or the eventual formation of weakly paired primer termini. Utilizing the known 3'----5' exonucleolytic activity of polymerases, in the absence of dNTPs, enabled us to conclude that, in contrast to the digestibility of poly[d(A-T)] which yielded the expected 3'-mononucleotides, the polymerizing enzymes did not digest O4-methyl dT or its neighbors. The presence of the resistant alpha-phosphorothionate linkage did not prevent measurable digestion of poly[d(A-T)] by the Klenow fragment. This, together with evidence that polymerization of O4-methyl dTTP is favored at low temperatures, supports the model proposed by Ollis et al. [Nature 313, 762-766, 1985] showing independent domains for the two activities in the Klenow fragment.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody R. S., Frey P. A. Unambiguous determination of the stereochemistry of nucleotidyl transfer catalyzed by DNA polymerase I from Escherichia coli. Biochemistry. 1981 Mar 3;20(5):1245–1252. doi: 10.1021/bi00508a030. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. A study of the mechanism of DNA polymerase I from Escherichia coli with diastereomeric phosphorothioate analogs of deoxyadenosine triphosphate. J Biol Chem. 1979 Aug 10;254(15):6889–6893. [PubMed] [Google Scholar]
- Dyroff M. C., Richardson F. C., Popp J. A., Bedell M. A., Swenberg J. A. Correlation of O4-ethyldeoxythymidine accumulation, hepatic initiation and hepatocellular carcinoma induction in rats continuously administered diethylnitrosamine. Carcinogenesis. 1986 Feb;7(2):241–246. doi: 10.1093/carcin/7.2.241. [DOI] [PubMed] [Google Scholar]
- Gupta A. P., Benkovic P. A., Benkovic S. J. The effect of the 3',5' thiophosphoryl linkage on the exonuclease activities of T4 polymerase and the Klenow fragment. Nucleic Acids Res. 1984 Jul 25;12(14):5897–5911. doi: 10.1093/nar/12.14.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes F. N., Hansbury E., Mitchell V. E., Ratliff R. L., Williams D. L. Synthesis of N-acetylated deoxyribonucleoside 5'-triphosphates and their utilization in enzymatic formation of single-stranded polydeoxyribonucleotides. Eur J Biochem. 1968 Dec 5;6(4):485–492. doi: 10.1111/j.1432-1033.1968.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Eckstein F., Mildvan A. S., Koplitz R. M., Loeb L. A. Deoxynucleoside [1-thio]triphosphates prevent proofreading during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6734–6738. doi: 10.1073/pnas.78.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Mizrahi V., Benkovic P. A., Benkovic S. J. Mechanism of the idling-turnover reaction of the large (Klenow) fragment of Escherichia coli DNA polymerase I. Proc Natl Acad Sci U S A. 1986 Jan;83(2):231–235. doi: 10.1073/pnas.83.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor P. J., Salisbury J. G., Margison G. P. Resistance of alkylated DNA to degradation by deoxyribonuclease II at neutral and acid pH. Chem Biol Interact. 1976 Aug;14(3-4):313–323. doi: 10.1016/0009-2797(76)90110-1. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Richardson F. C., Dyroff M. C., Boucheron J. A., Swenberg J. A. Differential repair of O4-alkylthymidine following exposure to methylating and ethylating hepatocarcinogens. Carcinogenesis. 1985 Apr;6(4):625–629. doi: 10.1093/carcin/6.4.625. [DOI] [PubMed] [Google Scholar]
- Saffhill R. In vitro reaction of N-n-butyl-N-nitrosourea and n-butyl methanesulphonate with guanine and thymine bases of DNA. Carcinogenesis. 1984 May;5(5):621–625. doi: 10.1093/carcin/5.5.621. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Singer B., Abbott L. G., Spengler S. J. Assessment of mutagenic efficiency of two carcinogen-modified nucleosides, 1,N6-ethenodeoxyadenosine and O4-methyldeoxythymidine, using polymerases of varying fidelity. Carcinogenesis. 1984 Sep;5(9):1165–1171. doi: 10.1093/carcin/5.9.1165. [DOI] [PubMed] [Google Scholar]
- Singer B., Chavez F., Spengler S. J. O4-Methyl-, O4-ethyl-, and O4-isopropylthymidine 5'-triphosphates as analogues of thymidine 5'-triphosphate: kinetics of incorporation by Escherichia coli DNA polymerase I. Biochemistry. 1986 Mar 25;25(6):1201–1205. doi: 10.1021/bi00354a001. [DOI] [PubMed] [Google Scholar]
- Singer B., Pergolizzi R. G., Grunberger D. Synthesis and coding properties of dinucleoside diphosphates containing alky pyrimidines which are formed by the action of carcinogens on nucleic acids. Nucleic Acids Res. 1979 Apr;6(4):1709–1719. doi: 10.1093/nar/6.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Spengler S. J., Fraenkel-Conrat H., Kuśmierek J. T. O4-Methyl, -ethyl, or -isopropyl substituents on thymidine in poly(dA-dT) all lead to transitions upon replication. Proc Natl Acad Sci U S A. 1986 Jan;83(1):28–32. doi: 10.1073/pnas.83.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Spengler S., Bodell W. J. Tissue-dependent enzyme-mediated repair or removal of O-ethyl pyrimidines and ethyl purines in carcinogen-treated rats. Carcinogenesis. 1981;2(10):1069–1073. doi: 10.1093/carcin/2.10.1069. [DOI] [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow E. T., Foote R. S., Mitra S. Kinetics of incorporation of O6-methyldeoxyguanosine monophosphate during in vitro DNA synthesis. Biochemistry. 1984 Sep 11;23(19):4289–4294. doi: 10.1021/bi00314a006. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Dyroff M. C., Bedell M. A., Popp J. A., Huh N., Kirstein U., Rajewsky M. F. O4-ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1692–1695. doi: 10.1073/pnas.81.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sági J., Czuppon A., Kajtár M., Szabolcs A., Szemzö A., Otvös L. Modified polynucleotides. VI. Properties of a synthetic DNA containing the anti-herpes agent (E)-5-(2-bromovinyl)-2'-deoxyuridine. Nucleic Acids Res. 1982 Oct 11;10(19):6051–6066. doi: 10.1093/nar/10.19.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]


