Abstract
Community-acquired pneumonia (CAP) presents both a diagnostic and therapeutic challenge to clinicians. The prognosis of CAP remains poor with a 4% to 33% fatal outcome. Most initial therapy is necessarily empiric because of the nonspecificity of clinical and radiographic findings and the limitations of diagnostic tests. Severity of illness at initial presentation and the presence of either coexisting illness or advanced age influence the spectrum of etiologic agents and the initial approach to therapy. Although an early etiologic diagnosis is optimal in the management of CAP, in as much as 50% of all patients the responsible pathogen is not defined, even with extensive diagnostic testing. The emphasis in the initial diagnostic approach is to not order extensive tests. The practice of obtaining blood cultures seldom leads to alteration in the therapeutic regimen, even if the cultures are positive. Lack of clinical improvement with empiric therapy, complications from unrelated underlying diseases, and allergic reactions are usually stronger forces for determining change in treatment. Requiring blood cultures only for patients with the greatest risk of mortality from bacteremia from pneumonia would save not only the cost of the blood cultures themselves but also costs resulting from contaminants. As a pragmatic approach, a strategy for microbial investigation linked to illness severity is suggested to replace the current haphazard approach.
Despite the availability of potent new antimicrobials and effective vaccines, community-acquired pneumonia (CAP) remains a common and serious illness that presents both a diagnostic and therapeutic challenge to clinicians (1). In the 1900s, Sir William Osler found pneumonia to be the “Captain of the men of death” by virtue of its associated mortality rate of about 200 persons per 100,000 per year. In the United States, pneumonia is the sixth leading cause of death and the number one cause of death from infectious diseases (2, 3). As many as 4 million cases of CAP may occur annually, and as much as 20% of these require hospitalization (800,000 patients/yr) (3).
In the outpatient setting, the mortality rate of pneumonia remains low, in the range of 1% to 5%. However, among patients with CAP who require hospitalization, the mortality rate approaches 30%, particularly if admission to the ICU is required (4–13).
Prognostic indicators for mortality related to CAP have been evaluated, and several studies have identified an increased risk of death in patients with pneumococcal bacteremia in the range of 28.6% to 66.7% (1). Because of the nonspecificity of clinical and radiographic findings and the limitations of diagnostic tests for identifying an etiologic pathogen, most initial therapy is necessarily empiric. The approach to such therapy is based on an assessment of the likelihood that a given pathogen is causing disease in a given patient, a determination guided by information from experience and literature. Three major variables that influence the spectrum of etiologic agents and the initial approach to therapy are the severity of illness at initial presentation and the presence of either coexisting illness or advanced age (1).
Etiology
In elderly patients, Streptococcus pneumoniae, Hemophilus influenzae, aerobic gram-negative bacillus, and viruses are the most common pathogens, while in young adults Streptococcus pneumonia and Mycoplasma pneumoniae are the most common pathogens. Although an early etiologic diagnosis is optimal in the management of CAP, the responsible pathogen is not defined in as much as 50% of all patients, even when extensive diagnostic testing is performed. No single test can presently identify all potential pathogens, and each diagnostic test has limitations (4, 5, 12).
Diagnosis
The usual work-up for hospitalized patients includes a standard posteroanterior and lateral chest x-ray as well as sputum culture, sputum Gram stain (which has a poor sensitivity and specificity), blood cultures, urine for Legionella antigen, and serology for Mycoplasma pneumonia. Invasive diagnostic techniques to obtain lower airway specimens uncontaminated by oropharyngeal flora are used if no etiologic agent is established and the patient continues to deteriorate despite empiric therapy.
The emphasis in the initial diagnostic approach is to not order extensive tests. It is quite clear that when patients have had multiple serologic tests, along with cultures of respiratory tract secretions, the yield is limited. In addition, identification of an etiologic agent through diagnostic tests results in a change of treatment for a small number of patients (1,14,15).
Objectives
This analysis examines the impact on clinical management of obtaining blood cultures in certain presumed immunocompetent adult patients with CAP admitted to the hospital and attempts to answer three questions:
What is the value of obtaining blood cultures in hospitalized patients with CAP?
Does it change the management?
What is the cost effectiveness in the management of CAP?
For this purpose, a brief review follows of several studies on CAP that were originally intended to determine etiology and treatment.
CAP Requiring Hospitalization: A 5-Year Prospective Study (Marrie et al) (5)
The goal was to determine the factors responsible for mortality from pneumonia in 719 patients admitted to the hospital. Pneumonia was defined as evidence of acute onset of respiratory symptoms and at least one of the following physical findings: crackles, ronchi, or consolidation associated with new radiographic findings. The study included private and nursing home patients, excluding those with no radiographic evidence of pneumonia (most common reason for rejection), previous hospitalization, congestive heart failure, pulmonary infarction, and carcinoma of the lung. The diagnostic work-up included blood culture in 75% of the patients, sputum culture in 36% (n=257), chest x-ray in 100% (n=719), lung biopsy in 3.8% (n=28), and acute and convalescent phase serum specimens in 92% (n=661). Of these patients, 98 were admitted with Nursing Home-Acquired Pneumonia (NHAP) and 441 patients with CAP. All isolates, with the exception of Staphylococcus. epidermidis and Corynebacterium, were considered the etiologic agents. Only 48 (8.9%) patients with pneumonia were bacteremic. Eight of the 98 (8.2%) patients with NHAP who had blood cultures done were bacteremic, compared with 40 (9.1%) patients with CAP. None of these patients received antibiotics before hospitalization. Some of the agents isolated from blood cultures of the 48 bacteremic patients are shown in Figure 1. The overall mortality from pneumonia was 21% with a 29% mortality rate among bacteremic patients. The primary or secondary causes of death were not reported.
Figure 1.
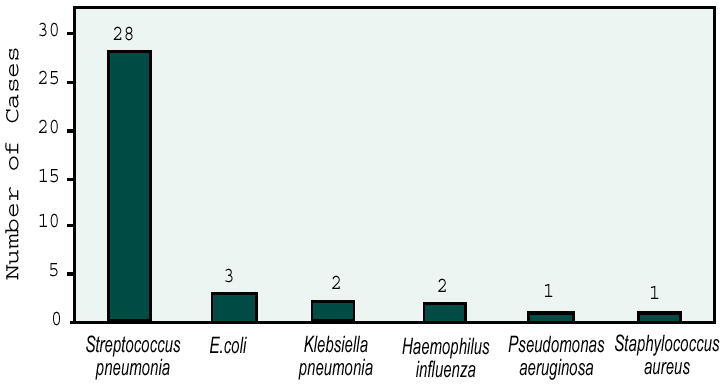
Pneumonia agents isolated by blood culture.
The authors concluded that blood cultures should be required for all patients, because even though the yield is low, a positive blood culture usually indicates the etiology of the pneumonia. A comprehensive serologic work-up, blood cultures, sputum cultures, and counterimmunoelectrophoresis (CIE) to detect pneumococcal antigen have a low yield of approximately 49% (16). One must rely on categorization of patients with CAP into various syndromes for which the best empiric antibiotic therapies have been defined (17).
A Hospital Study of CAP in the Elderly (Venkatesan et al) (18)
The goal was to determine the cause and outcome of pneumonia in elderly patients admitted to a district general hospital. Pneumonia was defined in this study as an acute lower respiratory tract infection with new, previously unrecorded shadowing on a chest radiograph. Patients older than 65 years were included, and those with chronic illnesses in which pneumonia was expected to be a terminal event (i.e. congestive heart failure, lung cancer, neurologic diseases, etc.) were excluded. The diagnostic work-up included sputum specimens and blood culture collection before the administration of antibiotics whenever possible, posterioanterior and lateral chest radiograph on admission and again at 10 days, and serologic tests for Mycoplasma, Chlamydia, Coxiella burnetii, adenovirus, and influenza A/B viruses. Sputum, urine, and serum samples were stored for later testing of pneumococcal capsular antigens.
A definite or probable pathogen was identified in 31 patients (43%), including viruses, bacteria, etc. Of these 31, evidence of bacterial infection was present only in 28, of which only six were bacteremic, representing a blood culture yield of 8.2%. The pattern of bacterial pathogens in the elderly was very similar to that reported in younger adults (Figure 2).
Figure 2.
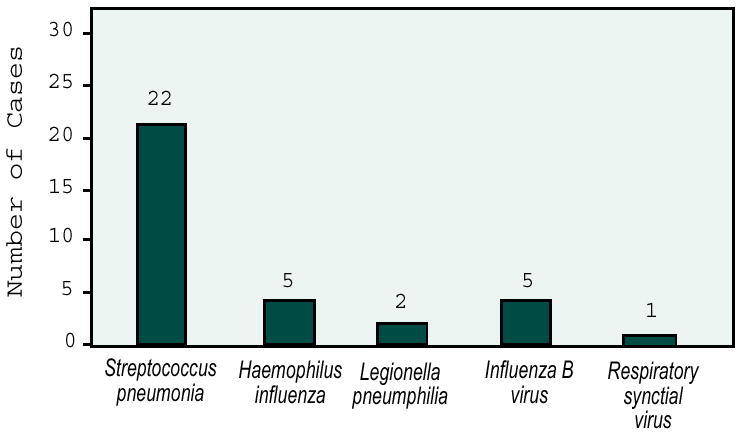
Etiologic agents reported in the elderly.
Streptococcus pneumoniae was the most common pathogen, diagnosed by detection of pneumococcal capsular antigen alone by CIE and by latex agglutination in half of the patients, indicating the sensitivity of this test. The second most common bacterial pathogen was H. influenzae identified by sputum culture and gram stains. The overall mortality rate from CAP was 14%; however, the number of bacteremic patients was not stated. The authors concluded that S. pneumoniae and H. influenzae are the major pathogens of CAP in both young and elderly patients; therefore, CAP should be treated primarily with antibiotics directed against S. pneumoniae and H. influenzae. Venkatesan et al demonstrated the high value of pneumococcal capsular antigen for the diagnosis of CAP and poor predictability of blood cultures.
Hospital Study of Adult CAP (Macfarlane et al) (19)
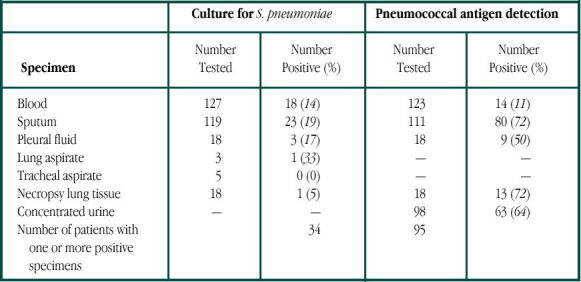
The goal was to determine the cause and outcome of pneumonia in 127 adults admitted to the hospital. Every patient presented with a history and clinical signs consistent with an acute lower respiratory tract infection associated with fresh pulmonary shadowing. Patients younger than 80 years were included; exclusion criteria were lung cancer, pulmonary infarction, cerebrovascular accident, and immunosuppressive therapy. The diagnostic work-up included blood culture (n=127), sputum cultures (n=119), tracheal aspirates (n=5), chest x-ray (n=127), complement fixation for viruses, and pneumococcal antigen by CIE. Antibiotic choice was not controlled. Fifty patients received antibiotics before admission but failed therapy. A diagnosis of the cause was established in 123 of 127 (97%) consecutive cases of adult CAP, with a bacterial infection implicated in 123 cases. Pneumococcal infection was diagnosed in 96 of 127 patients (76%); it was by far the most common cause of pneumonia. In 34 cases, the diagnosis was made by culture of the organism, with two-thirds diagnosed by CIE of the sputum. Only 18 (14%) patients had positive blood cultures (Table 1). The overall mortality was 15%. The authors concluded that more than two-thirds of these infections would have been missed if they had depended solely on bacterial cultures, since these were positive only in 34 of 127 (27%) patients compared with 95 of 127 (75%) patients using CIE.
Table 1.
Culture of S. pneumonia and detection of pneumoccoal antigen in different samples from pneumonia patients. Reprinted by permission of The Lancet Publishing Group from Macfarlane J T, et al, Lancet 1982; 2:256.
Importance of Noninvasive Bacteriologic and Radiographic Investigations (Levy et al) (20)
The goal was to evaluate the importance of initial noninvasive investigations in the bacteriological diagnosis of CAP and work out a diagnostic and therapeutic strategy based on pragmatic and economic considerations. Pneumonia was defined in this study as acute respiratory symptoms associated with fever and pulmonary radiographic densities. Patients older than 16 years were included. Excluded were patients in immunocompromised states or on immunosuppressive therapy, and those with radiographic evidence of pulmonary edema or pulmonary embolism, and nosocomial pneumonia. The diagnostic work-up included chest x-ray, three blood cultures within 12 hours before antibiotic administration, sputum Gram stain and culture, and serologic analysis when no bacteriologic diagnosis was made by other diagnostic methods. The choice of antibiotic was not controlled.
Thirty-five patients received antibiotics before admission but all of them were considered failures of initial treatment. Final microbiologic diagnosis was made in 75 of 116 (65%) patients. The distribution of the etiologic agents is shown in Figure 3. Results of blood culture were positive in 14 of 116 (12%) cases, mostly when S. pneumoniae was involved (Figure 4). The overall mortality was 13%.
Figure 3.
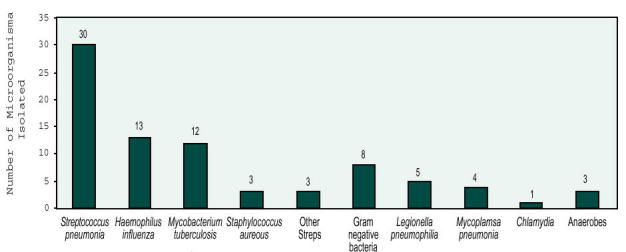
Distribution of etiologic agents.
Figure 4.
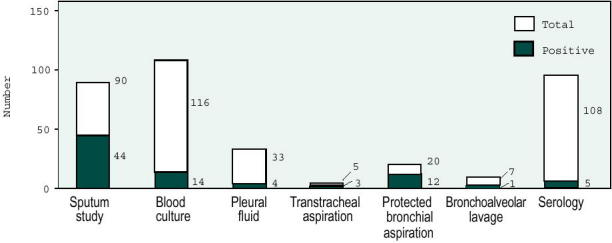
Results of different bacteriological investigations.
The authors concluded, as in many other studies, that S. pneumoniae was the most common pathogen. The prognosis was identical whether a bacteriologic diagnosis was made or not. Blood cultures contributed little in patients without severe infections, and the authors proposed blood cultures and invasive techniques only for patients with initially severe disease (defined by the presence of hypophosphatemia, hypoalbuminemia, or bilateral pulmonary infiltrates).
New and Emerging Etiologies for Community-Acquired Pneumonia and Implications for Therapy (Fang et al) (4)
The goal was to provide information on the frequency of microbial etiologies for CAP so that an updated approach to diagnosis and management could be rationally formulated. Pneumonia was defined as a syndrome associated with new pulmonary infiltrates on chest x-ray on admission or within 24 hours with at least one of the following “major” criteria: cough, sputum production, or fever > 37.8° C, or two of the following “minor” criteria: pleuritic chest pain, dyspnea, altered mental status, pulmonary consolidation by examination, or white blood cell count >12,000mm. Private and nursing home residents were included. Excluded were patients with chest x-ray abnormalities attributed to pulmonary edema, pulmonary embolism, or chronic pulmonary disease, and those previously hospitalized or transferred from another hospital within 7 days of the current diagnosis. The diagnostic work-up included blood cultures on admission, sputum Gram stains and culture, and serologic tests for Mycoplasma, Chlamydia, and Legionella. A diagnosis of the etiologic agent was established in 210 of 359 (58.5%) patients. Blood cultures were positive in only 28 (7.8%) (Figure 5). In the other cases, sputum studies and serology established the etiologic diagnosis.
Figure 5.
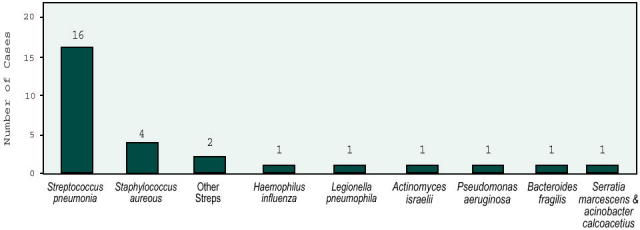
Etiologic agents diagnosed by blood culture.
The authors concluded that the clinical manifestations of CAP are rarely specific. S. pneumoniae and H. influenzae are the most common organisms; therefore, they should always be considered with empiric therapy. Even with dedicated microbiological methods, an etiologic agent could not be established in 33% of the cases. They emphasized the need for more rapid and sophisticated laboratory tests to pinpoint etiologies, given the low yield of the current diagnostic methods.
Clinical Utility of Blood Cultures in Adult Patients with CAP without Defined Underlying Risks (Chalasani et al) (14)
The goal was to examine the clinical utility of obtaining routine blood cultures before the administration of antibiotics to certain nonimmunosuppressed patients with CAP admitted to the hospital. CAP patients over 18 years old were included. Excluded were patients with HIV infection, hematologic malignancies, metastatic solid neoplasm receiving chemotherapy, and sickle cell disease; those receiving immunosuppressive therapy; nursing home residents; and patients discharged from the hospital less than 14 days prior to admission with pneumonia.
Of 517 patients, only 34 (6.6%) had positive blood cultures, 25 (4.8%) of which were considered contaminants (coagulase-negative staphylococci and corynebacteria). The antibiotic regimen was changed in only 56 of the study patients, (blood cultures were negative in 42 and positive in 14 (Figure 6).
Figure 6.
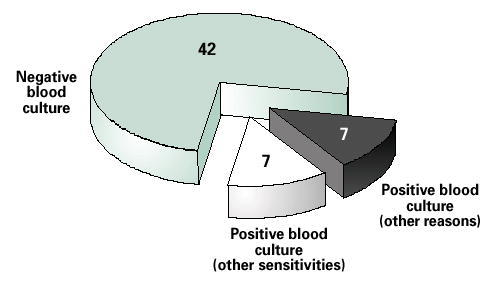
Blood culture results among patients whose antibiotics were changed. Data from Chalasani (14).
In six of the 14 patients, treatment was changed from a cephalosporin to penicillin, but the isolated bacteria were sensitive to both. In one case, the antibiotic was changed from trimethoprin/sulfamethoxazole to penicillin, but the isolate was sensitive to both drugs. Antibiotic therapy was changed in the other seven patients with positive blood cultures because of allergic reactions, continued elevated temperature after 72 hours, or positive sputum culture results that were available before blood culture results. In the remaining 42 patients who had negative blood cultures, the antibiotic therapy was changed secondarily due to allergic reactions or if no improvement in clinical status was demonstrated after 48–72 hours.
The authors concluded that few blood cultures were positive for organisms likely to infect adult patients with CAP without defined underlying risk factors (HIV/AIDS, sickle cell disease, nursing home residents, cancer, immunosuppressives, previous pneumonia). Positive blood cultures seldom lead to alteration in the therapeutic regimen, and in a carefully defined group of patients, blood culture may have limited clinical utility and questionable cost-effectiveness.
Overview
Figure 7 summarizes the previously discussed studies and a number of others. The first four studies have similar inclusion criteria and outcomes. Studies with different inclusion criteria are included to demonstrate a similar low incidence of positive blood cultures.
Figure 7.
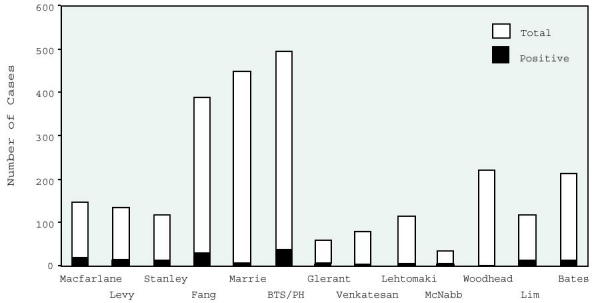
Frequency of positive blood cultures across studies.
How Can We Put Everything Together?
Despite substantial progress in the detection of pathogens and in therapeutic options, there continue to be major controversies in the clinical management of CAP. Few medical conditions are so controversial in terms of management. Pneumonia is one of the most difficult conditions to study, made more difficult by the inherent weaknesses in all studies. The prognosis of CAP remains poor with a 4% to 33% fatal outcome despite newer antibiotics, diagnostic techniques, and intensive care units. Ostergard and Anderson (21) concluded that bacteremia is a poor prognostic sign, raising the mortality up to 67% in patients with pneumococcal bacteremia, by far the most common cause of pneumonia in all reported studies (4, 5, 14, 18–22).
An ample body of evidence supports the blood culture yield ranging from 0.5% to 14% in the diagnosis of pneumonia and identification of specific pathogens (6, 13, 19–23). Marrie et al concluded that blood cultures should be done on all patients, because even though the yield is low, a positive blood culture usually indicates the etiology of the pneumonia (5). However, they also recognized the need for and value of empiric antibiotic therapy in the treatment of pneumonia.
What, then, is the true value of blood cultures in CAP? Does it change the management? And if not, what is the cost-effectiveness of blood cultures in the management of CAP? Chalasani et al found that blood cultures were infrequently positive in adult patients without defined underlying risk factors (immunosupression, HIV, sickle cell disease, renal failure, nursing home patients, etc) (Figure 6). In addition to yielding a low incidence of pathogens, the practice of obtaining blood cultures seldom leads to alteration in the therapeutic regimen, even if the cultures are positive (14). Other reasons, such as a lack of clinical improvement with empiric therapy, complications from unrelated underlying diseases, and allergic reactions, were usually stronger forces for determining change in treatment. Woodhead et al reported that the results of microbial tests directed a change in antibiotic therapy in only 8% of cases (6). They concluded that routine microbial investigations of all adults admitted to the hospital with CAP are not helpful and probably unnecessary (6). In addition, Glerant et al (22), reporting a 9% yield of positive blood cultures in patients previously treated for CAP as outpatients, concluded that the utilization of blood cultures in patients with moderate CAP (as defined by the American Thoracic Society guidelines) has reduced clinical usefulness and low cost-benefit. These results, however, are not surprising considering that a comprehensive serologic work-up, blood cultures, and CIE to detect pneumococcal antigens yield a specific etiology for pneumonia in only 50% of patients, as reported by Kettula et al (16).
Several studies have shown an increased propensity for bacteremia in certain immunosuppressed states (23, 24). Fine et al found an increased risk of complicated course in these patients (25). In studies by Levy et al (20), Lim et al (23), Macfarlane et al (19), and Chalasani et al (14), blood cultures had limited clinical utility in the treatment of patients without underlying risk factors as described above. Requiring blood cultures only for patients with the greatest risk of mortality from bacteremia from pneumonia would have saved not only the cost of the blood cultures themselves but also costs resulting from contaminants.
Bates et al concluded that false positive blood cultures are associated with higher laboratory and microbiologic charges and may be associated with an increased length of stay and a subsequent increase in total charges (12) (i.e. Chalasani found a total of $34,122.00 was spent on blood cultures at $66.00 per patient; the current cost for one set of blood cultures in our institution is $87.50).
Waterer et al (11) reported that despite the availability of sensitivities, physicians were reluctant to narrow antibiotic therapy, potentially adding to treatment cost and reducing the impact of blood culture results on management. The blood culture yield in their study was 74 of 1805 (4%) (11).
In April of 1998 and August of 2000, The Infectious Diseases Society of America released practice guidelines to assist clinicians who diagnose and manage CAP in immunocompetent patients. The recommendations are given an alphabetical ranking to reflect their strength and a roman numeral ranking to reflect the quality of supporting evidence. Despite their recommendation for obtaining blood cultures (one set) in hospitalized patients with CAP, they have given them a B-III ranking, which means moderate evidence to support the recommendations for use and quality of evidence based on expert opinion but not trials (26).
Therefore, as a pragmatic approach, we suggest a strategy for microbial investigation linked to illness severity to replace the current haphazard approach. An initial noninvasive investigation in patients without defined underlying risk factors (Table 2) could be reduced only to a Gram stain on a valid sputum sample and empiric therapy to cover the most common pathogens causing pneumonia. A reassessment in 48–72 hours after initiation of therapy will be necessary to appreciate the clinical status and the advisability of new invasive investigations (polymerase chain reaction [PCR], CIE, trans-thoracic needle aspiration [TNA], bronchoalveolar lavage, etc.) and therapeutic modifications as necessary. In a study by Lorente et al, PCR for the diagnosis of CAP had a sensitivity of 55% and a specificity of 100% as compared with blood culture, with 0.5% to 14% sensitivity (27). Ruiz-Gonzalez et al demonstrated that PCR of TNA of sputum had a specificity of 89.1% for S. pneumonia (28).
Table 2.
Poor prognostic factors in patients with CAP.
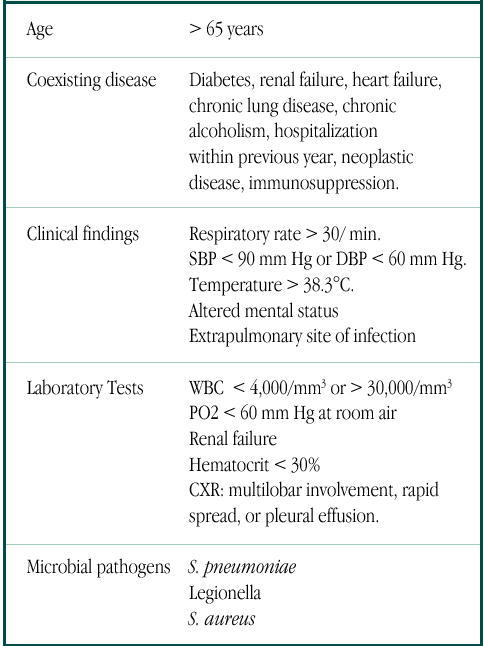
Conclusion
At a time when the cost-effectiveness of medical procedures is a major consideration, the usefulness of blood cultures in CAP gains special importance. The utilization of blood cultures for the diagnosis and treatment of outpatient CAP is rarely useful and results in increased cost of care without an impact on treatment strategy or patient outcome. One major problem is that there are no prospective studies on the usefulness of blood cultures in CAP; however, the reported sensitivity of blood cultures has ranged between 0.5% and 14% (6, 13, 19–23). Because of this low yield, the old belief that blood cultures represent the "gold standard" for the diagnosis of pneumococcal bacteremia (29) must be challenged, and new diagnostic and more cost-effective techniques should be developed, standardized, and implemented. Until that time, we recomment blood cultures for patients with CAP who require admission to the hospital.

Dr. Rodriguez, a former Ochsner Internal Medicine Resident, is a staff internist/hospitalist at Good Samaritan Hospital and Saint Joseph Hospital and a clinical researcher at Mountain Park Health Center, in Phoenix, AZ.

Dr. Pankey is Director of Infectious Diseases Research at the Alton Ochsner Medical Foundation. He was formerly Head of the Section on Infectious Diseases of the Ochsner Clinic. His main research interest concerns novel approaches to therapy for antimicrobial resistance.
References
- Niederman M. S., Bass J. B., Jr, Campbell G. D. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity and initial antimicrobial therapy. American Thoracic Society. Medical Section of the American Lung Association. Adopted July, 1993. Am Rev Respir Dis. 1993;148:1418–1426. doi: 10.1164/ajrccm/148.5.1418. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Commerce. Bureau of the Census. Statistical Abstract of the United States. 104th ed. Washington DC: USGPO, 1984. [Google Scholar]
- Garibaldi R. A. Epidemiology of community-acquired respiratory tract infections in adults. Incidence, etiology, and impact. Am J Med. 1985;78(6):32–37. doi: 10.1016/0002-9343(85)90361-4. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G. D., Fine M., Orloff J. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69:307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Durant H., Yates L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis. 1989;11:586–599. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- Woodhead M. A., MacFarlane J. T., McCracken J. S. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;1:671–674. doi: 10.1016/s0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Ortqvist A., Sterner G., Nilsson J. A. Severe community-acquired pneumonia: factors influencing need of intensive care treatment and prognosis. Scand J Infect Dis. 1985;17:377–386. doi: 10.3109/13813458509058778. [DOI] [PubMed] [Google Scholar]
- Torres A., Serra-Batlles J., Ferrer A. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Respir Dis. 1991;144:312–318. doi: 10.1164/ajrccm/144.2.312. [DOI] [PubMed] [Google Scholar]
- Pachon J., Prados M. D., Capote F. Severe community-acquired pneumonia. Etiology, prognosis, and treatment. Am J Respir Dis. 1990;142:369–373. doi: 10.1164/ajrccm/142.2.369. [DOI] [PubMed] [Google Scholar]
- Marston B. J., Plouffe J. F., File T. M., Jr Incidence of community-acquired pneumonia requiring hospitalizations. Results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157:1709–1718. [PubMed] [Google Scholar]
- Waterer G. W., Jennings S. G., Wunderink R. G. The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia. Chest. 1999;116:1278–1281. doi: 10.1378/chest.116.5.1278. [DOI] [PubMed] [Google Scholar]
- Bates J. H., Campbell G. D., Barron A. L. Microbial etiology of acute pneumonia in hospitalized patients. Chest. 1992;101:1005–1012. doi: 10.1378/chest.101.4.1005. [DOI] [PubMed] [Google Scholar]
- Community-acquired pneumonia in adults in British hospitals in 1982–1983: a survey of aetiology, mortality, prognostic factors and outcome. The British Thoracic Society and the Public Health Laboratory Service. Q J Med. 1987;62:195–220. [PubMed] [Google Scholar]
- Chalasani N. P., Valdecanas M. A. L., Gopal A. K. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932–936. doi: 10.1378/chest.108.4.932. [DOI] [PubMed] [Google Scholar]
- Ortqvist A. Prognosis of community-acquired pneumonia requiring treatment in hospital. Importance of predisposing and complicating factors, and of diagnostic procedures. Scand J Infect Dis Suppl. 1990;65:1–62. [PubMed] [Google Scholar]
- Kerttulla Y., Leinonen M., Koskela M. The aetiology of pneumonia. Application of bacterial serology and basic laboratory methods. J Infect. 1987;14:21–30. doi: 10.1016/s0163-4453(87)90730-4. [DOI] [PubMed] [Google Scholar]
- Donowitz G. R., Mandell G. L. Empiric therapy for pneumonia. Rev Inf Dis. 1983;5:40–41. doi: 10.1093/clinids/5.supplement_1.s40. Suppl 1. S. [DOI] [PubMed] [Google Scholar]
- Venkatesan P., Gladman J., Macfarlane J. T. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45:254–258. doi: 10.1136/thx.45.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane J. T., Finch R. G., Ward M. J. Hospital study of community-acquired pneumonia. Lancet. 1982;2:255–258. doi: 10.1016/s0140-6736(82)90334-8. [DOI] [PubMed] [Google Scholar]
- Levy M., Dromer F., Brion N. Community-acquired pneumonia. Importance of initial noninvasive bacteriologic and radiographic investigations. Chest. 1988;92:43–48. doi: 10.1378/chest.93.1.43. [DOI] [PubMed] [Google Scholar]
- Ostergaard L., Andersen P. L. Etiology of community-acquired pneumonia. Evaluation by transtracheal aspiration, blood culture, or serology. Chest. 1993;104:1400–1407. doi: 10.1378/chest.104.5.1400. [DOI] [PubMed] [Google Scholar]
- Glerant J. C., Hellmuth D., Schmit J. L. Utility of blood cultures in community-acquired pneumonia requiring hospitalization: influence of antibiotic treatment before admission. Respir Med. 1999;93:208–212. doi: 10.1016/s0954-6111(99)90010-0. [DOI] [PubMed] [Google Scholar]
- Lim I., Shaw D. R., Stanley D. P. A prospective hospital study of the aetiology of community-acquired pneumonia. Med J Aust. 1989;151:87–91. doi: 10.5694/j.1326-5377.1989.tb101168.x. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L., Lyons R. W., Verilli M. Infection with the immunodeficiency virus (HIV) as a risk factor for bacteremic illness due to Streptococcus pneumoniae. Conn Med. 1988;52:703–704. [PubMed] [Google Scholar]
- Fine M. J., Smith D. N., Singer D. E. Hospitalization decision in patients with community-acquired pneumonia: a prospective cohort study. AmJ Med. 1990;89:713–721. doi: 10.1016/0002-9343(90)90211-u. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Breiman R. F., Mandell L. A. Community-acquired pneumonia in adults: guidelines for management. The Infectious Diseases Society of America. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- Lorente M. L., Falguera M., Nogues A. Diagnosis of pneumococcal pneumonia by polymerase chain reaction (PCR) in whole blood: a prospective clinical study. Thorax. 2000;55(2):133–137. doi: 10.1136/thorax.55.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gonzalez A., Nogues A., Falguera M. Rapid detection of pneumococcal antigen in lung aspirates: comparison with culture and PCR technique. Respir Med. 1997;91(4):201–206. doi: 10.1016/s0954-6111(97)90039-1. [DOI] [PubMed] [Google Scholar]
- Austrian R. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]


