Abstract
Purpose:
The risks and benefits of continuing bisphosphonate therapy beyond 5 years in patients with primary osteoporosis have not been well established.
Methods:
We searched MedLine, EMBase, CENTRAL, CINAHL, and AgeLine prior to February 2010. Bibliographies were also searched and experts in the field contacted. The ProQuest Dissertations and Theses database and relevant conference proceedings were searched to identify unpublished or ongoing studies. Two authors independently reviewed search results. Randomized controlled trials and comparative nonrandomized controlled trials examining post-menopausal women or men ≥50 years of age with primary osteoporosis assigned to continue versus discontinue bisphosphonate therapy after ≥5 years of therapy were included. Of 1188 identified articles, three studies (n = 1443) met criteria for inclusion in data synthesis. Data were extracted and risk of bias assessed by two independent reviewers using predefined criteria.
Results:
No statistically significant association was found between fracture incidence and the discontinuation of therapy beyond 5 years for any type of fracture: clinical nonvertebral fracture (relative risk [RR] = 0.97; 95% confidence interval [CI] 0.77–1.23), clinical vertebral fracture (RR = 0.61; 95% CI 0.32–1.19), or morphometric vertebral fracture (RR = 0.90; 95% CI 0.5–1.64). No differences in adverse events were identified between the two groups.
Conclusion:
We found no significant difference in fracture risk or adverse events between postmenopausal women with primary osteoporosis who continued bisphosphonate therapy versus those who discontinued bisphosphonate therapy after 5 years of treatment. However, given the small number and limited quality of available studies, no firm conclusions or recommendations can be made.
Keywords: bisphosphonates, fracture, long-term follow-up, bone mineral density
Introduction
Osteoporosis is a common condition in Canada, affecting up to 1 in 4 women and 1 in 8 men, with numbers expected to rise as the population ages.1 Currently, bisphosphonates are considered first-line therapy for both prevention and treatment of osteoporosis in men and postmenopausal women.2,3 Multiple studies support the efficacy of bisphosphonate therapy in preventing bone loss and decreasing fracture risk for up to 4 years of treatment, but few studies have examined the effects of bisphosphonate therapy beyond 5 years.4,5
Bisphosphonates work by inhibiting bone resorption and decreasing bone formation. Unlike other medications, bisphosphonates remain in bone for long periods of time after therapy is discontinued, with a calculated elimination half-life for some bisphosphonates of up to 10 years.6 With continued use, the amount of drug deposited in bone accumulates.7 Therefore, it is thought that the actions of bisphosphonates may continue for some time after they have been discontinued, as they are slowly released from bone during bone turnover.8 There has been some concern about the cumulative effect of prolonged bisphosphonate use on bone integrity.7 With continued increased mineralization, bone may become brittle and fracture risk may increase. Although causality has not been established, multiple reports of unexpected, low-energy subtrochanteric fractures in patients on prolonged bisphosphonate (alendronate) therapy have made many clinicians question the wisdom of suppressing bone turnover with bisphosphonates for extended periods of time, as it may lead to decreased repair of bone microdamage and an increased risk of stress fractures.9–11 Other potential, but serious, adverse effects associated with bisphosphonate therapy include osteonecrosis of the jaw and a possible link to increased rates of atrial fibrillation.12,13
Few studies have examined the effects of bisphosphonate therapy beyond 5 years, leaving many clinicians questioning what the next most appropriate step is. Osteoporotic fractures cause significant morbidity and mortality. Hip fracture leads to nursing home placement in approximately 58% of patients, and death, within 1 year of fracture, occurs in 22%–29% of patients.14,15 Hence, identifying the optimal course of bisphosphonate treatment for patients with osteoporosis, while avoiding needless (and potentially dangerous) over-treatment, is of utmost importance.
We performed a systematic review to examine whether continuing bisphosphonates after 5 years of therapy for primary osteoporosis is associated with a decreased fracture risk, increased bone mineral density (BMD), decreased rates of bone turnover, and increased mortality and adverse events compared with similar individuals who discontinue therapy. Our goal was to include all published randomized controlled trials and nonrandomized controlled trials that measured the effect of long-term (beyond 5 years) bisphosphonate therapy used for the prevention and treatment of primary osteoporosis on vertebral (clinical and morphometric) and nonvertebral fractures or BMD. No prior systematic reviews have been performed on this topic. We aim, by summarizing the current available literature, to define the risks and benefits of continuation versus discontinuation of therapy after 5 years and to identify areas where future research is needed.
Methods
Eligibility criteria
All clinical trials (randomized and nonrandomized) examining discontinuation of bisphosphonate therapy in patients with primary osteoporosis after ≥5 years of treatment were included. No language, publication date, or publication status restrictions were imposed. Postmenopausal women and men ≥50 years of age with primary osteoporosis were included. Osteoporosis was defined as a pretreatment BMD T-score of ≤–2.5 at either the hip or spine, or a minimal trauma fracture occurring after the age of 45 years. Participants who started another osteoporosis medication after discontinuing their bisphosphonate (selective estrogen receptor modulator, calcitonin, hormone replacement therapy, teriparatide) were excluded. We included all types of bisphosphonates regardless of mode of administration (intravenous or oral) or dose. The primary outcome measure for this review was fragility fracture (both clinical and morphometric). Secondary outcomes included change in BMD (at any site), change in bone turnover markers, mortality, and adverse events. All adverse events were included, but particular attention was paid to osteonecrosis of the jaw, new atrial fibrillation, renal dysfunction, subtrochanteric fractures, and gastrointestinal events. Studies included in the review had to have a minimal length of follow-up of 1 year after discontinuation of bisphosphonate therapy.
Information sources and search
An electronic search was conducted using the following electronic databases (date of search: February 8, 2010): OVID MedLine (1950–2010 week 3), OVID EMBase (1980–2010 week 4), the Cochrane Central Register of Controlled Trials (CENTRAL), CSA-AgeLine (1978–present), and the Cumulative Index to Nursing and Allied Health Literature (EBSCO-CINAHL) (1982–present). To review potentially relevant grey literature, the ProQuest Dissertations and Theses database was searched and conference proceedings from the International Osteoporosis Foundation (IOF) and American Society of Bone Mineral Research (ASBMR) for the past 3 years were hand searched. Studies identified as relevant during the review were cross-referenced for relevant citations. Experts in the field were contacted for relevant citations and unpublished or ongoing studies. Keywords and search strategies were reviewed by a research librarian who is experienced in systematic reviews of healthcare literature. Variations in search terms were used in different databases to reflect the differences in indexing used by different databases. The full search strategy used in OVID MedLine is presented in Appendix 1.
Study selection and data collection
Two reviewers independently screened all titles and abstracts retrieved from searching. Agreement between the two reviewers for this initial screen was fair (κ statistic = 0.44 [95% confidence interval (CI) 0.33–0.55]). Any titles or abstracts deemed to be potentially relevant by either reviewer were then reviewed independently in full text form by both reviewers using predefined inclusion criteria. There was perfect agreement between the two reviewers during full text screening (κ statistic = 1.0). Data were then independently abstracted by each reviewer using a single comprehensive data extraction form. In studies with multiple reports, all prior publications were collected and integrated with the final report to make the data available for extraction as complete as possible. Disagreements were resolved by consensus.
The two reviewers also independently assessed each eligible study for risk of bias during the data extraction phase using the Cochrane “risk of bias tool”.16 Inter-rater agreement was calculated by means of κ statistics for each of the six items in the tool (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other bias).
Summary measures and synthesis
Relative risk (RR) of fracture was the primary measure of treatment effect. For all dichotomous outcomes (fracture, mortality, and adverse events), RR and 95% CI were calculated using the Mantel–Haenszel method. For continuous outcomes (BMD and bone turnover markers) we planned to calculate mean difference and standard error using the generic inverse variance method. Random effects models were used for all calculations to account for between-study variability. All calculations were performed using RevMan 5 software (The Nordic Cochrane Centre, Copenhagen, Denmark).
In trials where discontinuation (placebo) was compared with more than one dose of bisphosphonate continuation, the different dose groups were combined to create a single comparison (where sufficient data were available) of continuation (bisphosphonate) versus discontinuation (placebo). Similarly, different trials examining different bisphosphonates were included in the analysis. Sensitivity and subgroup analyses were planned a priori to explore excess heterogeneity that this combining of trials may cause. Where not stated by trial authors, numbers of events per group were calculated from percentage of events per group and number of patients per group. Only studies that reported a specified outcome were included for that particular effect estimate. We tested for heterogeneity by calculating a test of heterogeneity and an I2 statistic. We hypothesized a priori that possible causes of heterogeneity of study results would include type of bisphosphonate used, severity of osteoporosis in trial subjects, male versus female subjects, duration of bisphosphonate use before discontinuation, length of follow-up, and quality of trials. Subgroup analyses were planned a priori for these variables. We planned to use funnel plots for each outcome to assess for risk of publication bias based on plot asymmetry. Broad search inclusion criteria and examination of grey literature during the search phase of this review were meant to decrease bias in our results. Both data abstractors attempted to estimate reporting bias in individual studies.
Results
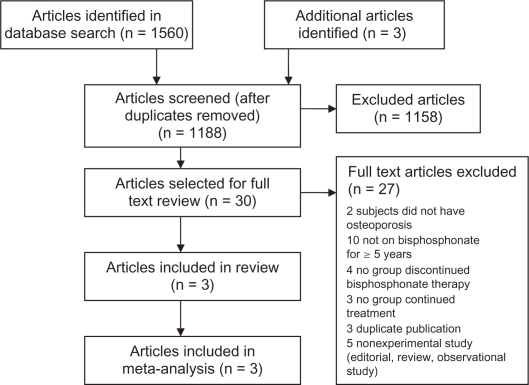
Figure 1 shows the flow of studies selected for this review. After duplicate studies were removed, 1188 studies (from all sources) were included in the title and abstract screening. Of those, 1158 studies were identified as being irrelevant or inappropriate to our review topic and therefore were excluded. The remaining 30 studies were included in formal full text review. Three studies met inclusion criteria and were included in the systematic review and meta-analysis.
Figure 1.
Flow diagram of study selection.
The three included studies were all extension studies of prior randomized controlled trials. For the FLEX (Fracture Intervention Trial Long Term Extension) 1996 study (Black et al17) and the 1997 study by Miller et al,18 participants were rerandomized into bisphosphonate (continuation) versus placebo (discontinuation) groups after a mean of 5 years of bisphosphonate therapy. In contrast, in the 2004 study by Bone et al,19 subjects were not rerandomized after 5 years of bisphosphonate therapy but remained in their original randomized groups with one group changed from bisphosphonate to placebo at year 5. A summary of study characteristics is presented in Table 1.
Table 1.
Characteristics of included studies
| Study | Participants | Intervention | Outcomes | Methodological quality | Notes |
|---|---|---|---|---|---|
| Black et al17 | 1099 postmenopausal women from the US, originally with BMD ≤ 0.68 g/cm2, who received alendronate | Alendronate 5 mg/day (n = 329) or 10 mg/day (n = 333) vs placebo (n = 437) for 5 years | Change in BMD at hip and other sites Changes in bone turnover markers Fracture incidencea Outcomes measured at 5-year follow-up |
AS adequately generated AC not clear Blinding: patients, physicians, data collectors, and outcome assessors Incomplete data adequately addressed No selective outcome reporting, or other threats to validity |
Funded by Merck and Co. (makers of alendronate, the study drug) Merck involved with study design, editorial input, and approval of final manuscript Not all participants had “osteoporosis” by WHO definition, inclusion criterion was BMD ≤ 0.68 g/cm2 |
| 5 mg/day for 2 years and then 10 mg/day for a total mean duration of therapy of 5 years’ treatment during the Fracture Intervention Trial | |||||
| Bone et al19 | 804 postmenopausal women from multiple countries with BMD T-scores <–2.5 underwent initial randomization; 247 included in the year 8–10 extension study (used for this review) | Alendronate 5 mg/day (n = 78) vs 10 mg/day (n = 86) for 10 yrs vs 20 mg/day for 2 years then 5 mg/day for 3 years then placebo for 5 years (n = 83) | Change in BMD at lumbar spine and other sites Changes in bone turnover markers Morphometric and clinical vertebral fracture incidenceb Height change Safety Outcomes measured at either 2- or 5-year follow-up |
AS and AC not clear “Double blinded”, also data assessors blinded Incomplete data not adequately addressed All outcomes not fully reported No other threats to validity |
Funded by Merck Research Laboratories (makers of alendronate, the study drug) First author with honorariums and grant support from Merck |
| Miller et al18 | 97 postmenopausal women from the US with a history of fragility fracture completed 7 years of follow-up with at least 5 years of etidronate therapy | Continued cyclical etidronate (n = 51) vs placebo (n = 46) (subgroups of larger extension study) | Change in BMD at lumbar spine and other sites Morphometric vertebral fracture incidence Safety Outcomes measured at 2-year follow-up |
AS adequately generated AC not clear “Double blinded” Incomplete data not adequately addressed All outcomes not fully reported No other threats to validity |
Funded by Procter and Gamble Pharmaceuticals (makers of etidronate, the study drug) |
Notes:
Fracture incidence was considered an exploratory outcome;
Fracture incidence collected as safety endpoint, no formal analysis planned. No attempt made to exclude trauma-related fractures.
Abbreviations: AC, allocation concealment; AS, allocation sequence; BMD, bone mineral density; WHO, World Health Organization.
Study characteristics
Participants
The included studies involved 1443 postmenopausal women of different ethnicities mostly from the US. A total of 877 women were assigned to the continuation of bisphosphonate group and 566 women were in the discontinuation (placebo) group. However, for the outcome clinical vertebral fractures, results were not available in the Bone et al19 study and therefore an earlier published extension study of this same cohort was used, which included slightly more patients, thereby inflating the total number of participants for this one outcome.20 Included women were postmenopausal and generally had primary osteoporosis, defined either by a history of fragility fracture or a BMD T-score of <–2.5, at the start of the original drug study (ie, not at the beginning of the extension study). The exception was the FLEX trial,17 which included women with a femoral neck BMD of <0.68 g/cm2 as part of the original study inclusion criteria.21 Mean age of participants in the three studies was between 63 years and 74 years. All subjects received 500 mg of calcium per day, and the FLEX trial also offered 250 IU of vitamin D to both treatment groups.
Interventions
All trials included participants who had been on bisphosphonate therapy for a mean of 5 years. The Bone et al19 and Miller et al18 trials studied other groups of patients as well, but for the purposes of this systematic review these other groups were not examined. In the “continuation” group in the FLEX17 and Bone et al trials, participants were divided into two subgroups (5 mg or 10 mg per day of oral alendronate). The Miller et al trial looked at 90- to 91-day cycles of etidronate 400 mg per day for 14 days followed by 500 mg of elemental calcium per day. A placebo was given to the “discontinuation” group in all trials. The Bone et al and FLEX trials reported outcomes at 5 years after discontinuation (or continuation) of alendronate therapy, whereas the Miller et al trial reported outcomes at 2 years.
Outcomes
The primary outcome in the Miller et al18 and Bone et al19 studies was percentage change in lumbar spine BMD; in FLEX17 it was change in total hip BMD. BMD was analyzed on an intention-to-treat (or “modified” intention-to-treat) basis in the FLEX and Bone et al studies. Loss to follow-up was dealt with by carrying forward the last known value. Changes in bone turnover markers were analyzed on a per protocol basis in both these studies, and none of the three studies stated clearly how fracture data were assessed. All studies reported adverse events. Change in bone turnover markers was included as a secondary outcome in the FLEX and Bone et al trials. Fracture was not a primary outcome in any trial.
Risk of bias
Specific components of methodological quality are listed in Table 1. All three studies were sponsored by the drug company making the bisphosphonate used in the study, and therefore potential conflict of interest existed in all cases.
Outcomes
Fracture
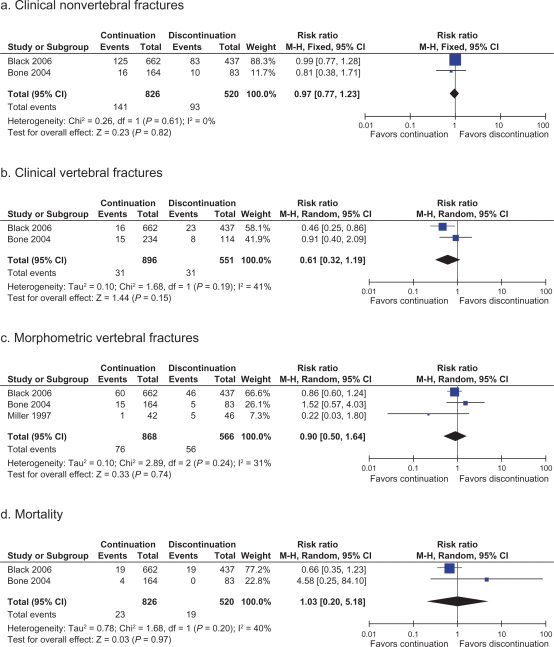
The forest plots presented in Figure 2 (panels a, b, and c) show trial-level fracture rates for nonvertebral, clinical vertebral, and morphometric vertebral fractures in bisphosphonate continuation and discontinuation groups. Hip fracture rates were also reported in the FLEX trial with RR 1.02 (95% CI 0.51–2.10) in the continuation group (20 of 662 patients) versus discontinuation group (13 of 437 patients).
Figure 2.
Forest plots showing pooled estimates for fracture risk and mortality in patients who continue bisphosphonates after 5 years of therapy versus those who discontinue after 5 years.
Notes: aClinical nonvertebral fractures; bClinical vertebral fractures; cMorphometric vertebral fractures; dMortality.
Two trials reported rates of clinical nonvertebral fractures at 5 years. The pooled analysis showed no difference in rates between the continuation and discontinuation groups (RR = 0.91, 95% CI 0.77–1.23, I2 = 0%). Pooled analysis of the same two trials showed that rates of clinical vertebral fractures were no different between groups (RR = 0.61, 95% CI 0.32–2.09, I2 = 41%). However, for this outcome, results for one study were reported from 2-year follow-up, whereas the other was 5-year follow-up, which may contribute to the higher I2 value, although the results were not found to be heterogeneous (χ2 = 1.68, degree of freedom [df] = 1, P = 0.19). It should be noted that before pooling, the Black et al17 study did show a significant increase in clinical vertebral fractures in those discontinuing bisphosphonate therapy after 5 years. However, this result became statistically nonsignificant when pooled. Morphometric vertebral fractures were reported in all three studies. However, results from Miller et al18 were reported at 2-year follow-up, whereas the results from the other two trials were from 5-year follow-up. Pooled analyses showed no difference between groups (RR = 0.9, 95% CI 0.5–1.64, I2 = 31%).
Change in BMD
Although all three trials reported change in BMD as their primary outcome, the number of subjects analyzed and the standard deviation for each outcome was not provided in two of the three studies, and therefore results were not able to be pooled. As seen in Table 2, bone gain was consistently higher (or bone loss was less) in the continuation group compared with in the discontinuation group at all sites (lumbar spine, femoral neck, and trochanter) in all three studies. Two of the studies did not perform direct between-group comparisons, and therefore statistical significance is not stated. However, comparisons were performed in the reporting of the FLEX trial with decreased BMD in the discontinuation (versus continuation) group of −2.4% for total hip (P < 0.001) and −3.7% for the lumbar spine (P < 0.001), indicating that significant losses in BMD do occur in patients discontinuing bisphosphonate therapy after 5 years.
Table 2.
Mean percentage change (standard error) in bone mineral density over 5 years in continuation versus discontinuation of bisphosphonate groups
| Trial | Continuation/discontinuation | Lumbar spine | Femoral neck | Trochanter |
|---|---|---|---|---|
| Alendronate | ||||
| Black et al17 (FLEX) | Continuation | 5.26 (0.24) | 0.46 (0.24) | −0.08 (0.22) |
| Discontinuation | 1.52 (0.29) | −1.48 (0.30) | −3.25 (0.27) | |
| Alendronate | ||||
| Bone et al19,a | Continuation | |||
| 5 mg group | 2.5 (0.56) | 1.0 (0.87) | 0.0 (0.87) | |
| 10 mg group | 3.7 (0.56) | 0.9 (0.87) | 1.0 (0.82) | |
| Discontinuation | 0.3 (0.61) | −2.2 (0.87) | −1.0 (0.82) | |
| Etidronate | ||||
| Miller et al18,b | Continuation | 1.8 (0.71) | 0.5 (0.82) | 0.4 (0.76) |
| Discontinuation | 1.4 (1.11) | –0.9 (0.96) | –0.6 (0.91) |
Notes:
No combined continuation group data available. Standard error calculated from confidence intervals provided in paper;
Results are from 2-year follow-up.
Change in bone turnover markers
Change in bone turnover markers from the time of continuation/discontinuation to 5-year follow-up were not described in the reports from Bone et al19 (only shown graphically) or Miller et al.18 In a select subset of FLEX17 patients (n = 236), increased bone turnover was found 5 years later in those discontinuing bisphosphonate therapy compared with those continuing therapy. Markers were elevated by 55.6% (P < 0.001) for c-telopeptide of type 1 collagen, 59.5% (P < 0.001) for serum n-propeptide of type 1 collagen, and 28.1% (P < 0.001) for bone-specific alkaline phosphatase.
Mortality
Two of the three studies reported death rates. Pooled analysis showed no difference between discontinuation and continuation groups (RR = 1.03, 95% CI 0.2–5.18, I2 = 40%) Heterogeneity χ2 = 1.68, df = 1, P = 0.20. A wide confidence interval is probably the result of very small numbers of reported deaths in all groups.
Adverse effects
All three studies comment that no significant differences in adverse events were found between treatment and placebo groups. Numerical comparisons of specific adverse events between groups were not reported for most outcomes.
Risk of bias across studies
Due to the small number of studies included in our review, funnel plots were not appropriate or helpful. Although none of the outcomes demonstrated statistical heterogeneity, all outcomes should be viewed with caution, given the small sample size. Similarly, with the very small number of studies included in this review, prespecified subgroup analyses were not able to be performed. Results from the Miller et al18 trial, in particular, were suspected of being different from the other studies, as Miller et al reported outcomes after 2 years of bisphosphonate discontinuation (versus 5 years in the other trials) and studied etidronate (versus alendronate in the other trials). The only pooled fracture result that included the Miller et al study was that of morphometric fracture. A sensitivity analysis was therefore performed, with the Miller et al study removed. The RR of morphometric vertebral fracture was found to be 0.95 (95% CI 0.62–1.44, I2 = 12%), which was a minimally changed effect estimate.
Discussion
This systematic review found no difference in fracture incidences in patients with primary osteoporosis who continued bisphosphonate therapy for an additional 2–5 years versus those who discontinued treatment at 5 years. Incidence of nonvertebral fractures, clinical vertebral fractures, and morphometric vertebral fractures were all found to be similar in the two groups. However, the evidence presented here is not robust enough to make definitive conclusions, due to the small number of studies: three studies including 1443 patients (1447 for clinical vertebral fractures) with only one to two studies with available data for meta-analysis for most outcomes. Also, all three trials were sponsored by the pharmaceutical company that manufactures the bisphosphonate under study in the trial, resulting in the possibility of a significant conflict of interest.
None of the three trials sought to examine fracture incidence as a primary outcome, probably due to the fact that all three trials were designed as follow-ups to larger trials. These extensions were designed to look at the effects over time on BMD and bone markers and were not powered to look at fracture risk. All fracture-related data were therefore either exploratory or considered together with safety outcomes. Pooled fracture effect estimates presented here had wide confidence intervals, and all crossed 0, indicating that the true effect of continued bisphosphonate therapy could be negligible or, in many cases, could provide significant benefit or significant harm. No differences in adverse effects or mortality were identified between the two groups. Specifically, there were no reported cases of subtrochanteric fractures, osteonecrosis of the jaw, or atrial fibrillation (the most common reasons for concern with long-term bisphosphonate use). However, whether these events were not specifically looked for, or whether the number of subjects was just too small to capture them, is unclear.
Although we were unable to pool BMD results, all three trials showed a clear trend toward ongoing BMD gain (or less BMD loss) in groups where bisphosphonate therapy was continued. These BMD differences were found to be significant in the FLEX trial. Similarly, in a subset of subjects studied in the FLEX trial, bone turnover markers were elevated 5 years after discontinuation of alendronate compared with levels in those who continued alendronate.
Studies included in this review examined postmenopausal women with a BMD of ≤0.68 g/cm2 or a history of fragility fracture before initiation of bisphosphonate therapy. Therefore, findings cannot be applied to individuals outside these criteria. Similarly, conclusions in patients taking bisphosphonate therapies other than aldendronate or etitdronate cannot be assumed. In particular, zoledronic acid, which is the most potent bisphosphonate available and the longest lasting, may have different effects on long-term fracture risk after discontinuation.22
We found no effect on fracture risk after discontinuation of bisphosphonate therapy after 5 years of treatment. However, treatment for a shorter interval followed by discontinuation may result in different risks as bisphosphonates have less time to accumulate in bone. Previous studies have shown that residual BMD benefits after treatment withdrawal are proportional to the original duration of bisphosphonate therapy.23,24 Similarly, the studies in this review followed patients for a maximum of 5 years after discontinuation. The implications of discontinuation beyond this 5-year follow-up period are unknown.
Strengths of this review include the rigorous search strategy used to identify articles. All study selection and data abstraction were performed in duplicate in order to minimize errors and bias, and excellent agreement was found between reviewers. Similarly, quality of studies was assessed in duplicate using a formal “risk of bias” tool, with excellent agreement.
As mentioned previously, the quality and volume of the evidence retrieved limit its interpretation. We were also limited by the reporting of included trials. For instance, some outcome data (eg, bone turnover markers) were reported in graphical format only in some studies, and we were unable to obtain the original trial data to incorporate into our meta-analysis. Another limitation is that studies varied in terms of bisphosphonate type and dose used in the 5 years prior to discontinuation. Given the different half-life properties of different bisphosphonates, this may affect bone-protective properties after discontinuation. This also limits applicability of results to current clinical practice where once-weekly (or once-monthly) oral bisphosphonate preparations are standard and etidronate therapy is generally no longer considered a first-line therapy.3,25
No previous systematic reviews have been carried out on this particular topic, but multiple narrative summaries and editorials have been written. It has been suggested, based on the limited amount of data available in this area, that all patients should have their fracture risk reassessed after 5 years of therapy to see whether ongoing treatment with bisphosphonate therapy is warranted. Continuing therapy may be appropriate in those who continue to be at high risk, whereas discontinuing treatment may be more appropriate in lower-risk patients.26 This is similar to the conclusions offered by the authors of the FLEX trial, who suggested that women at very high risk of clinical vertebral fractures continue therapy, whereas in other women discontinuation does not increase fracture risk.17 Our study adds support to those who argue that there is currently no proof that continued bisphosphonate therapy beyond 5 years has any effect on fracture outcomes.27,28 Similar to our study, other papers have questioned the fracture results of current randomized controlled trials citing insufficient power and significant loss to follow-up in these trials.29 Thus, in general, most of the literature to date comments on the lack of current antifracture evidence in this area and the need for further studies. The findings of our review are in keeping with these comments.
Conclusion
Implications for practice
Between 1997 and 2006, extension studies of three randomized controlled trials looked at the impact of continuation versus discontinuation of bisphosphonate therapy after 5 years of treatment in postmenopausal women with primary osteoporosis. All three trials found improved BMD with continuation of bisphosphonate therapy and, when it was examined, increased suppression of bone turnover markers. None of the trials found excess burden of continued long-term bisphosphonates in terms of extra adverse events or mortality. However, BMD and bone turnover markers are surrogate outcomes, and when pooled data were examined, the patient-important outcome of fracture risk was found to be no different between the continuation and discontinuation groups. Due to the small number and limited quality of included studies as well as differences in treatment provided (compared with that routinely used in current clinical practice), no firm conclusions or recommendations can be made.
Implications for research
This review highlights the importance, and urgency, of further research in this area. Considering how common osteoporosis is in the general population and that bisphosphonate therapy is considered first-line treatment, it is imperative that we be able to offer clinicians clear, evidence-based guidance on how to manage bisphosphonate therapy in the long term (beyond 5 years). Randomized controlled trials, using current modes of bisphosphonate therapy and specifically powered for fracture outcome, need to be performed. Similarly, to inform policy makers, cost-effectiveness studies looking at treatment beyond 5 years need to be performed.
Acknowledgments
Dr Fraser is supported by the University of Western Ontario Resident Research Career Development Program.
Appendix 1.
OVID MedLine search strategy (1950 to January week 4 2010)
| No. | Searches | Results |
|---|---|---|
| 1 | Osteoporosis/ | 27,944 |
| 2 | Osteoporosis, Postmenopausal/ | 8648 |
| 3 | Osteoporosis.mp. | 45,270 |
| 4 | 1 or 2 or 3 | 45,270 |
| 5 | Bisphosphonate$.mp. | 6888 |
| 6 | Diphosphonates/or alendronate/or clodronic acid/or etidronic acid/ | 12,926 |
| 7 | Alendronate.mp. | 2624 |
| 8 | Risedronate.mp. | 838 |
| 9 | Pamidronate.mp. | 2175 |
| 10 | Zoledronic acid.mp. | 1348 |
| 11 | Etidronate.mp. | 1048 |
| 12 | Ibandronate.mp. | 487 |
| 13 | Diphosphonate$.mp. | 11,655 |
| 14 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 16,843 |
| 15 | 4 and 14 | 5044 |
| 16 | Time factors/ | 830,953 |
| 17 | Follow-up studies/ | 393,252 |
| 18 | Follow up studie$.mp. | 397,885 |
| 19 | (stop$ or hold$ or finish$ or withold$ or withdraw$ or withdrew$ or drug holiday$ or skip$ or halt$ or cessat$ or discontinue$ or held or cease$ or suspend$).mp. [mp = title, original title, abstract, name of substance word, subject heading word, unique identifier] | 391,767 |
| 20 | 16 or 17 or 18 or 19 | 1,512,880 |
| 21 | 15 and 20 | 836 |
| 22 | Limit 21 to (clinical trial, all or clinical trial, phase iii or clinical trial, phase iv or clinical trial or comparative | 361 |
| study or controlled clinical trial or randomized controlled trial) | ||
| 23 | Limit 22 to humans | 353 |
Footnotes
Disclosure
Drs Fraser, Vogt, and Thabane have nothing to disclose. Dr Adachi has been a consultant/speaker for Amgen, Astra Zeneca, Eli Lilly, GSK, Merck, Novartis, Nycomed, Pfizer, Procter and Gamble, Roche, Sanofi-aventis, Servier, Wyeth, and Bristol-Myers Squibb and has performed clinical trials for Amgen, Eli Lilly, GSK, Merck, Novartis, Pfizer, Procter and Gamble, Sanofi-aventis, Roche, Wyeth, and Bristol-Myers Squibb.
References
- 1.Hanley DA, Josse RG. Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada: 1. Introduction. CMAJ. 1996;155(7):921–933. [PMC free article] [PubMed] [Google Scholar]
- 2.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358(14):1474–1482. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 3.Brown JP, Josse RG. Scientific Advisory Council of the Osteoporosis Society of Canada. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167(10 Suppl):1–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359(9322):2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- 5.Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23(4):508–516. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- 6.Papapoulos SE. Bisphosphonates for postmenopausal osteoporosis. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7th ed. Washington: ASBMR; 2008. p. 237. [Google Scholar]
- 7.Ott SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90(3):1897–1899. doi: 10.1210/jc.2005-0057. [DOI] [PubMed] [Google Scholar]
- 8.Shebba A. Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obs. 2008;15(6):502–507. doi: 10.1097/MED.0b013e328317ca83. [DOI] [PubMed] [Google Scholar]
- 9.Neviaser AS, Lane JM, Lenart BA, et al. Low-energy femoral shaft fractures associated with alendronate use. J Ortho Trauma. 2008;22(5):346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 10.Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover; a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 11.Goddard MS, Reid KR, Johnston JC, Khanuja HS. Atraumatic bilateral femur fracture in long-term bisphosphonate use. Orthopedics. 2009;32(8):pii. doi: 10.3928/01477447-20090624-27.. orthosupersite.com/view.asp?rID=41933 [DOI] [PubMed] [Google Scholar]
- 12.Khan AA, Sandor GK, Dore E, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36(3):478–509. doi: 10.3899/jrheum.080759. [DOI] [PubMed] [Google Scholar]
- 13.Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf. 2009;32(3):219–228. doi: 10.2165/00002018-200932030-00004. [DOI] [PubMed] [Google Scholar]
- 14.Haleem S, Lutchman L, Mayahi R, et al. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39(10):1157–1163. doi: 10.1016/j.injury.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290–1299. doi: 10.1093/aje/kwp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S, The Cochrane Collaboration, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated September 2009]. http://www.cochrane-handbook.org. Accessed March 30, 2011.
- 17.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment. The fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 18.Miller PD, Watts NB, Licata AA, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: Efficacy and safety after seven years of treatment. Am J Med. 1997;103(6):468–476. doi: 10.1016/s0002-9343(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 19.Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 20.Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2000;85(9):3109–3115. doi: 10.1210/jcem.85.9.6777. [DOI] [PubMed] [Google Scholar]
- 21.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 22.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 23.Bagger YZ, Tanko LB, Alexandersen P, et al. Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone. 2003;33(3):301–307. doi: 10.1016/s8756-3282(03)00112-1. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JR, Westfall AO, Cheng H, et al. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19(11):1613–1620. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper C. Beyond daily dosing: clinical experience. Bone. 2006;38(4 Suppl 1):S13–S17. doi: 10.1016/j.bone.2006.01.152. [DOI] [PubMed] [Google Scholar]
- 26.Geusens P. Bisphosphonates for postmenopausal osteoporosis: determining duration of treatment. Curr Osteoporos Rep. 2009;7(1):12–17. doi: 10.1007/s11914-009-0003-6. [DOI] [PubMed] [Google Scholar]
- 27.Briot K, Tremollieres F, Thomas T, Roux C. How long should patients take medications for postmenopausal osteoporosis? Joint Bone Spine. 2007;74(1):24–31. doi: 10.1016/j.jbspin.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Rosen CJ, Brown SA. A rational approach to evidence gaps in the management of osteoporosis. Am J Med. 2005;118(11):1183–1189. doi: 10.1016/j.amjmed.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Laroche M. Treatment of osteoporosis: all the questions we still cannot answer. Am J Med. 2008;121(9):744–747. doi: 10.1016/j.amjmed.2008.03.048. [DOI] [PubMed] [Google Scholar]