Abstract
Osteoporosis is a growing problem worldwide, linked to an increasingly aging population. Despite the availability of a wide variety of treatments for osteoporosis, a significant number of patients are either not being prescribed treatment or discontinue therapy as early as 6 months after initiation. The reasons for a lack of adherence are many but poor adherence increases the risk of fracture and, therefore, the disease burden to the patient and society. Results from large-scale, randomized clinical studies have shown that different osteoporosis treatments are efficacious in reducing the risk of fracture. Studies assessing the effects of discontinuing osteoporosis therapies show that some treatments appear to continue to protect patients from the risk of future fracture even when treatment is stopped. However, these trials involve patients who have been compliant with treatment for between 2 and 5 years, a situation not reflective of real-world clinical practice. In reality, patients who discontinue therapy within the first 6 months may never achieve the optimum protection from fracture regardless of which treatment they have been prescribed. Clinicians need to develop management strategies to enable patients to adhere to their treatment. This will ultimately result in better prevention of fracture and a lower burden of disease to society and patients.
Keywords: osteoporosis, adherence, treatment, persistence, compliance, fracture
Introduction
Osteoporosis is a growing problem worldwide, linked to an aging population.1 Currently, a number of options are available for the treatment of postmenopausal osteoporosis (PMO). These include oral and intravenous (IV) bisphosphonates, hormone replacement therapy (HRT), selective estrogen receptor modulators (SERMs), human parathyroid hormone (PTH) preparations, strontium ranelate, and denosumab, an inhibitor of RANKL (Receptor Activator of Nuclear Factor κB Ligand).
Despite the availability of a number of efficacious treatments for osteoporosis, the issues of how to achieve effective protection from future fracture are more complex than the availability of such treatments. Barriers to achieving maximum fracture protection include diagnosis, appropriate follow-up after fracture, and adherence of patients to prescribed treatments. Ultimately, patients who do not receive or adhere to treatment will be at risk for future fracture, something that carries a significant cost to the patient in terms of their quality of life, increased morbidity and even mortality following some fragility fractures.2 Nonadherence to treatment also increases a patient’s utilization of healthcare resources, thereby incurring a financial cost to both patient and healthcare provider. Understanding the factors which contribute to treatment effectiveness in clinical practice is, therefore, essential in order to minimize the disease burden. The aim of this review is to examine some of the barriers to diagnosis and patient adherence and to discuss the results from studies examining the consequences of discontinuing treatment.
Barriers to treatment
Lack of diagnosis
A significant barrier to achieving optimum fracture protection for an individual patient is the lack of a diagnosis of osteoporosis. Osteoporosis is often termed a ‘silent disease’ due to the lack of symptoms patients experience prior to the occurrence of a fracture. The presentation of a low-impact fracture should be a trigger to clinicians to assess fracture risk. However, a number of studies have indicated that this does not always happen. In a retrospective cohort study, 1162 women 55 years of age or older who had a distal radial fracture were identified from a claims database that includes more than 3 million patients in the USA, enrolled in multiple health plans. Of these women, only 33 (2.8%) underwent a bone-density scan and 266 (22.9%) were treated with at least one approved medication for the treatment of osteoporosis. This means that 67% of women in this study were not even assessed for their risk of fracturing. Overall, only 279 (24.0%) of the women who sustained a distal radial fracture underwent either diagnostic evaluation or treatment of osteoporosis.3 The rate of diagnosis among men with a fragility fracture is even lower than for women. In a study of community-dwelling men aged 50 years and older, only 10.3% of men with a fragility fracture reported a diagnosis of osteoporosis within 5 years of the fracture event and less than 10% were receiving treatment.4 These data highlight a clear diagnostic and treatment care gap for patients with osteoporosis.
Physician perception and recognition of osteoporosis
Examination of how physicians view osteoporosis might provide some indication of why patients are not diagnosed and treated following a fracture. Orthopedic surgeons are often the first clinicians to see a patient with a fragility fracture and as such may be best placed to make an assessment of whether a patient has osteoporosis and requires treatment. A study of the attitudes of 107 orthopedic surgeons in the USA showed that 68% thought that the orthopedic surgeon should expand their practice to include the prescribing of pharmacological treatment for osteoporosis. Up to 70% of the respondents said they would favor a program in which they initiated medical treatment and/or full assessment of patients with an apparent osteoporotic fracture, provided that a primary care physician assumed the continuation of pharmacological management and the assessment of secondary causes of osteoporosis. Despite this, only 52% of respondents stated that they routinely notify the patient’s primary care physician after seeing a patient with a probable osteoporotic fracture. This indicates that for some surgeons there is discordance between how they view their role in the identification of patients with osteoporosis and how they follow-up with primary care physicians. Most of the surgeons who responded to the survey (94%) were concerned about the adverse events (AEs) associated with treatments for osteoporosis and 47% were concerned enough about AEs that they would rather avoid prescribing them altogether.5 The study did not examine whether there were specific AEs that prevented surgeons from wanting to prescribe osteoporosis treatments. This perception by clinicians regarding the safety of treatments for osteoporosis represents another potential barrier to treatment.
Within primary care, recognition of osteoporosis by physicians has proven to be low. For example, in a large survey of more than 85,000 Australian women aged 60 years and older who visited a primary care physician, approximately 57,000 reported symptoms and/or risk factors for osteoporosis. Of the 85,000 women who took part in the survey, 29% reported fractures. Despite this, only 8% of the women reporting symptoms and or/risk factors for osteoporosis had undergone bone mineral density (BMD) testing and only 25% of women reported a diagnosis of osteoporosis.6
Rates of treatment with osteoporosis therapies
Although there is still a gap in care between the number of patients who require treatment and the number who receive it, treatment with osteoporosis therapies was shown to have increased from 7% to 31% between 1995 and 2004, in patients who had experienced a hip fracture.7 During this period a number of bisphosphonates such as alendronate and risedronate were approved for use in the treatment of osteoporosis. Although the rates of prescribing of osteoporosis medications have increased over time,8 there is evidence that prescribing of bisphosphonates is currently not increasing or is even on the decline.
The problem of noncompliance, nonpersistence and treatment discontinuation
Compliance and persistence with osteoporosis treatments
A lack of adherence (compliance and persistence) by patients to their treatment is an issue for most chronic diseases, including osteoporosis. Evidence for the efficacy of osteoporosis treatments comes from randomized, controlled clinical trials (RCTs) in which patients are carefully monitored and are in regular contact with healthcare professionals.9–16 This results in compliance and persistence rates of between 60% and 81%.11,16–18 However, in the real-world clinical practice setting, numerous studies have shown that compliance and persistence by patients to osteoporosis treatment is poor.19–26
Data from a study by Siris et al of more than 35,000 women who received a bisphosphonate prescription showed that 57% of those prescribed alendronate or risedronate (daily or weekly doses) were noncompliant and 80% were nonpersistent within 2 years of initiating treatment.25 In this study, compliance was defined using the medication possession ratio (MPR), calculated as the sum of the days’ supply divided by the follow-up time. Patients were deemed compliant if their MPR was ≥0.80. Persistence was defined as the length of time a patient received continuous therapy without a gap in refills >30 days. The study showed that noncompliance or nonpersistence with treatment led to a significant reduction in treatment benefit such that patients who only took half their medication over 2 years achieved no better efficacy than if they had never taken any medication.25 Another study by Downey et al used a managed care administration claims database from the USA to examine the adherence and persistence of 10,566 women, newly diagnosed with PMO to treatment with alendronate, risedronate or raloxifene.20 Adherence rates to treatment with both daily and weekly alendronate and risedronate were 60.7%, and 58.4% respectively, and with raloxifene were 53.9%. Persistence rates at 12 months were 21.3% for alendronate, 19.4% for risedronate and 16.2% for raloxifene. The study showed that persistence rapidly declined in the first 3 months of treatment, with a slower, more gradual decline over the following 9 months. The lack of persistence at 12 months was comparable in this study to that found by Siris et al at 2 years.25 A recent meta-analysis of 15 studies assessing adherence to osteoporosis treatment showed that the mean number of days patients persisted with alendronate, risedronate or ibandronate treatment (n = 236,540) was 184 (95% CI 164–204). The mean MPR for patients treated with alendronate or risedronate (n = 234,737) was 66.9% (95% CI 63–70).27 The results from this meta-analysis show that across multiple studies, patients are discontinuing their treatment within 6 months and that, even within this timeframe, they are not taking their medication on a regular basis.
A lack of persistence to treatment by a patient may also not be permanent or irreversible. A study of Australian veterans who were prescribed oral bisphosphonates following a fracture showed that although the majority of users (68%) did not persist with treatment, 19% of users had two treatment episodes and 13% had three or more episodes of use. In this study an episode of use was determined as the number of treatment days between the first prescription and the date of the last prescription plus 35 days.28 These data indicate that there may be opportunities for clinicians to restart their patients on treatment even when they have previously stopped taking it and that finding out the root causes of why a patient has desisted are important in increasing the length of time on treatment.
In addition, clinicians may also have a misconception of how adherent their patients are. In a survey of 412 clinicians, it was estimated that 69.2% of patients were adherent after 12 months of treatment. Pharmacy claims for the same patients showed that only 48.7% were adherent after 12 months. Clinicians’ perceptions did not significantly differ according to medication class or frequency of administration.29
Reasons for lack of compliance and persistence with osteoporosis therapies
Determining why patients are noncompliant and nonpersistent is important in enabling clinicians to select the most appropriate treatment. Numerous studies have examined the reasons why patients fail to comply with instructions on how to take their medication or stop taking their medication altogether. Common reasons for nonadherence include AEs, either real or perceived, complicated dosing regimens and a lack of knowledge surrounding osteoporosis and the importance of fracture prevention.30 AEs are an issue for all osteoporosis treatments, but events such as GI irritation, which may be associated with oral bisphosphonates, can often be made worse if patients do not take their medication according to the instructions (sufficient water, upright position and fasting state).31 Other reasons for a lack of adherence to treatment include the dosing intervals of different treatments. Studies which have examined the adherence of patients have shown that patients prefer and are more adherent to weekly versus daily alendronate32,33 and monthly versus weekly bisphosphonates.34 However, even with weekly treatment, patients still had suboptimal adherence.35 The availability of intravenous (IV) bisphosphonates such as ibandronate (quarterly dosing) and zoledronic acid (annual dosing) may lead to improved adherence by patients. A preference study of patients recently diagnosed with osteoporosis showed that out of oral bisphosphonates (daily or weekly), IV bisphosphonates every 3 months, or IV bisphosphonates once-yearly, 65% of patients preferred annual over weekly dosing.36
Adherence may also be influenced by the use of generic versions of branded medication. A recent study by Ringe and Moller of the differences in patient persistence between generic and original branded once-weekly bisphosphonates, using the clinical records of 204 women, showed clear differences in the persistence rates between generic and branded oral bisphosphonates.33 At 12 months, 68% of patients were still taking generic alendronate compared with 84% of patients taking branded alendronate (Fosamax®). The persistence rates for this study are much higher than has been seen in other clinical practice studies;19,20,25 however, it does suggest that patients are less likely to persist with generic alendronate compared with branded. Examination of the number of GI AEs experienced by patients in this study showed that significantly more patients taking generic alendronate experienced a GI AE than patients taking branded alendronate (32 patients versus 15 patients, respectively; P < 0.05).33 These data are further supported by a report showing that patients who were previously stable on doses of branded alendronate experienced an increase in AEs which resulted in treatment discontinuation after switching to a generic version of alendronate.37
Belief in the importance of taking medication for the treatment of osteoporosis and the relationship between the patient and healthcare provider are two important considerations when trying to achieve maximum adherence.30 Patients must understand the nature of the disease and how it progresses in order to compensate for the asymptomatic nature of osteoporosis. A close relationship between the healthcare provider and the patient has been shown to improve adherence by 57% among postmenopausal women with osteopenia.38
Consequences of a lack of compliance and persistence with osteoporosis therapies
A lack of adherence to osteoporosis treatment will ultimately have an effect on the degree of fracture protection patients might achieve. In a study of over 38,000 women with PMO who received a prescription for a bisphosphonate, low compliance (MPR < 80%) was found to be associated with a 31% higher risk of fracture (P < 0.0001). Hospitalization rates were 47% higher in patients with poor compliance (MPR < 50%) than in those patients with excellent compliance (MPR > 90%).22 In a later study by Penning-van Beest et al of 8,822 female bisphosphonate users, a MPR of <20% was associated with an 80% increased fracture risk compared with patients with a MPR of ≥90%.39 Results from a meta-analysis of adherence to osteoporosis treatments by Danese et al showed that the risk of fracture was 46% greater for patients with poor compliance to bisphosphonates (MPR < 80%) compared with highly compliant patients (MPR ≥ 80%), over 1–2.5 years. Examination of the fracture risk for different fracture sites showed that lack of compliance affected the risk of clinical vertebral fractures most (43%), followed by hip (28%) and nonvertebral fractures (16%).40 These findings have clear implications for wasted healthcare expenditures on unused medication, and increased outlays for hospital and other direct and indirect medical costs. Danese et al have modeled the effect of adherence on lifetime fractures in osteoporotic women who receive treatment with oral bisphosphonates.40 They compared the benefits of patients being highly compliant with treatment, both in terms of fracture rates and monetary cost, with the current state of adherence with daily and weekly bisphosphonate therapies in the USA. Optimal adherence was defined as a MPR > 90%. Using this model, the overall fracture rates per 1000 patient years for usual adherence were calculated to be 58 (95% CI 51–64) compared with 44 (95% CI 36–53) for optimal adherence. The increase in fracture costs between usual and optimal adherence was estimated to be USD $2100 (95% CI 400–7300) based on site-specific United States Department of Labor Consumer Price Index-based estimates for the 1-year direct medical cost of fracture. Despite the increase in fracture costs between usual and optimum adherence, the study showed that optimal adherence was more expensive overall, compared with usual adherence (USD $1700 [95% CI 4100 to −3300]). When this was examined further, the authors calculated that the increase in cost was due to the cost of medication (generic and branded). In this study, a number of assumptions were made in the process of assessing cost. For example, patients were assumed to be free of fracture at the time treatment was initiated and the cost of fracture after 1 year and the quality of life decrements associated with fracture were not incorporated. The authors also assumed that all patients with a T score ≤–2.5 were treated with bisphosphonates. It is clear that in clinical practice not all of these assumptions will be true, and so the estimates of the cost of adherence, both usual and optimum, should be interpreted with caution.40
Consequences of discontinuing osteoporosis treatment
The efficacy of different osteoporosis treatments has been demonstrated in a number of large-scale clinical trials (Tables 1–4). Follow-up studies have examined the clinical consequences of discontinuing treatment, information that is important given the previously discussed issues of poor persistence.
Table 1.
Summary of patient populations and treatments examined in trials of antiresorptive osteoporosis treatments
| Study | Patients | Number of patients |
Treatments |
|
|---|---|---|---|---|
| Active | Comparator | |||
| Postmenopausal Estrogen/Progestin Interventions (PEPI)18 | Healthy PM women | 875 | Conjugated equine estrogen (CEE) 0.625 mg/day CEE 0.625 mg/day and medroxyprogesterone acetate (MPA) 10 mg/day (days 1–12) CEE 0.625 mg/day and MPA 2.5 mg/day CEE 0.625 mg/day and micronised progesterone 200 mg/day (days 1–12) |
Placebo |
| Multiple Outcomes of Raloxifene Evaluation (MORE)9 | PM women 31–80 years with lumbar spine or femoral neck BMD T score ≤–2.5 or low BMD and ≥1 moderate vertebral fracture or ≥2 mild vertebral fracture, or ≥2 moderate vertebral fracture regardless of BMD | 7705 | Raloxifene 60 mg/day Raloxifene 120 mg/day |
Placebo |
| Fracture Intervention Trial (FIT)10 | PM women 55–80 years | 6459 | Alendronate 5 mg/day (for 2 years) then | Placebo |
| Clinical fracture arm | Femoral neck BMD ≤0.68 g/cm2 | 4438 | 10 mg/day (for 1 year) | |
| Vertebral fracture arm | ≥1 vertebral fracture | 2027 | ||
| Alendronate Phase III osteoporosis studies63 | PM women 45–80 years with lumbar spine BMD T score ≤–2.5 | 804 | Alendronate 5 mg/day Alendronate 10 mg/day Alendronate 20 mg/day (for 2 years then switched to 5 mg/day) |
Placebo |
| Fosamax® Interventional Trial Study (FOSIT)a,64 | PM women ≤85 years with lumbar spine BMD T score ≤–2 |
1908 | Alendronate 10 mg/day | Placebo |
| Vertebral Efficacy With Risedronate Therapy-National (VERT-NA) (USA based)13 | PM women <85 years with ≥1 vertebral fracture | 2458 | Risedronate 2.5 mg* Risedronate 5 mg |
Placebo |
| Vertebral Efficacy With Risedronate Therapy-Multinational (VERT-MN) (Worldwide)65 | PM women <85 years with ≥2 vertebral fractures | 1226 | Risedronate 2.5 mg† Risedronate 5 mg |
Placebo |
| Hip Intervention Program Study66 | PM women 70–79 years with femoral neck BMD T score ≤–4 or femoral neck T score ≤–3 and one risk factor for hip fracture PM women ≥80 years with ≥1 risk factor for hip fracture and a femoral neck BMD T score ≤–4 or femoral neck BMD T score ≤–3 and hip axis length ≥11.1 cm or greater |
5445 | Risedronate 2.5 mg‡ | Placebo |
| 3886 | Risedronate 5 mg‡ | |||
| Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE)12 | PM women with lumbar spine BMD | 2946 | Ibandronate 2.5 mg/day | Placebo |
| T score ≤–2.0 and 1–4 vertebral fractures | Ibandronate 20 mg every other day for 12 doses every 3 months | |||
| Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Pivotal Fracture Trial11 | PM women with femoral neck BMD T score ≤–2.5 or T score ≤–1.5 and ≥2 mild vertebral fractures or 1 moderate vertebral fracture | 7765 | Zoledronic acid 5 mg once yearly | Placebo |
| Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Recurrent Fracture Trial59 | Men and women with a surgically repaired hip fracture (within previous 90 days) | 1065 | Zoledronic acid 5 mg once yearly | Placebo |
| Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM)17 | PM women 60–90 years with lumbar spine or total hip BMD T score ≤–2.5 | 7868 | Denosumab 60 mg/3 months | Placebo |
| Spinal Osteoporosis Therapeutic Intervention (SOTI)15 | PM women ≥50 years with lumbar spine BMD ≤0.840 g/cm2 and ≥1 vertebral fracture |
1649 | Strontium ranelate 2 g/day | Placebo |
| Treatment of Peripheral Osteoporosis (TROPOS)b,16 | PM women ≥74 years with femoral neck BMD T score ≤–2.5 or 70–74 years and one additional risk factor |
5091 | Strontium ranelate 2 g/day | Placebo |
Notes: All trials were 3-year, randomized, double-blind, placebo-controlled with the exception of:
1-year trial;
5-year trial;
This dose was discontinued after 1 year;
This dose was discontinued after 2 years;
Doses were combined for analysis of efficacy.
Abbreviations: PM, postmenopausal; BMD, bone mineral density.
Table 4.
Summary of results from trials of anabolic osteoporosis treatments
| Study | Treatments |
Fracture reduction versus placebo over 18 months (RR [95% CI]) |
Change from baseline over 18 months (percentage) |
|||||
|---|---|---|---|---|---|---|---|---|
| Vertebral | Nonvertebral | Hip | Lumbar spine BMD | Femoral neck BMD | Serum CTX | Serum P1NP | ||
| Fracture Prevention Trial (FPT)14 | Placebo | 1.1% | –0.7% | N/R | N/R | |||
| Teriparatide 20 μg | 0.35 (0.22–0.55), P ≤ 0.001 | 0.47 (0.25–0.88) | N/R | 9.7%, P < 0.001 versus placebo | 2.8%, P < 0.001 versus placebo | N/R | N/R | |
| Teriparatide 40 μg | 0.31 (0.19–0.50), P ≤ 0.001 | 0.46 (0.25–0.86) | N/R | 13.7%, P < 0.001 versus placebo | 5.1%, P < 0.001 versus placebo | N/R | N/R | |
| Treatment of Osteoporosis with Parathyroid Hormone Study (TOPS)71 | Placebo | –0.323% | –0.69 | N/R | N/R | |||
| PTH (1–84) 100 μg/day | 0.42 (0.24–0.72), P = 0.001 | P = NS | N/R | 6.53%, P < 0.001 versus placebo | 1.78, P < 0.001 versus placebo | Urinary NTX 85% | 80% | |
Note: All trials were 18-month, randomized, double-blind, placebo-controlled.
Abbreviations: CI, confidence interval; N/R, not reported; RR, risk reduction; BMD, bone mineral density.
HRT and SERMs
In a study of older women aged 65–77 years who received 3 years of treatment with estrogen or placebo and 2 years of no further treatment, overall spine BMD was not significantly different to baseline at the end of 5 years. The lack of overall difference in BMD was the result of a loss of BMD during the 2 years of no treatment that counteracted the gains made during active treatment.41 The fall in BMD after discontinuation of estrogen was also accompanied by a rapid increase (rebound) back to baseline in the bone turnover marker (BTM) urinary crosslinked N-telopeptide of type I collagen (urinary NTX), indicating a significant increase in bone remodeling in this population of women.41 Although this study has shown a rapid loss of BMD after discontinuation of treatment, a follow-up study (5, 11 or 15 years) of healthy postmenopausal women who had received 2–3 years of treatment with HRT in their early postmenopausal years showed that despite a loss of BMD once treatment was stopped, patients previously treated with HRT had a significantly lower risk of vertebral fracture at the time of follow-up compared with women on placebo (odds ratio 0.47 [95% CI 0.24–0.93]; P = 0.03). The risk of nonvertebral fractures was 32% lower in patients treated with HRT, compared with patients in the placebo group, but did not reach statistical significance.42
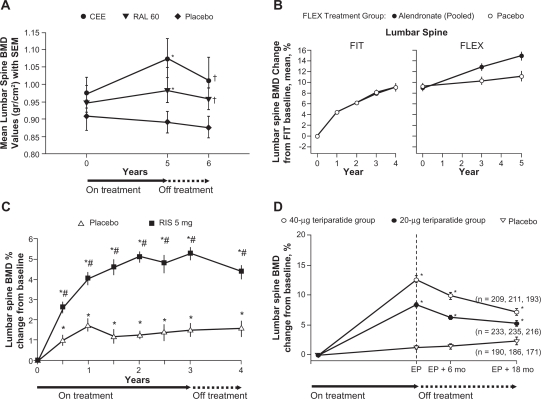
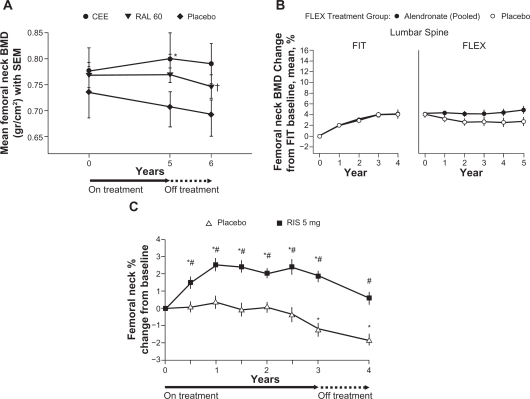
Data from a study of postmenopausal women who were randomized to receive treatment with raloxifene 60 mg or estrogen for 5 years showed that despite significant increases in lumbar spine BMD versus baseline (P = 0.024 and P = 0.045, respectively), within 1 year of discontinuation of treatment lumbar spine BMD was significantly lower compared with the end-of-treatment values (P = 0.001 and P = 0.012, respectively) (Figure 1A). A significant decline in femoral neck BMD was also observed after discontinuation of raloxifene treatment (Figure 2A).43
Figure 1.
Changes in lumbar spine BMD: A) during, and after treatment with raloxifene (mean ± SEM [g/cm2]);43 B) during alendronate treatment in the FIT trial and alendronate or placebo treatment in the FLEX trial (mean percent change from baseline);44 C) during 3 years of blinded treatment with placebo or risedronate 5 mg daily, followed by 1 year of open label treatment with calcium (and vitamin D, if needed) [mean percent change from baseline];46 D) during and after treatment with teriparatide in women who did not use any osteoporosis drugs during the 18 month follow-up period (mean percent change from baseline).51
Notes: A) *P < 0.05 for within-group analysis (baseline versus. treatment); †P < 0.05 for within-group analysis (treatment versus. post-treatment); B) BMD = bone mineral density, FIT = Fracture Intervention Trial, FLEX = Fracture Intervention Trial Long-term Extension. Error bars indicate 95% confidence intervals. Data are shown for the period spanning the beginning of FIT through the completion of FLEX, a total of 10 years; C) *P < 0.05 from baseline based upon a paired t-test; #P < 0.05 from placebo; D) EP = end point of the Fracture prevention Trial (end of teriparatide treatment), *P < 0.001.
Figure 2.
Changes in femoral neck BMD: A) during and after treatment with raloxifene (mean ± SEM [g/cm2]);43 B) during alendronate treatment in the FIT trial and alendronate or placebo treatment in the FLEX trial (mean percent change from baseline);44 C) during 3 years of blinded treatment with placebo or risedronate 5 mg daily, followed by 1 year of open label treatment with calcium (and vitamin D, if needed) [mean percent change from baseline].46
Notes: A) *P < 0.05 for within-group analysis (baseline versus treatment); †P < 0.05 for within-group analysis (treatment versus post-treatment); B) BMD = bone mineral density, FIT = Fracture Intervention Trial, FLEX = Fracture Intervention Trial Long-term Extension. Error bars indicate 95% confidence intervals. Data are shown for the period spanning the beginning of FIT through the completion of FLEX, a total of 10 years; C) *P < 0.05 from baseline based upon a paired t-test’ #P < 0.05 from placebo.
Bisphosphonates
Alendronate
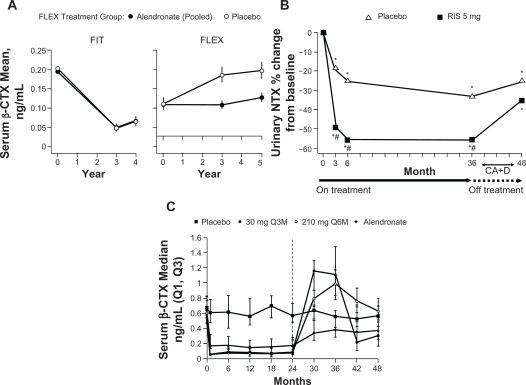
In the FIT Long-term Extension (FLEX) follow-up study women who had received treatment with alendronate for 5 years during the FIT trial were re-randomized to receive alendronate 5 or 10 mg/day or placebo for a further 5 years.44 Women randomized to placebo, and who therefore discontinued treatment with alendronate, showed a moderate decline in BMD at the total hip (–3.38%), femoral neck (–1.48%) (Figure 2B) and trochanter (–3.25%) at the end of 5 years of placebo treatment, compared with the end of treatment with alendronate. In contrast, lumbar spine BMD increased for the same women during this period (Figure 1B). Mean levels of BTMs gradually increased in women who discontinued treatment with alendronate compared with women who continued taking alendronate. However, when compared with pretreatment levels in the FIT study 10 years previously, levels of serum C-telopeptide of type I collagen (CTX) (Figure 3A) and bone alkaline phosphatase (ALP) at the end of 5 years of placebo treatment had not returned to baseline pretreatment levels.44 Despite the changes in BMD and BTMs there were no significant differences in RR of all clinical fractures or nonvertebral fractures between women who continued with alendronate treatment and those who received placebo treatment over the 5-year follow-up. A significant difference in risk of clinical vertebral fractures was observed, with a RR of a clinical vertebral fracture of 0.45 (95% CI 0.24–0.85) for women who continued with alendronate treatment versus those who switched to placebo. A post-hoc analysis of the data showed that in women who did not have a vertebral fracture at FLEX baseline, continuation of treatment with alendronate reduced nonvertebral fractures in women with FLEX baseline femoral neck T-scores <–2.5 (RR 0.50; 95% CI 0.26–0.96) but not with T-scores >–2.5 and <–2 (RR 0.79; 95% CI 0.37–1.66) or with T-scores >–2 (RR 1.41; 95% CI 0.75–2.66) (p for interaction = 0.019). In the group of women with a vertebral fracture at baseline, there were no significant interactions between FLEX baseline femoral neck BMD and the effect of continued treatment with alendronate on any fracture outcomes, although the numbers of women who experienced a fracture were small.45 In patients who received placebo, neither prevalent vertebral fracture at baseline or femoral neck T-score had a significant interaction on the risk of nonvertebral or clinical vertebral fractures. However, data from this group were not analyzed using both parameters together.44 It should perhaps be noted that in this study, only 72% of patients were still taking their assigned medication at the end of the 5-year study.44
Figure 3.
Changes in bone resorption markers: A) serum β-CTX during alendronate treatment in the FIT trial and alendronate or placebo treatment in the FLEX trial (mean);44 B) urinary NTX during 3 years of blinded treatment with placebo or risedronate 5 mg daily, followed by 1 year of open label treatment with calcium (and vitamin D, if needed) [median percent change from baseline];46 C) serum β-CTX during and after treatment with denosumab (median value, ng/mL).49
Notes: A) FIT = Fracture Intervention Trial, FLEX = Fracture Intervention Trial Long-term Extension. Error bars indicate 95% confidence intervals. Data are shown for the period spanning the beginning of FIT through the completion of FLEX, a total of 10 years; B) *P < 0.05 from baseline based upon a Signed Rank t-test, #P < 0.05 from placebo; C) Group receiving 30 mg Q3M discontinued denosumab treatment at Month 24 and were retreated with 60 mg Q6M denosumab at Month 36. Groups receiving 210 mg Q6M or alendronate discontinued treatment for the last 24 months. The dashed line at Month 24 indicates the time at which dosing was reallocated.
Risedronate
Follow-up assessment of 599 women with PMO who took part in the Vertebral Efficacy with Risedronate Therapy trials showed that 1 year after stopping treatment, lumbar spine BMD had significantly decreased compared with placebo at the end of 3 years of treatment (Figure 1C). However, the decline was gradual and BMD remained significantly above baseline levels and above the lumbar spine BMD of the original placebo group. A significant decline in femoral neck BMD also occurred in the year after treatment was discontinued (Figure 2C). Levels of urinary NTX and bone ALP gradually rose following discontinuation, as with treatment with alendronate, levels did not return to baseline within the follow-up period (Figure 3B). Despite gradual but significant declines in BMD, there remained a significant reduction in risk of vertebral fractures in the former risedronate group compared with the placebo group, 1 year after treatment was stopped.46
Ibandronate
There is currently a lack of published information regarding the clinical consequences of discontinuing treatment with ibandronate.
Zoledronic acid
Devogelaer et al showed that in a Phase II, dose-ranging study of treatment with zoledronic acid 4 mg over 5 years, women with PMO who received 3 years of treatment and were then followed up for 2 years showed an overall gain in BMD at the end of treatment which was maintained during the years without treatment.47 Lumbar spine BMD at 5 years had increased by 9.01% compared with baseline, proximal femur by 4.92%, distal radius by 2.60% and total body by 3.59%. Analysis of serum levels of CTX and bone ALP show that once treatment had been stopped, levels of both markers gradually rose but did not return to baseline during the follow-up period. Levels were also well within the normal range for the duration of the study and follow-up.47 Data from a 3-year extension to the HORIZON-PFT are expected shortly and should provide information about the effects of continuing versus discontinuing treatment with zoledronic acid.
RANKL inhibitors
Denosumab
Data on the effects of discontinuing treatment with denosumab are limited; however, in a Phase III trial, 332 postmenopausal women with low bone mass who received treatment with either denosumab 60 mg or placebo every 6 months for 2 years showed a rapid decrease in lumbar spine and total hip BMD back to baseline within 12 months of discontinuing treatment. Total hip BMD continued to decrease over the next 12 months.48 Data on changes in BTMs for this study are currently unavailable. However, in a phase II study, discontinuation of denosumab 30 mg administered every 3 months, resulted in an increase in serum CTX and bone ALP that went beyond baseline levels within 12 months (Figure 3C).49 There are no published data on the antifracture efficacy of denosumab after discontinuing treatment; however, due to the rapid loss of BMD and increase in BTMs observed thus far, data on how the risk of future fracture is affected once treatment is stopped is needed to determine whether antifracture efficacy with denosumab is long lasting.
Strontium ranelate
Strontium ranelate consists of two atoms of stable strontium and an organic moiety (ranelic acid) that when taken orally exerts modest antiresorptive and anabolic effects on bone.15 In the SOTI trial extension, after 4 years of treatment with strontium ranelate, patients crossed over from active treatment to placebo for a further year. During the year patients were taking placebo, lumbar spine BMD decreased compared with patients who remained on treatment who experienced an increase in lumbar spine BMD. BTMs also changed during the year of treatment with placebo; within 3 months of moving to placebo, significant decreases in bone ALP and significant increases in serum CTX were observed. Despite changes in both lumbar spine BMD and BTMs, the difference in annual vertebral fracture incidence between patients who continued on active treatment and those who moved to placebo was nonsignificant. This was also true for the proportion of patients with new nonvertebral fractures.50
Bone-forming agents
Parathyroid hormone (PTH)
Both teriparatide (PTH 1–34) and full length PTH (1–84) are anabolic agents for bone, which stimulate osteoblastic new bone formation and hence an increase in bone mass.
Teriparatide is a recombinant form of human PTH (1–34). In a follow-up to the Fracture Prevention Trial, discontinuation of treatment resulted in a gradual reduction in BMD over 18 months, which did not fall below baseline (Figure 1D).51 This was associated with a sustained reduction in vertebral fracture risk over 18 months of follow-up.
In the Parathyroid Hormone and Alendronate (PaTH) study, postmenopausal women with osteoporosis were randomized to receive treatment with PTH (1–84) for 1 year followed by placebo for 1 year, PTH (1–84) and alendronate for 1 year followed by alendronate for 1 year or alendronate for 2 years. During the year of treatment with placebo that followed 1 year of treatment with PTH (1–84), BMD at the lumbar spine declined but did not return to baseline levels. Femoral neck BMD increased and total hip BMD did not change during the same period. Both P1NP and serum CTX decreased during the year of treatment with placebo with levels of P1NP returning to baseline at the end of 12 months and levels of serum CTX returning close to baseline. The proportion of fractures did not differ between any of the treatment groups over the 2-year period of the study.52
Osteoporosis treatments in clinical trials: summary
Clinical studies of different classes of osteoporosis treatments show that whilst all approved treatments increase bone density and provide a degree of fracture protection, the effects of discontinuing treatment differ both between classes and within them. The consequences of discontinuing treatment with estrogen or raloxifene are a rapid loss of BMD and an increase in BTMs. Despite this, there is some evidence that for healthy postmenopausal women who received treatment with HRT during their early postmenopausal years, a degree of vertebral fracture protection remained for a number of years after treatment was stopped.
For bisphosphonates, studies have shown that the antifracture efficacy may continue even when treatment is discontinued. This could be explained by the fact that bisphosphonates bind tightly to bone and once bound are released slowly during the process of bone turnover,53 potentially making the bisphosphonate continuously available for some time after the patient has stopped taking the treatment. Overall, even when treatment for 3 years with alendronate and risedronate is discontinued, some anti-fracture efficacy remains. Decreases in BMD were gradual and did not return to baseline within 1 year of discontinuing risedronate treatment46 and 3 years of discontinuing alendronate.44 Changes in BTM upon discontinuation of treatment do differ between alendronate and risedronate. For both drugs, BTMs increased after discontinuation; however, the degree of increase and the timeframe over which the increase occurs differ between the two drugs.44,46 The clinical significance of these differences is currently unknown. What is also unclear from the available discontinuation data is which patients are likely to achieve the most comprehensive antifracture efficacy from continued treatment. Subgroup analysis of the FLEX study indicates that it is patients who remain at high risk for fracture after 5 years of treatment who benefit from continuing with treatment; however, numbers of fractures in this study were small and so the results should be interpreted with caution.44 Analysis of data from the HORIZON-PFT extension study will be required to determine if a similar pattern exists following treatment with zoledronic acid.
Data on the effects of discontinuing treatment with RANKL inhibitors are limited. A phase III study of denosumab showed that when treatment was halted there was a rapid decrease in lumbar spine and total hip BMD back to baseline within 12 months48 and a phase II study showed an increase in BTMs to beyond baseline levels within the same period.49 There are currently no data on whether antifracture efficacy continues when patients stop treatment with denosumab.
When treatment with strontium ranelate is stopped, BMD decreases and BTMs increase although within the first 12 months after treatment is halted, there is no reduction in antifracture efficacy.50 Halting treatment with PTH results in a gradual decline in BMD but without a loss in vertebral fracture risk reduction during the first 18 months after discontinuing treatment.51
Drug holidays
The issue of whether physicians should consider a drug holiday for patients taking osteoporosis treatments is closely related to the consequences of discontinuing versus continuing treatment over the long term. The FLEX study, as previously discussed, showed that an additional 5 years of alendronate treatment following an initial 5 years did not significantly reduce the risk of morphometric vertebral fractures or nonvertebral fractures in the overall population.44 However, in women with low femoral neck BMD, there was a significant reduction in risk of nonvertebral fractures with continued treatment.45 In addition, the results from the follow-up to the VERT studies of risedronate showed that fracture risk remained reduced 1 year after treatment was discontinued.46 These data suggest that patients may be able to take a break from treatment without incurring additional fracture risk. However, the post-hoc analysis of women with low femoral neck BMD in the FLEX study suggest that patients who may be considered to still be at high risk for fracture after 5 years of treatment should continue with treatment.
The issue of which patients may be suitable for a drug holiday, and the length of that holiday, is one that requires substantially more research on the long-term benefits versus the risks of specific treatments from well-designed, placebo-controlled trials. It is likely that only certain subgroups of low risk patients should be considered for a break in treatment with patients at high risk remaining on treatment.
Drug holidays also need to be carefully considered in light of the issues of poor treatment adherence. If patients have not adhered to treatment, then the expected gains in BMD, decreased bone turnover and ultimately, protection from fracture, are unlikely to exist. Therefore, when a patient is assessed after several years of treatment, the assumption that they have received the equivalent benefit to that observed by patients participating in clinical trials may be false. Given that studies have shown that physicians can overestimate the adherence of their patients, reassessment of the patient’s risk factors for fracture is critical to ensure that discontinuing treatment is the appropriate course of action for a specific patient. Ongoing reassessment of patients who stop treatment should also be considered.
Discussion
Osteoporosis and the fragility fractures associated with the disease are an increasing problem and pose an ever-growing burden to a society with an increasing elderly population. However, there are still a number of barriers to achieving effective fracture protection for patients, despite the availability of a wide range of efficacious treatments. These include obtaining a diagnosis, being prescribed an efficacious treatment and compliance with the treatment regimen.
Optimum fracture protection for patients with osteoporosis requires that they adhere to treatment. Despite this, numerous papers report that many patients do not continue with treatment after diagnosis.24,25,27,29–31,54 Factors affecting adherence include a) a belief in the importance of taking medication for osteoporosis,30 b) beliefs regarding medications and health in general,55,56 c) doctor and other healthcare provider relationships38,55 and d) information exchange between healthcare professionals and patients.38 A wide variety of interventions have been studied to improve adherence. These include: monitoring a patient’s BTMs and providing feedback to them based on their response to therapy, structured patient education presented face-to-face or via brochures, letters, or telephone calls, telephone patient counseling in order to address individual barriers to medication adherence and use of a weekly dose versus a monthly dose together with a patient support program.57 The authors of a systematic review of such interventions concluded that one-dimensional strategies for improving adherence were ineffective and that, as with other chronic long-term diseases, the most effective strategies were likely to be multifaceted.57 Results from several studies and a recent meta-analysis suggest that osteoporosis therapy also reduces mortality risk in women and possibly men.58–61 This reported mortality benefit should have an impact on the way osteoporosis treatment is viewed and used, and should help to encourage patients to be more compliant.
If clinicians are aware that even with interventions to improve adherence to treatment, some patients will still continue to be nonadherent, it is important that they know what happens when treatment is discontinued. Differences in the design of follow-up or discontinuation studies of individual osteoporosis treatments mean that it is not possible to make direct comparisons between different treatments. Although all the treatments discussed have shown BMD declining once treatment is discontinued, the rate of loss of bone density differs according to treatment. Markers of bone turnover have also increased once treatment has been discontinued; however, the rate at which BTMs rise also differs between treatments. Examination of the reduction in fracture risk in patients once they have stopped treatments such as alendronate, risedronate or strontium indicates that some degree of fracture risk reduction may remain; although these data are mostly uncontrolled and therefore must be interpreted with caution. Patients in these studies were taking active treatment for between 3 and 5 years prior to treatment being stopped and it is therefore likely that these patients had achieved and were maintaining the maximum fracture protection from each of the respective treatments when it was halted. This level of fracture protection may not be achieved in patients in the real-world as adherence studies indicate that a large number of patients discontinue treatment within the first 6 months. It is also important to examine which patient subgroups maintained their fracture protection once treatment had been discontinued. This is highlighted by the results from the FLEX trial of alendronate which showed that only patients with a low femoral neck BMD T-score after 5 years of alendronate treatment continued to benefit from an additional 5 years of treatment. The lack of increased efficacy and the potential safety concerns associated with long-term treatment have led to questions regarding whether clinicians should instigate a drug holiday for patients who have taken osteoporosis treatments, specifically bisphosphonates for 5 years or more. Although data on this topic are limited, it may be appropriate for patients who continue to be at high risk for fracture to remain on treatment and patients at low risk for fracture to cease treatment and continue to be monitored.
Overall, the consequences of not being treated or not adhering to treatment are high for patients with osteoporosis. Without effective treatment and adherence to treatment, patients will continue to be at high risk for fracture and the associated morbidity and mortality. Clinicians must ensure that their patients understand the significance of the disease, the importance of their treatment and are able to discuss with them any issues they face whilst taking a specific treatment.
Conclusion
Patients who are not diagnosed or followed-up after a fragility fracture are at high risk of experiencing another fracture in the future. Once diagnosed, patients who fail to comply with treatment for osteoporosis are known to increase their risk of future fracture regardless of the efficacy of the treatment prescribed.62 Despite the availability of a range of efficacious treatments, a lack of adherence is a well recognized issue. Failure to tackle this has consequences both for the patient, in terms of avoidable morbidity and mortality, and for healthcare providers who are required to invest more time and fund additional treatment. Given that osteoporosis is an ever-growing burden, improving adherence to treatment will require clinicians to develop multifactorial approaches to adherence and to tackle issues specific to individual patients. Additional data are required on the long-term changes in fracture risk when treatments are discontinued to help clinicians determine how to provide their patients with optimum protection from fracture.
Table 2.
Summary of results from trials of antiresorptive osteoporosis treatments
| Study | Treatments |
Fracture reduction versus placebo over 3 years (RR [95% CI]) |
Mean change from baseline over 3 years (percentage) |
|||||
|---|---|---|---|---|---|---|---|---|
| Vertebral | Nonvertebral | Hip | Lumbar spine BMD | Femoral neck BMD | Serum CTX | Serum bone ALP | ||
| Postmenopausal Estrogen/Progestin Interventions (PEPI)18 | Placebo | –2.8% | N/R | N/R | N/R | |||
| Conjugated equine estrogen (CEE) 0.625 mg/day | N/R | N/R | N/R | All active treatments = 5.1% | N/R | N/R | ||
| CEE 0.625 mg/day and medroxyprogesterone acetate (MPA) 10 mg/day (days 1–12) | ||||||||
| CEE 0.625 mg/day and MPA 2.5 mg/day | ||||||||
| CEE 0.625 mg/day and micronised progesterone 200 mg/day (days 1–12) | ||||||||
| Multiple Outcomes of Raloxifene Evaluation (MORE)9 | Placebo | N/R | N/R | Urinary CTX –8.1% | N/R | |||
| Raloxifene 60 mg/day | 0.7 (0.5–0.8) | Raloxifene combined group = 0.9 (0.8–1.1) | N/R | Change versus placebo 2.1%, P < 0.001 | Change versus placebo 2.65, P < 0.001 | Urinary CTX –34.0%, P < 0.001 versus placebo | N/R | |
| Raloxifene 120 mg/day | 0.5 (0.4–0.7) | N/R | N/R | Change from placebo 2.7%, P < 0.001 | Change from placebo 2.7%, P < 0.001 | Urinary CTX –31.5%, P < 0.001 versus placebo | N/R | |
| Fracture Intervention Trial (FIT)10 | Placebo | N/R | N/R | N/R | N/R | |||
| Combined population | Alendronate 5 mg/day (for 2 years) then 10 mg/day (for 1 year) | 0.52 (0.42–0.66), P < 0.001 | 0.73 (0.61–0.87), P < 0.001 | 0.47 (0.26–0.79), P = 0.005 | N/R | N/R | N/R | N/R |
| Clinical fracture arm67 | Placebo | 1.5% | –0.8% | N/R | N/R | |||
| Alendronate 5 mg/day (for 2 years) then 10 mg/day (for 2 years)a | 0.56 (0.39–0.80) | 0.88 (0.74–1.04) | 0.79 (0.43–1.44) | 8.3%, P < 0.001 versus placebo | 3.8%, P < 0.001 versus placebo | N/R | N/R | |
| Vertebral fracture arm68 | Placebo | N/R | N/R | |||||
| Alendronate 5 mg/day (for 2 years) then 10 mg/day (for 1 year) | 0.45 (0.27–0.72) | 0.80 (0.63–1.01) | 0.49 (0.23–0.99) | Change versus placebo 6.2%, P < 0.001 versus placebo | Change versus placebo 4.1%, P < 0.001 versus placebo | N/R | N/R | |
| Alendronate Phase III Osteoporosis Studies63 | Placebo | ∼–0.8%† | ∼–1.2%† | N/R | N/R | |||
| Alendronate 5 mg/day | Pooled alendronate data = 0.52 (0.28–0.95) | P = NS | N/R | ∼5.0, P = NS versus placebo† | ∼1.7, P = NS versus placebo† | N/R | N/R | |
| Alendronate 10 mg/day | N/R | N/R | N/R | ∼8.2%, P < 0.001 versus placebo† | ∼3.2%, P < 0.001 versus placebo† | N/R | N/R | |
| Alendronate 20 mg/day (for 2 years then switched to 5 mg/day) | N/R | N/R | N/R | ∼7.8%, P = NS versus placebo† | ∼4.7%, P = NS versus placebo† | N/R | N/R | |
| Fosamax® Interventional Trial Study (FOSIT)b,64 | Placebo | 0.1%, P = NS versus baseline | –0.2, P = NS versus baseline | Urinary NTX –21%, | –11% | |||
| Alendronate 10 mg/day | N/R | After 1 year 0.53 (0.30–0.90) | N/R | 5.0%, P < 0.001 versus baseline 1.1%, P < 0.05 versus baseline |
2.3%, P < 0.001 versus baseline –1.2%, P < 0.05 versus baseline |
Urinary NTX –74%, P < 0.001 versus placebo N/R |
–52%, P < 0.001 versus placebo Median change –7% |
|
| Vertebral Efficacy With Risedronate Therapy-National (VERT-NA)13 | Placebo | |||||||
| Risedronate 5 mg | 0.59 (0.43–0.82), P = 0.001 | 0.60 (0.39–0.94), P = 0.02 | N/R | 5.4%, P < 0.05 versus baseline | 1.6%, P < 0.05 versus baseline | N/R | Median change –33% | |
| Vertebral Efficacy With Risedronate Therapy-Multinational (VERT-MN)69 | Placebo | N/R | N/R | N/R | N/R | |||
| Risedronate 5 mg | 0.51 (0.36–0.73), P < 0.001 | P = NS | N/R | Change versus placebo 5.9% (95% CI 4.5–7.3), P < 0.001 | Change versus placebo 3.1% (95% CI 1.8–4.5), P < 0.001) | N/R | N/R | |
| Hip Intervention Program Study66 | Placebo | N/R | N/R | N/R | N/R | |||
| Risedronate 5 mg* | N/R | N/R | All women = 0.7 (0.6–0.9), P = 0.02 Women aged 70–79 years = 0.6 (0.4–0.9), P = 0.009 Women aged ≥80 years = no significant reduction |
N/R Change versus placebo 3.4%, P < 0.001 N/R |
N/R | N/R | N/R | |
| Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE)12,70 | Placebo | 1.3% | –0.6% | N/R | N/R | |||
| Ibandronate 2.5 mg/day | 0.38 (41–75), P = 0.0001 | P = NS | N/R | 6.5% | 2.8% | Urinary CTX –65.3%, P < 0.001 versus placebo | N/R | |
| Ibandronate 20 mg every other day for 12 doses every 3 months | 0.50 (26–66), P = 0.0006 | P = NS | N/R | 5.7% | 2.4% |
Urinary CTX –52.7%, P < 0.001 versus placebo Mean value at 36 months 0.473 ng/mL |
N/R Mean value at 36 months 53.43 ng/mL |
|
| Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Pivotal Fracture Trial11 | Placebo | |||||||
| Zoledronic acid 5 mg once yearly | 0.30 (0.24–0.38), P < 0.001 | 0.75 (0.64–0.87), P < 0.001 | 0.59 (0.42–0.83), P = 0.002 | Change versus placebo 6.71% (95% CI 5.69–7.74) | Change versus placebo 5.06% (95% CI 4.76–5.36) | Mean value at 36 months 0.205 ng/mL | Mean value at 36 months 10.61 ng/mL | |
| Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Recurrent Fracture Trial59 | Placebo | N/R | 0.7% | N/R | N/R | |||
| Zoledronic acid 5 mg once yearly | 0.54 (0.32–0.92), P = 0.02 | 0.73 (0.55–0.98), P = 0.03 | P = NS | N/R | 3.6%, P < 0.001 versus placebo | N/R | N/R | |
| Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM)17 | Placebo | N/R | N/R | N/R | N/R | |||
| Denosumab 60 mg/3 months | 0.32 (0.26–0.41), P < 0.001 | 0.80 (0.67–0.95), P = 0.01 | 0.60 (0.37–0.97), P = 0.04 | Change versus placebo 9.2%, P < 0.001 versus placebo | N/R | Change versus placebo –72% | N/R | |
| Spinal Osteoporosis Therapeutic Intervention (SOTI)15 | Placebo | N/R | N/R | ∼520 pmol/L‡ | ∼1.4 ng/mL‡ | |||
| Strontium ranelate 2 g/day | 0.59 (0.48–0.73), P < 0.001 | P = NS | N/R | Change versus placebo 14.4%, P < 0.001 versus placebo | Change versus placebo 8.3%, P < 0.001 versus placebo | ∼290 pmol/L‡ | ∼2.35 ng/mL‡ | |
| Treatment of Peripheral Osteoporosis (TROPOS)c16 | Placebo | N/R | N/R | N/R | N/R | |||
| Strontium ranelate 2 g/day | 0.61 (0.51–0.73), P < 0.001 | 0.84 (0.702–0.995), P = 0.04 | P = NS | N/R | Change versus placebo 8.2%, P < 0.001 versus placebo | N/R | N/R | |
Notes: All trials were 3-year, randomized, double-blind, placebo-controlled with the exception of:
Mean treatment duration was 4.2 years;
1-year trial;
5-year trial;
Risedronate 2.5 mg and 5 mg doses were combined for analysis of efficacy;
Estimated from Figure 1 from Liberman UA, et al. (1995) N Engl J Med. 333:1437–1443;
Estimated from Figure 4 from Meunier PJ, et al (2004). N Engl J Med. 350:459–468.
Abbreviations: CI, confidence interval; N/R, not reported; RR, risk reduction; NS, not significant; BMD, bone mineral density; CTX, serum C-telopeptide of type I collagen; ALP, alkaline phosphatase.
Table 3.
Summary of patient populations and treatments examined in trials of anabolic osteoporosis treatments
| Study | Patients | Number of patients |
Treatments |
|
|---|---|---|---|---|
| Active | Comparative | |||
| Fracture Prevention Trial (FPT)14 | PM women with ≥1 moderate or ≥2 mild vertebral fractures | 1637 | Teriparatide 20 μg Teriparatide 40 μg |
Placebo |
| Treatment of Osteoporosis with Parathyroid Hormone Study (TOPS)71 | PM women 45–54 years with lumbar spine, femoral neck or total hip BMD T score ≤–3.0 or BMD T score = −2.5 and 1–4 vertebral fractures PM women aged ≥55 years with BMD T score ≤–2.5 or BMD T score ≤–2.0 and 1–4 vertebral fractures |
2532 | PTH (1–84) 100 μg/day | Placebo |
Note: All trials were 18-month, randomized, double-blind, placebo-controlled.
Abbreviations: PM, postmenopausal; BMD, bone mineral density; PTH, parathyroid hormone.
Footnotes
Disclosure
J.D. Adachi, Research support and consultant: Amgen, Astra Zeneca, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Nycomed, Pfizer, Procter and Gamble, Roche, Sanofi Aventis, Servier, Warner Chilcott, Wyeth.
R.G.G. Russell, Research support from Warner Chilcott Pharmaceuticals, Consultant/speaker activities Amgen, GlaxoSmithKline, Novartis, Lilly, Roche, Warner Chilcott Pharmaceuticals.
R.G. Josse, Advisory board member or consultant/speaker activities: Amgen, GlaxoSmithKline, Novartis, Lilly, Warner Chilcott, Pfizer.
References
- 1.Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 2006;32(4):617–629. doi: 10.1016/j.rdc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6(2):99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 3.Freedman KB, Kaplan FS, Bilker WB, Strom BL, Lowe RA. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am. 2000;82-A(8):1063–1070. doi: 10.2106/00004623-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Papaioannou A, Kennedy CC, Ioannidis G, et al. The osteoporosis care gap in men with fragility fractures: the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2008;19(4):581–587. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skedros JG, Holyoak JD, Pitts TC. Knowledge and opinions of orthopaedic surgeons concerning medical evaluation and treatment of patients with osteoporotic fracture. J Bone Joint Surg Am. 2006;88(1):18–24. doi: 10.2106/JBJS.D.02949. [DOI] [PubMed] [Google Scholar]
- 6.Eisman J, Clapham S, Kehoe L. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res. 2004;19(12):1969–1975. doi: 10.1359/JBMR.040905. [DOI] [PubMed] [Google Scholar]
- 7.Cadarette SM, Katz JN, Brookhart MA, et al. Trends in drug prescribing for osteoporosis after hip fracture; 1995–2004. J Rheumatol. 2008;35(2):319–326. [PMC free article] [PubMed] [Google Scholar]
- 8.Stafford RS, Drieling RL, Hersh AL. National trends in osteoporosis visits and osteoporosis treatment; 1988–2003. Arch Intern Med. 2004;164(14):1525–1530. doi: 10.1001/archinte.164.14.1525. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 10.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11):4118–4124. doi: 10.1210/jcem.85.11.6953. [DOI] [PubMed] [Google Scholar]
- 11.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 12.Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 13.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 14.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 15.Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med. 2004;350(5):459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 16.Reginster JY, Seeman E, de Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90(5):2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, San MJ, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 18.The Writing Group for the PEPI Trial Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276(17):1389–1396. [PubMed] [Google Scholar]
- 19.Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19(6):811–818. doi: 10.1007/s00198-007-0506-x. [DOI] [PubMed] [Google Scholar]
- 20.Downey TW, Foltz SH, Boccuzzi SJ, Omar MA, Kahler KH. Adherence and persistence associated with the pharmacologic treatment of osteoporosis in a managed care setting. South Med J. 2006;99(6):570–575. doi: 10.1097/01.smj.0000221637.90495.66. [DOI] [PubMed] [Google Scholar]
- 21.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int. 2006;17(11):1645–1652. doi: 10.1007/s00198-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 22.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38(6):922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Jones TJ, Petrella RJ, Crilly R. Determinants of persistence with weekly bisphosphonates in patients with osteoporosis. J Rheumatol. 2008;35(9):1865–1873. [PubMed] [Google Scholar]
- 24.Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28(2):236–242. doi: 10.1016/j.clinthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81(8):1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 26.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18(8):1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 27.Imaz I, Zegarra P, Gonzalez-Enriquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–1951. doi: 10.1007/s00198-009-1134-4. [DOI] [PubMed] [Google Scholar]
- 28.Roughead EE, Ramsay E, Priess K, Barratt J, Ryan P, Gilbert AL. Medication adherence, first episode duration, overall duration and time without therapy: the example of bisphosphonates. Pharmacoepidemiol Drug Saf. 2009;18(1):69–75. doi: 10.1002/pds.1687. [DOI] [PubMed] [Google Scholar]
- 29.Copher R, Buzinec P, Zarotsky V, Kazis L, Iqbal SU, Macarios D. Physician perception of patient adherence compared to patient adherence of osteoporosis medications from pharmacy claims. Curr Med Res Opin. 2010;26(4):777–785. doi: 10.1185/03007990903579171. [DOI] [PubMed] [Google Scholar]
- 30.Kamatari M, Koto S, Ozawa N, et al. Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metab. 2007;25(5):302–309. doi: 10.1007/s00774-007-0768-6. [DOI] [PubMed] [Google Scholar]
- 31.Aki S, Eskiyurt N, Akarirmak U, et al. Gastrointestinal side effect profile due to the use of alendronate in the treatment of osteoporosis. Yonsei Med J. 2003;44(6):961–967. doi: 10.3349/ymj.2003.44.6.961. [DOI] [PubMed] [Google Scholar]
- 32.Kendler D, Kung AW, Fuleihan G, et al. Patients with osteoporosis prefer once weekly to once daily dosing with alendronate. Maturitas. 2004;48(3):243–251. doi: 10.1016/j.maturitas.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Ringe JD, Moller G. Differences in persistence, safety and efficacy of generic and original branded once weekly bisphosphonates in patients with postmenopausal osteoporosis: 1-year results of a retrospective patient chart review analysis. Rheumatol Int. 2009;3(2):213–221. doi: 10.1007/s00296-009-0940-5. [DOI] [PubMed] [Google Scholar]
- 34.Cotte FE, Fardellone P, Mercier F, Gaudin AF, Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21(1):145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80(7):856–861. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 36.Fraenkel L, Gulanski B, Wittink D. Patient treatment preferences for osteoporosis. Arthritis Rheum. 2006;55(5):729–735. doi: 10.1002/art.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grima DT, Papaioannou A, Airia P, Ioannidis G, Adachi JD. Adverse events, bone mineral density and discontinuation associated with generic alendronate among postmenopausal women previously tolerant of brand alendronate: a retrospective cohort study. BMC Musculoskelet Disord. 2010;11:68. doi: 10.1186/1471-2474-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–1123. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 39.Penning-van Beest FJ, Erkens JA, Olson M, Herings RM. Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int. 2008;19(4):511–517. doi: 10.1007/s00198-007-0466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danese MD, Badamgarav E, Bauer DC. Effect of adherence on lifetime fractures in osteoporotic women treated with daily and weekly bisphosphonates. J Bone Miner Res. 2009;24(11):1819–1826. doi: 10.1359/jbmr.090506. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher JC, Rapuri PB, Haynatzki G, Detter JR. Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab. 2002;87(11):4914–4923. doi: 10.1210/jc.2002-020727. [DOI] [PubMed] [Google Scholar]
- 42.Bagger YZ, Tanko LB, Alexandersen P, et al. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone. 2004;34(4):728–735. doi: 10.1016/j.bone.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Neele SJ, Evertz R, De Valk-De RG, Roos JC, Netelenbos JC. Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone. 2002;30(4):599–603. doi: 10.1016/s8756-3282(01)00706-2. [DOI] [PubMed] [Google Scholar]
- 44.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz AV, Bauer DC, Cummings SR, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: The FLEX trial. J Bone Miner Res. 2010;25(5):976–982. doi: 10.1002/jbmr.11. [DOI] [PubMed] [Google Scholar]
- 46.Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19(3):365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- 47.Devogelaer JP, Brown JP, Burckhardt P, et al. Zoledronic acid efficacy and safety over five years in postmenopausal osteoporosis. Osteoporos Int. 2007;18(9):1211–1218. doi: 10.1007/s00198-007-0367-3. [DOI] [PubMed] [Google Scholar]
- 48.Popat V. Denosumab efficacy FDA analysis. Proceedings of the August 13, 2009 Meeting of the Reproductive Health Drugs Advisory Committee; Gaithersburg, Maryland. 2009. [Google Scholar]
- 49.Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Kendler DL, Adachi JD, Josse RG, Slosman DO. Monitoring strontium ranelate therapy in patients with osteoporosis. Osteoporos Int. 2009;20(7):1101–1106. doi: 10.1007/s00198-009-0886-1. [DOI] [PubMed] [Google Scholar]
- 51.Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164(18):2024–2030. doi: 10.1001/archinte.164.18.2024. [DOI] [PubMed] [Google Scholar]
- 52.Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353(6):555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 53.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 54.Roughead EE, Ramsay E, Priess K, Barratt J, Ryan P, Gilbert AL. Medication adherence, first episode duration, overall duration and time without therapy: the example of bisphosphonates. Pharmacoepidemiol Drug Saf. 2009;18(1):69–75. doi: 10.1002/pds.1687. [DOI] [PubMed] [Google Scholar]
- 55.Lau E, Papaioannou A, Dolovich L, et al. Patients’ adherence to osteoporosis therapy: exploring the perceptions of postmenopausal women. Can Fam Physician. 2008;54(3):394–402. [PMC free article] [PubMed] [Google Scholar]
- 56.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009;20(12):2127–2134. doi: 10.1007/s00198-009-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Center JR, Bliuc D, Nguyen ND, Nguyen TV, Eisman JA. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011;96(4):1006–1014. doi: 10.1210/jc.2010-2730. [DOI] [PubMed] [Google Scholar]
- 59.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolland MJ, Grey AB, Gamble GD, Reid IR. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95(3):1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 61.Colon-Emeric CS, Mesenbrink P, Lyles KW, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res. 2010;25(1):91–97. doi: 10.1359/jbmr.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siris ES, Selby PL, Saag KG, Borgstrom F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122(2 Suppl):S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333(22):1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 64.Pols HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9(5):461–468. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 65.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 66.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 67.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 68.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 69.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 70.Delmas PD, Recker RR, Chesnut CH, III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15(10):792–798. doi: 10.1007/s00198-004-1602-9. [DOI] [PubMed] [Google Scholar]
- 71.Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146(5):326–339. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]