Abstract
Cerebral palsy (CP) is the main cause of physical disability in childhood and is an important health issue that has a strong socioeconomic impact. There is no effective treatment for CP and therapeutic approaches report only partial benefits for affected people. In this study we assessed the effects of growth hormone (GH) treatment combined with psychomotor and cognitive stimulation in the neurodevelopment of children with CP and GH deficiency (GHD). The study was carried out in 11 patients (7 boys and 4 girls; 4.12 ± 1.31 years) with GHD and CP who were treated with recombinant GH (rGH) and psychomotor and cognitive stimulation during 2 months. Battelle Developmental Inventory Screening Test (BDIST) was performed 2 months before commencing GH treatment, just before commencing GH administration, and after 2 months of combined treatment involving GH and cognitive stimulation. Psychomotor and cognitive status did not change during the period in which only cognitive stimulation was performed; however, significant improvements in personal and social skills, adaptive behavior, gross motor skills and total psychomotor abilities, receptive and total communication, cognitive skills and in the total score of the test (P < 0.01), and in fine motor skills and expressive communication (P < 0.02) were observed after the combined treatment period. Therefore, GH replacement together with psychomotor and cognitive stimulation seem to be useful for the appropriate neurodevelopment of children with GHD and CP.
Keywords: growth hormone, cerebral palsy, cognitive functions, Battelle’s test, neurodevelopment
Introduction
Nervous system plasticity and regeneration of the brain in response to both neurological injury and repetitive practice of different neurological stimulations have been widely demonstrated.1 However, achievements obtained with such stimulations usually are incomplete. Results are better when brain injuries occur at an early age and rehabilitation intervention begins promptly after the injury, since there is a greater plasticity when the central nervous system (CNS) is developing.2
Cerebral palsy (CP) is the most common cause of physical disability in childhood3 and the estimated prevalence of CP in the general population is 2/1000.4 CP is defined as a persistent but not progressive disorder of posture and movement system, associated with functional activity limitations and sensorial, cognitive, communications problems, epilepsy, and musculoskeletal system problems.4 Major causes for CP involve prematurity (40% to 50% of cases of CP), abnormal intrauterine developments due to fetal–maternal infections, asphyxia during delivery, brain trauma during labor, and delivery and complications in the perinatal period.5 Currently there is no cure for CP and therapeutical approaches show only partial benefits for affected individuals.1
It has been shown that most CP children have poor linear growth during childhood resulting in a diminished final adult height.6 We demonstrated that in a population of CP children, 70% showed impaired GH secretion.5 This phenomenon, could have a great importance since the GH-plasma insulin-like growth factor (IGF-1) system plays an important role during CNS development, and also in inducing neurogenesis and increasing brain plasticity.1,7–9 In this sense, we demonstrated that combined treatment involving GH replacement and adequate physical or cognitive stimulation is effective in the motor rehabilitation of children with CP or adult patients with cognitive disorders after traumatic brain injury, respectively.1,10
This pilot study assessed the effects of GH replacement combined with psychomotor and cognitive stimulation in the cognitive rehabilitation of children with CP and GHD.
Method
Participants
Eleven patients with GHD and CP admitted for cognitive and motor rehabilitation were included in the study (7 males and 4 females; 4.12 ± 1.31 years; range: 3 to 7 years old). According to the Gross Motor Function Classification System (GMFCS) patients’ levels ranged from I to V.11 Table 1 shows the main characteristics of patients studied.
Table 1.
Main characteristics of the patients studied
| Patient | Age (years) | Gender | PC cause | Radiologic findings | Motor impairment | GMFCS level |
|---|---|---|---|---|---|---|
| 1 | 3 | M | Perinatal hypoxia | PVL; plagiocephaly; corpus callosum hypoplasia; macrocephaly | PI | III |
| 2 | 4 | F | Perinatal hypoxia | PVL | PI | I |
| 3 | 5,4 | F | Perinatal hypoxia | PVL | PI | I |
| 4 | 4,8 | M | Postsurgical iatrogenic injury | Decreased activity and volume in PO cortex; bilateral PO ischemia; diffuse cerebral edema | Spastic TP | V |
| 5 | 3,5 | M | Perinatal hypoxia | PVL; bilateral parietal pachygyria; hydrocephalus; microcephaly | Flaccid TP | V |
| 6 | 3,1 | M | Premature twins; single umbilical artery | PVL; plagiocephaly; polymicrogyria | Spastic TP | V |
| 7 | 3 | F | Premature twins | PVL; right occipital cortex and bilateral frontal cortex ischemic injuries | Distonic TP | V |
| 8 | 3 | M | Prematurity | PVL; microcephaly | PI | III |
| 9 | 3,2 | M | Perinatal hypoxia | PVL | Distonic TP | V |
| 10 | 5,7 | F | Postsurgical iatrogenic injury | PVL; diffuse multicystic leukoencephalopathy; focal hemorrhage in right brain hemisphere and cerebellar hemispheres deep white matter; microcephaly | Distonic TP | V |
| 11 | 6,7 | M | Postvaccine iatrogenic injury | PVL | PI | I |
Abbreviations: GMFCS, Gross Motor Function Classification System; F, female; M, male; PC, cerebral palsy; PI, psychomotor impairment; PO, parieto-occipital; PVl, periventricular leucomalacia; TP, tetraplegia.
GHD
The existence of GHD was detected previously in the CP children included in the study.5 The existence of GHD was established according to auxological parameters and the GH peak response to the insulin-induced hypoglycemia test. IGF-1 and plasma insulin-like growth factor binding protein 3 (IGFBP-3) plasma levels were measured by a solid-phase, enzyme-labeled chemiluminiscent immunometric assay (Inmulite 2000, Siemens). Other pituitary hormone deficiencies were excluded by chemiluminiscent immunometric assays (data not shown). Routine blood analyses (hematology and chemistry) were carried out at 2-month intervals (Coulter HmX, Beckman and AU400, Beckman, respectively).
Clinical assessments
To assess neurodevelopment in patients under study we used the Battelle Developmental Inventory Screening Test (BDIST).12 The BDIST assesses key developmental skills in children from 6 months to 8 years of age. The reliability and validity of the BDIST has been previously demonstrated.13 The test comprises 96 items assessing the following 5 domains: personal and social skills (including adult interaction, expression of feelings, self-concept, peer interaction, copying, and social roles), adaptive behavior (including attention, eating, dressing, toileting, and personal responsibility), psychomotor ability, communication and cognition (including perceptual discrimination, memory, reasoning and academic skills, and conceptual development). Subdomain scores can be computed for psychomotor ability (fine motor skills and gross motor skills) and communication (expressive and receptive communication). Scores for each domain are expressed as age-equivalent scores that indicate the age (in months) at which a raw score is average. Total test score is obtained after combining the scores of all domains.
Study timing
BDIST was during 2 study periods: first, a pretreatment period (from 2 months before starting GH treatment until just before commencing it); second, a treatment period (from just before the beginning of GH treatment until 2 months after GH administration commenced) (Figure 1).
Figure 1.
Study timing.
Notes: Arrowheads: assessments by using the BDIST carried out: 2 months before commencing GH treatment (1), just before the beginning of the treatment (2) and after 2 months of GH treatment (3).
During the pretreatment period only psychomotor and cognitive stimulation was performed. During the treatment period all patients received GH replacement together with psychomotor and cognitive stimulation.
It is remarkable that before admission at our center all these CP children had been intensively stimulated in other centers, most of them since they were 1 year old.
Intervention
GH treatment
The patients were treated with recombinant human GH (rhGH; Omnitrope, Sandoz) (subcutaneously; 30 μg/kg/day, 5 days/week, during 2 months; after 2 months of psychomotor and cognitive stimulation). Fasting blood samples were analyzed for routine hematology and chemistry parameters at 2-month intervals and plasma thyroid stimulating hormone (TSH), IGF-I, and IGFPB3 levels were also measured every 2 months.
Psychomotor and cognitive stimulation
Patients received psychomotor and cognitive stimulation; these therapies were carried out for 45 minutes per day, 5 days per week during 4 months.
Psychomotor and cognitive stimulation were adapted to the specific needs of each patient. Psychomotor stimulation involved tasks aimed at improving tonic–postural control, laterality, breathing and relaxation, static and dynamic balance, motor coordination and dissociation, body image, oculomotor coordination, spatial and temporal orientation, and gross and fine motor skills. Cognitive stimulation involved tasks directed at improving interaction with the environment, communication, attention, perception, memory, reasoning, and concept learning.
Data analysis
Data from each BDIST assessment were compared using a nonparametric test for 2 related samples (Wilcoxon signed-rank test). The analysis was done by comparing data obtained before and after the 2 study periods (pretreatment and treatment periods). The improvement means relative to baseline during the pretreatment period and during the treatment period were compared in the same way (Wilcoxon signed-rank test) (**P < 0.02 and ***P < 0.01). Similar statistical analysis was used for evaluating plasma changes in IGF-I, IGFBP3 changes, total cholesterol, triglycerides, and glucose levels. Results are shown as the mean ± standard deviation (SD) of the mean.
Results
No undesirable side-effects were observed during or after the study. Plasma cholesterol and triglyceride values decreased significantly at the end of treatment period (Table 2); plasma IGF-I and IGFBP3 increased significantly (Table 2), but their values were not higher than the mean for the patient’s age (data not shown). No changes were observed in plasma glucose levels (Table 2) or in plasma TSH levels (data not shown).
Table 2.
Main plasma chemistry parameters analyzed
| Patient | GH peak |
TC |
TG |
G |
IGF-1 |
IGFBP3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| 1 | 4.5 | 205 | 182 | 75 | 74 | 82 | 75 | 34.2 | 89 | 2.33 | 3.1 |
| 2 | 6.3 | 181 | 169 | 102 | 65 | 89 | 73 | 28.1 | 256 | 1.7 | 2.9 |
| 3 | 2.8 | 194 | 171 | 41 | 37 | 70 | 77 | 53 | 200 | 3.61 | 4.3 |
| 4 | 5.3 | 175 | 168 | 125 | 104 | 76 | 85 | 67.2 | 103.3 | 2.5 | 3.8 |
| 5 | 1.5 | 225 | 165 | 186 | 85 | 72 | 77 | 25 | 69.5 | 2.2 | 3.6 |
| 6 | 6.9 | 193 | 166 | 125 | 97 | 75 | 83 | 31 | 75 | 1.8 | 3.03 |
| 7 | 3.4 | 215 | 171 | 157 | 110 | 81 | 79 | 35 | 125 | 2.1 | 3.1 |
| 8 | 4.7 | 186 | 155 | 114 | 65 | 92 | 84 | 69.5 | 89.5 | 3 | 4.2 |
| 9 | 7.1 | 177 | 165 | 145 | 75 | 74 | 72 | 25 | 78 | 1.4 | 1.76 |
| 10 | 5.2 | 191 | 164 | 165 | 108 | 84 | 96 | 78 | 162 | 2.9 | 4.06 |
| 11 | 4.8 | 187 | 155 | 128 | 90 | 74 | 101 | 67 | 225 | 1.96 | 3.03 |
| Mean (SD) | 4.77 (1.71) | 193.54 (15.69) | 166.45 (7.51) | 123.90 (41.16) | 82.72 (22.25) | 79 (7.15) | 82 (9.27) | 46.63 (20.49) | 133.84 (66.41) | 2.31 (0.64) | 3.35 (0.73) |
| SS | 0.003*** | 0.003*** | ns | 0.003*** | 0.003*** | ||||||
Note:
P < 0.01 (Wilcoxon signed-rank test).
Abbreviations: GH peak, peak growth hormone (ng/mL) after insulin-induced hypoglycemia; TC, plasma total cholesterol (mg/dL); TG, plasma triglycerides (mg/dL); G, plasma glucose (mg/dL); IGF-1, plasma insulin-like growth factor (ng/mL); IGFBP3, plasma insulin-like growth factor binding protein 3 (μg/mL); SD, standard deviation of the mean; SS, statistical significance; ns, not significant; pre, pretreatment; post, post-treatment.
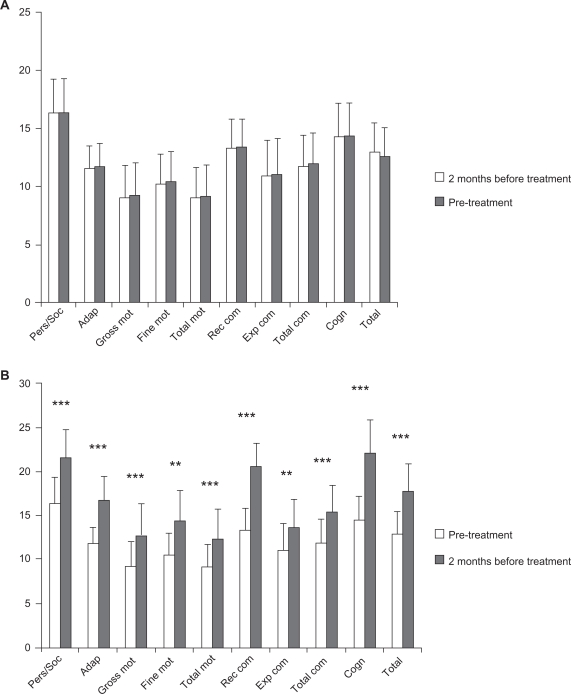
Patients did not improve their psychomotor and cognitive status during the pretreatment period, during which only psychomotor and cognitive stimulation were performed. However, significant improvements in all BDIST domains were observed when GH was administered together with psychomotor and cognitive stimulation; specifically, patients improved in personal and social skills, adaptive behavior, gross motor skills and total psychomotor abilities, receptive and total communication, cognitive skills, and in the total score of the scale (P < 0.01), and in fine motor skills and expressive communication (P < 0.02) (Figure 2 and Table 3).
Figure 2.
Clinical data from the BDIST during pre-treatment and treatment periods.
Notes: A) Pre-treatment period; (B) Treatment period. Horizontal axis: the different domains of the BDIS T and total score of it (TOTAL) are described. Vertical axis: score achived. Notice that different scales are used for (A) and (B). A) means and SD of the mean before (white bars) and after (grey bars) for each specific assessment. B) means and SD of the mean before (white bars) and after (grey bars) treatment period for each specific assessment. Statistical significance was calculated by comparing the data from the BDIST before and after the pre-treatment period (A) and the treatment period (B) (Wilcoxon signed-rank test). **P < 0.02 and ***P < 0.01.
Table 3.
Results obtained from the Battelle Developmental Inventory Screening Test (BDIST)
| BDIST | Pretreatment period mean (months) (SD) | After treatment period mean (months) (SD) | Statistical significance from improvements during the treatment period (Wilcoxon) | Improvement mean relative to baseline during the pretreatment period (%) (SD) | Improvement mean relative to baseline during the treatment period (%) (SD) | Statistical significance from the comparison of improvements during the pretreatment and treatment periods (Wilcoxon) |
|---|---|---|---|---|---|---|
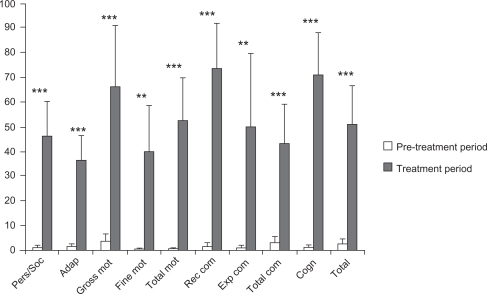
| Personal/social | 16.36 (9.71) | 21.64 (10.22) | 0.003*** | 1.01 (3.35) | 46.26 (46.54) | 0.003*** |
| Adaptive | 11.73 (6.45) | 16.73 (8.84) | 0.005*** | 1.38 (3.25) | 36.49 (31.57) | 0.008*** |
| Gross motor | 9.18 (9.32) | 12.64 (12.29) | 0.008*** | 3.63 (10.05) | 65.91 (84.29) | 0.008*** |
| Fine motor | 10.45 (8.26) | 14.36 (11.35) | 0.018** | 0.43 (1.43) | 39.91 (62.18) | 0.018** |
| Total motor | 9.18 (8.61) | 12.36 (11.03) | 0.007*** | 0.47 (1.58) | 52.31 (59.18) | 0.008*** |
| Receptive communication | 13.36 (8.06) | 20.55 (8.41) | 0.003*** | 1.51 (5.02) | 73.67 (60.73) | 0.003*** |
| Expressive communication | 11 (10.14) | 13.55 (10.78) | 0.018** | 1.01 (3.35) | 50 (98.69) | 0.018** |
| Total communication | 11.91 (8.81) | 15.45 (9.66) | 0.003*** | 3.18 (7.83) | 43.24 (51.51) | 0.006*** |
| Cognitive | 14.36 (9.36) | 22.09 (12.46) | 0.003*** | 0.9 (3.01) | 70.95 (56.26) | 0.003*** |
| Total score | 12.91 (8.51) | 17.73 (10.46) | 0.003*** | 2.61 (6.13) | 51.2 (51.34) | 0.004*** |
Notes: The first column shows the different domains involved in the BDIST. The third column shows the statistical significance from data obtained in the BDIST during the treatment period (Wilcoxon signed-rank test). The fourth, fifth, and sixth columns show, respectively, the mean improvement relative to baseline during pretreatment period, treatment period and the statistical significance obtained after comparing these 2 values (Wilcoxon signed-rank test) (
P < 0.02 and
P < 0.01).
Discussion
Results from this study show that the combined treatment involving rhGH treatment and psychomotor and cognitive stimulation led to significant improvements in all BDIST domains during the treatment period. This indicates that the combined treatment clearly improved the neurodevelopment process of CP children by increasing their psychomotor and cognitive capacities and abilities. Specifically, the patients improved their potential to interact with their environment (both with adults and with their peers), affectivity, self-concept, collaboration, and understanding of their social role. Moreover, patients improved their attention and abilities to carry out their daily living activities. In the motor area patients specifically improved their motor control, body coordination, locomotion, performing precision activities, and, in general, their movement functionality. Patients increased their communication too (both receptive and expressive) and their cognitive abilities and capacities (perceptual discrimination, memory, reasoning, and concept development).
Although we did not use any control group to compare the results of the treatment used, we think that the pretreatment period can be considered as a control, despite the limitations of this strategy. Moreover, patients had received intense stimulation before admission, without any significant achievements.
It has been demonstrated that early intervention in low-birth-weight (LBW) premature infants leads to significant improvements in diverse cognitive parameters only in the heavier LBW stratum.14,15 Moreover, a systematic review of the literature shows that early interventions for preterm infants have a positive influence on cognitive outcomes in the short to medium term but not on motor outcomes.16 Few studies have described the effects of GH replacement in GHD CP children and most report only growth data without assessing any possible neurologic functional recovery.6,17,18 GH replacement therapy in GH-deficient children has been reported recently to significantly improve some areas such as the motor-component scale and performance IQ.19 This agrees with our data in this study and with our previous report demonstrating that the combined treatment involving GH replacement and adequate physical therapy is effective in the gross motor function rehabilitation of patients with CP and GHD.1
The neurotrophic role of GH is now well known.7,8,20–22 Moreover, the GH–IGF-I axis is involved in the regulation of brain growth, development, and metabolism.23 Recent data from our group demonstrate that exogenous growth hormone may combine with locally produced GH in increasing the proliferative response of hippocampal progenitors to brain injury in rats.9 Thus the possibility exists that most of the changes we observed in our study are a consequence of GH inducing newborn neural cells. However, some of these changes seem not to be related to this putative effect of GH, but to an enhanced or modified metabolic turnover of exciters neurotransmitters, such as noradrenaline (NA) and dopamine (DA). The prompt appearance of some positive responses in our CP children treated with GHD, such as alertness and increased interaction with the environment, cannot be explained by a putative neural stem cell proliferation induced by the hormone. GH neuroregulation is complex and a number of neurotransmitters participate in it. The pituitary release of the hormone is tonically inhibited by hypothalamic somatostatin (SS).24 In turn, NA negatively controls hypothalamic SS release.25 We previously demonstrated that GH administration increases hypothalamic SS release, which in turns triggers cerebral NA and DA synthesis and release for negatively controlling SS.24 Thus, it is tempting to speculate that in the absence of a pituitary GH secretion, as occurs in GH deficiency, hypothalamic SS secretion is weak or also deficient. The exogenous administration of the hormone would lead to increased SS tone, therefore to increased DA and NA turnover for maintaining the physiological feedback circuits involved in GH control.24 This might explain some of the early responses observed after GH administration in this study.
The effect of GH on plasma lipids profile is well known. GHD patients usually display an abnormal lipid profile characterized by elevated total cholesterol, elevated low-density lipoprotein cholesterol, and elevated triglycerides.26 GHD adolescents present an abnormal fasting and postprandial lipid profile that, in addition to increased fibrinogen and homocysteine levels, suggest the accumulation of cardiovascular risk factors early in life.27 In our study, GH replacement therapy significantly decreased fasting plasma total cholesterol and triglyceride levels, in agreement with that observed in other studies.26 Fasting glycemia was not significantly modified after 2 months of GH administration. As expected, plasma IGF-1 and IGFBP3 significantly increased too after GH treatment, indicating that GHD in our patients did not occur as a consequence of any mutation in GH receptor, but as a result of an altered hypothalamic control of pituitary GH release. This agrees with the fact that no clear anatomical abnormalities were observed in hypothalamic and pituitary MRI studies carried out before admission.
In summary, we have demonstrated that the combined therapy involving GH replacement and psychomotor and cognitive stimulation seems to be useful for the appropriate neurodevelopment of children with GHD and CP. Since these children had received an intensive neurostimulation without significant improvements before being treated with GH, it seems to be clear that the hormone, and/or IGF-1, was the main factor responsible for the results obtained. Although this was a pilot study, and new and larger studies are needed to understand more precisely the effects of GH treatment in patients with CP and GHD, accumulating evidence (this and our previous studies) shows that GH administration improves both motor and cognitive abilities in GHD CP children. Since exogenous GH combines with locally produced GH for repairing brain injuries,9 it is feasible to assume that GH treatment may be used too in CP children without GHD. Because GH treatments last a few months, and GH doses are not higher than those used for treating GH deficiency, no significant risks seem to be associated with GH treatment in CP children in whom GH secretion is normal.
Figure 3.
Comparison of improvements during pre-treatment and treatment periods.
Notes: The horizontal axis shows the different domains of the BDIST and the total (TOTAL) score of the test. The vertical axis shows the percentage improvements observed during the pre-treatment period (white bars) and treatment period (grey bars) over baseline values for each specific assessment (mean + SD). Statistical significance was calculated by comparing the improvements relative to baseline from the BDIST during pre-treatment periods (Wilcoxon signed-rank test). **P < 0.02 and ***P < 0.01.
Acknowledgments
This study was supported by Fundación Foltra and Fundación Repsol.
Footnotes
Disclosure
The authors report no conflicts of interest in this research.
References
- 1.Reimunde P, Rodicio C, Lopez N, Alonso A, Devesa P, Devesa J. Effects of recombinant growth hormone replacement and physical rehabilitation in recovery of gross motor function in children with cerebral palsy. Ther Clin Risk Manag. 2010;6:585–592. doi: 10.2147/TCRM.S14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15(2):94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 3.Krageloh-Mann I, Cans C. Cerebral palsy update. Brain Dev. 2009;31(7):537–544. doi: 10.1016/j.braindev.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Kerem Gunel M. Rehabilitation of children with cerebral palsy from a physiotherapist’s perspective. Acta Orthop Traumatol Turc. 2009;43(2):173–180. doi: 10.3944/AOTT.2009.173. [DOI] [PubMed] [Google Scholar]
- 5.Devesa J, Casteleiro N, Rodicio C, Lopez N, Reimunde P. Growth hormone deficiency and cerebral palsy. Ther Clin Risk Manag. 2010;6:413–418. doi: 10.2147/tcrm.s12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim ML, Moshang T, Jr, Oppenheim WL, Cohen P. Is treatment with growth hormone effective in children with cerebral palsy? Dev Med Child Neurol. 2004;46(8):569–571. doi: 10.1017/s0012162204000945. [DOI] [PubMed] [Google Scholar]
- 7.Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Scientific World Journal. 2006;18(6):53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devesa J, Devesa P, Reimunde P. Growth hormone revisited. Med Clin (Barc) 2010;135(14):665–670. doi: 10.1016/j.medcli.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Devesa P, Reimunde P, Gallego R, Devesa J, Arce VM. Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Inj. 2011;25(5):503–510. doi: 10.3109/02699052.2011.559611. [DOI] [PubMed] [Google Scholar]
- 10.Reimunde P, Quintana A, Castanon B, et al. Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 2011;25(1):65–73. doi: 10.3109/02699052.2010.536196. [DOI] [PubMed] [Google Scholar]
- 11.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 12.Newborg J, Stock JR, Wnek L. Spanish adaptation. In: Cruz López MV, González Criado M, editors. Battelle: Inventario de desarrollo. 1st ed. Madrid: TEA Ediciones; 1996. [Google Scholar]
- 13.Newborg J, Stock JR, Wnek L, et al. Battelle Developmental Inventory with Recalibrated Technical Data and Norms: Screening Test Examiner’s Manual. 2nd ed. Allen, TX: DLM, Inc; 1988. [Google Scholar]
- 14.Brooks-Gunn J, McCarton CM, Casey PH, et al. Early intervention in low-birth-weight premature infants. Results through age 5 years from the Infant Health and Development Program. JAMA. 1994;272(16):1257–1262. [PubMed] [Google Scholar]
- 15.McCarton CM, Brooks-Gunn J, Wallace IF, et al. Results at age 8 years of early intervention for low-birth-weight premature infants. The Infant Health and Development Program. JAMA. 1997;277(2):126–132. [PubMed] [Google Scholar]
- 16.Spittle AJ, Orton J, Doyle LW, Boyd R. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev. 2007;2(2):CD005495. doi: 10.1002/14651858.CD005495.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Coniglio SJ, Stevenson RD. Growth hormone deficiency in two children with cerebral palsy. Dev Med Child Neurol. 1995;37(11):1013–1015. doi: 10.1111/j.1469-8749.1995.tb11957.x. [DOI] [PubMed] [Google Scholar]
- 18.Ali O, Shim M, Fowler E, et al. Growth hormone therapy improves bone mineral density in children with cerebral palsy: a preliminary pilot study. J Clin Endocrinol Metab. 2007;92(3):932–937. doi: 10.1210/jc.2006-0385. [DOI] [PubMed] [Google Scholar]
- 19.Puga González B, Ferrández Longás A, Oyarzábal M, Nosas R, Grupo Colaborativo Español The effects of growth hormone deficiency and growth hormone replacement therapy on intellectual ability, personality and adjustment in children. Pediatr Endocrinol Rev. 2010;7(4):328–338. [PubMed] [Google Scholar]
- 20.Scheepens A, Williams CE, Breier BH, Guan J, Gluckman PD. A role for the somatotropic axis in neural development, injury and disease. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1483–1491. doi: 10.1515/jpem-2000-s623. [DOI] [PubMed] [Google Scholar]
- 21.Aberg ND, Johansson I, Aberg MA, et al. Peripheral administration of GH induces cell proliferation in the brain of adult hypophysectomized rats. J Endocrinol. 2009;201(1):141–150. doi: 10.1677/JOE-08-0495. [DOI] [PubMed] [Google Scholar]
- 22.Christophidis LJ, Gorba T, Gustavsson M, et al. Growth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: A potential role for growth hormone in injury-induced neurogenesis. Growth Horm IGF Res. 2009;19(6):497–506. doi: 10.1016/j.ghir.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Gasperi M, Castellano AE. Growth hormone/insulin-like growth factor I axis in neurodegenerative diseases. J Endocrinol Invest. 2010;33(8):587–591. doi: 10.1007/BF03346653. [DOI] [PubMed] [Google Scholar]
- 24.Devesa J, Lima L, Tresguerres JA. Neuroendocrine control of growth hormone secretion in humans. Trends Endocrinol Metab. 1992;3(5):175–183. doi: 10.1016/1043-2760(92)90168-z. [DOI] [PubMed] [Google Scholar]
- 25.Devesa J, Arce V, Lois N, Tresguerres JA, Lima L. Alpha 2-adrenergic agonism enhances the growth hormone (GH) response to GH-releasing hormone through an inhibition of hypothalamic somatostatin release in normal men. J Clin Endocrinol Metab. 1990;71(6):1581–1588. doi: 10.1210/jcem-71-6-1581. [DOI] [PubMed] [Google Scholar]
- 26.Gleeson H, Barreto ES, Salvatori R, et al. Metabolic effects of growth hormone (GH) replacement in children and adolescents with severe isolated deficiency due to a GHRH receptor mutation. Clin Endocrinol (Oxf) 2007;66(4):466–474. doi: 10.1111/j.1365-2265.2007.02753.x. [DOI] [PubMed] [Google Scholar]
- 27.Lanes R, Paoli M, Carrillo E, Villaroel O, Palacios A. Cardiovascular risk of young growth-hormone-deficient adolescents. Differences in growth-hormone-treated and untreated patients. Horm Res. 2003;60(6):291–296. doi: 10.1159/000074247. [DOI] [PubMed] [Google Scholar]