Abstract
Background and purpose
Treatment options for stroke related dysphagia are currently limited. In this study we investigated whether non-invasive brain stimulation in combination with swallowing maneuvers facilitates swallowing recovery in dysphagic stroke patients during early stroke convalescence.
Methods
Fourteen patients with subacute unilateral hemispheric infarction were randomized to anodal transcranial direct current stimulation (tDCS) versus sham stimulation to the sensorimotor cortical representation of swallowing in the unaffected hemisphere over 5 consecutive days with concurrent standardized swallowing maneuvers. Severity of dysphagia was measured using a validated swallowing scale, Dysphagia Outcome and Severity Scale (DOSS), before the first and after the last session of tDCS or sham. The effect of tDCS was analyzed in a multivariate linear regression model using changes in DOSS as the outcome variable, after adjusting for the effects of other potential confounding variables such as the NIH Stroke Scale (NIHSS) and DOSS scores at baseline, acute ischemic lesion volumes, patient’s age and time from stroke onset to stimulation.
Results
Patients who received anodal tDCS gained 2.60 points improvement in DOSS scores compared to patients in the sham stimulation group who showed an improvement of 1.25 points (P=0.019) after controlling for the effects of other aforementioned variables. 6 out 7 (86%) patients in tDCS stimulation group gained at least 2 points improvement compared with 3 out 7 (43%) patients in sham group (P=0.107).
Conclusion
Since brainstem swallowing centers have bilateral cortical innervations, measures that enhance cortical input and sensorimotor control of brainstem swallowing may be beneficial for dysphagia recovery.
Keywords: Dysphagia, stroke recovery, swallowing recovery, non-invasive brain stimulation, transcranial direct current stimulation
Introduction
Dysphagia is a potentially fatal complication of stroke [1]. It afflicts numerous patients with hemispheric strokes [1] and carries high rates of complications even after adjusting for stroke severity [2]. Since hemispheric infarcts are the major subtype of ischemic stroke in the population [3], it can be assumed that the magnitude of dysphagia burden attributable to such strokes is large. Despite its frequent occurrence, treatment of stroke related dysphagia remains limited. The usual practice is to provide nutritional support via alternative feeding methods, till swallowing functions recover; however, such methods fail to protect against complications of dysphagia such as aspiration pneumonia [4, 5]. Development of an effective intervention that improves swallowing in the early course of stroke recovery will be helpful in curtailing dysphagia related complications and improving swallowing functions.
Swallowing functions are subserved by a distributed brain network, though involvement of the inferior peri-rolandic sensorimotor cortex appears consistent across studies [6–8]. Disruption of projections from these cortical regions to the brainstem “swallowing centers” produces dysphagia with hemispheric strokes [9]. Different lines of evidence suggest that recovery of swallowing functions occurs via expansion of the pharyngeal representation in the uninvolved hemisphere possibly ensuring greater input to the brainstem swallowing centers [10, 11]. Cortical stimulation techniques may facilitate this process in patients with hemispheric lesions, where the brainstem and peripheral structures are intact but the upper echelons of the swallowing apparatus are dysfunctional. Repetitive Transcranial Magnetic Stimulation (rTMS) over the swallowing motor cortex in healthy volunteers induces a long-term effect on the excitability of corticobulbar projections to the pharynx [12] and may improve swallowing functions in dysphagic stroke patients [13].
Transcranial Direct Current Stimulation (tDCS) is another non-invasive brain stimulation technique that utilizes weak, direct current to produce shifts in neuronal excitability [14, 15] and can be combined with swallowing maneuvers or exercises. It has generated great interest recently for its ease of use, patient tolerability, and safety profile which is of particular importance during the acute/subacute phases of a stroke. It has been shown to improve motor functions in chronic stroke patients [16, 17]. Moreover, presence of a sham mode makes it possible to examine its effects it in a blinded trial paradigm [18]. More recently investigators have shown that application of anodal tDCS to the pharyngeal motor cortex in healthy human subjects increases pharyngeal excitability in an intensity dependent manner [19]. In this pilot study, we investigated the effects of anodal tDCS versus sham stimulation of the unaffected hemisphere for improving dysphagia in the acute-subacute stroke phase.
Materials and Methods
This was an investigator-initiated, prospective, single-center, blinded pilot trial. All participants were recruited from our inpatient stroke service, were between 24–168 hours after their first ischemic stroke at time of enrollment and had dysphagia secondary to a new unilateral hemispheric infarction. They were all evaluated by speech and language pathologists [SLP] specializing in dysphagia (C.W and C.F), who were blinded to study allocation, and rated swallowing impairments using a validated dysphagia scale, Dysphagia Outcome and Severity Scale (DOSS) [20]. DOSS scores range from 1 to 7, where 7 represents normal swallowing and 1 represents severe dysphagia. DOSS rates the functional severity of dysphagia and recommends a dietary level, independence level, and type of nutrition based on the level of impairment, thus conveying information about dysphagia severity and related disability. To qualify, a DOSS score of ≤ 5 (mild-severe dysphagia) was required. Patients, with difficulty following instructions due to obtundation or cognitive impairment, pre-existing swallowing problems or other contraindications to tDCS were excluded.
All swallowing evaluations were conducted using hospital based protocols that used different food consistencies representing the range of food consistencies consumed in real life (teaspoon, cup sip and straw sip of thin liquids and nectar thick liquids; honey; pureed solids and a cookie). Patients were monitored for bolus control, oropharyngeal delays and retention, overt signs of aspiration including coughing, change in voice quality or oxygen desaturation, with each consistency. In cases of ambiguity about assigning an appropriate DOSS score, a video-swallow evaluation using the following boluses was performed the same day: (3 ml) teaspoon of nectar-thick liquid × 1, (10 ml) cup sip of nectar-thick liquid ×1, straw sip of nectar-thick liquid x1 followed by (3 ml) a teaspoon of thin liquid ×2, (10 ml) cup sip followed by straw sips of thin liquid ×2, followed by 5 ml varibar pudding ×2, and ½ Vanilla Wafer Cookie ×2. Overall 7 patients required a video-fluoroscopic swallowing evaluation to record DOSS scores.
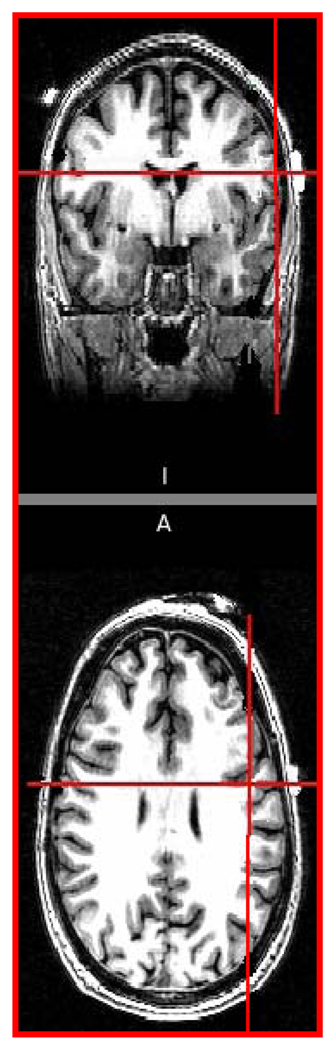
We recorded patient’s age, gender, lesion site, time in hours from stroke onset (time when patient was last seen normal if precise time of onset was unknown) to stimulation, lesion volume and NIH Stroke Scale (NIHSS) scores as measures of stroke severity prior to stimulation. Acute ischemic lesion volumes were computed on diffusion-weighted imaging sequences (DWI) on patient’s brain MRI using customized software routines. The details of specific MRI sequence parameters, imaging processing, and volumetric analysis are described elsewhere [21]. Two patients, unable to undergo an MRI, had their ischemic lesion volumes computed on a subacute head CT (obtained within 48–96 hours after symptoms onset). Patients were randomized to receive either anodal tDCS or sham stimulation to the unaffected hemisphere employing simple randomization, and were blinded to their stimulation allocations. Using the international 10–20 EEG electrode system for guidance [22], a saline soaked anodal electrode was placed over the undamaged hemisphere, mid-distance between C3 and T3 on the left or C4 and T4 on the right with a reference electrode over the contralateral supraorbital region. This montage was expected to generate maximal current density over the inferior sensorimotor cortex and the neighboring premotor brain regions critical for reorganization of the swallowing motor cortex after a dysphagic stroke [10, 11, 23]. We confirmed the location of the stimulating electrode and its proximity to the targeted regions by co-registering it with high-resolution T1-weighted MRI scans (figure 1). A DOSS score was obtained immediately prior to stimulation sessions (DOSS-pre) and after the 5th session (DOSS-post).
Figure 1.
Co-registration of anode mid-distance between C3/T3 using 10–20 EEG systems with a T1-weighted brain MRI demonstrates it to be centered over the caudal end of the primary motor cortex in a healthy volunteer.
TDCS/sham was applied in conjunction with standardized swallowing maneuvers to provide adequate sensory and motor activation of the swallowing cortex [24]. All participants sucked on a lemon flavored lollipop during these sessions. Patients complaining of dryness of mouth were provided with 1–2 small ice chips intermittently. Patients were instructed to “swallow hard” every 30 seconds, thereby generating approximately 60 effortful swallows during each session. We used gesticulations to encourage aphasic patients to swallow at regular intervals. Occurrence of a swallow response was assessed by observing the movement of the thyroid cartilage or by palpating its’ excursion in patients with thicker necks. All subjects were able to follow study swallowing instructions appropriately. Anodal tDCS (2 mA for 30 minutes) or sham was applied daily to the non-lesional hemisphere for 5 consecutive days. TDCS was delivered through a battery-driven, constant current stimulator [Phoresor, Iomed Inc., Salt Lake City, UT], with the following electrode dimensions: 3 × 5 cms for the anode and 5 × 6 cms for the reference electrode.
The study was approved by our institutional review board. A written, informed consent was obtained from the patients or their legal representative prior to enrollment.
Statistical Analysis
We analyzed the effect of stimulation (tDCS or sham, entered as a binary variable), as our exposure of interest, on improvement in dysphagia scores, as our outcome of interest, after adjusting for the potential confounding effects of other important variables, i.e. stroke severity as assessed by baseline NIHSS score, ischemic lesion volume, baseline DOSS score, patient age and time from stroke onset to stimulation. A correlation analysis and collinearity assessment among all independent variables was checked before the final model. A responder variable (Yes or No) was defined as at least ≥ 2 point improvement on DOSS. A logistic regression was applied with the same covariates from the general linear regression model as a secondary outcome analysis. All statistical analyses were performed using SAS V9.1 (SAS Institute Inc., Cary, NC).
Results
14 patients were recruited and randomized to anodal tDCS or sham stimulation group in a 1:1 fashion. The important characteristic of our patient sample is tabulated in table 1. All patients who were consented participated in this study and tolerated the sessions well; stimulation was not curtailed in anyone because of discomfort or fatigue. No adverse events, such as seizures, headaches, visual disturbances or significant skin irritation were observed. Two patients in the sham group but none in the tDCS group underwent PEG placement after their trial participation.
Table 1.
Clinical, radiological characteristics and swallowing measures of enrolled patients.
| Age | Sex | NIHSS | NIHSS Subscale 1a; 1 |
Infarct Location |
Lesion Volume (cc) |
Time to stimulation (hours) |
Dietary Status (baseline) |
DOSS pre |
DOSS post |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Anodal | tDCS | Group | ||||||||
| 1. | 92 | M | 6 | 0;1 | Frontal, parietal, temporal lobes |
20.6 | 40 | NPO | 1 | 5 |
| 2. | 81 | F | 21 | 1; 2 | Basal ganglia, internal capsule, parietal lobe |
122.2 | 82 | NPO | 1 | 3 |
| 3. | 90 | F | 10 | 0; 1 | Insula, frontal lobe |
43.9 | 50 | Nectar- thick and pureed solids only |
3 | 5 |
| 4. | 70 | F | 9 | 0; 2 | Insula, frontal lobe |
36.48 | 30 | NPO | 1 | 6 |
| 5. | 77 | F | 17 | 1; 2 | Insula, frontal lobe, basal ganglia, internal capsule |
58.06 | 97 | Thin liquids, pureed solids |
5 | 7 |
| 6. | 84 | M | 20 | 1; 4 | Internal capsule, frontal, temporal, parietal lobes |
120 | 140 | NPO | 1 | 2 |
| 7. | 64 | M | 12 | 1; 0 | Basal ganglia, internal capsule |
20.1 | 123 | Nectar thick liquids, pureed solids |
3 | 5 |
| 79.7* | 13.6* | 0.6*; | 60.2* | 80.3* | 2.1* | 4.7* | ||||
| 1.7* | ||||||||||
| Sham | Group | |||||||||
| 1. | 57 | F | 16 | 0; 2 | Insula, frontal lobe, basal ganglia |
84.65 | 42 | Nectar thick and pureed solids |
4 | 5 |
| 2. | 83 | M | 12 | 0; 1 | Insula, frontal lobe, basal ganglia |
63.06 | 52 | Ground solids and nectar thick |
3 | 4 |
| 3. | 50 | M | 16 | 1; 5 | Insula, frontal, temporal, parietal lobe, basal ganglia |
135.24 | 75 | NPO | 1 | 3 |
| 4. | 74 | M | 6 | 0; 0 | Insula, frontal lobe |
22.5 | 76 | Nectar thick and pureed solids |
3 | 5 |
| 5. | 72 | M | 11 | 1; 1 | Insula, basal ganglia, internal capsule |
40.32 | 146 | NPO | 1 | 2 |
| 6. | 78 | F | 15 | 1;2 | Insula, basal ganglia, internal capsule |
54.9 | 148 | NPO | 1 | 3 |
| 7. | 76 | F | 16 | 0; 2 | Insula, basal ganglia, internal capsule |
84.56 | 138 | Nectar thick and ground solids |
4 | 4 |
| 70* | 13.14* | 0.4*; 2* | 69.46* | 96.71* | 2.4* | 3.7* |
Average values for each column
Multivariate analysis
NIHSS scores and DOSS scores at baseline, acute ischemic lesion volume, time-to-stimulation and age were initially included for a generalized linear model; however, NIHSS and lesion volume were highly correlated (r=0.84 and p=0.0002), and further collinearity diagnostics revealed a significant collinearity (tolerance>0.1; VIF<10) between the 2 variables. Thus the latter was eliminated from the model. In summary, (table 2), our results shows that patients who received anodal tDCS gained 2.60 (1.91, 3.29; 95% CI) points on DOSS while patients in sham stimulation group improved by 1.25 (0.57, 1.95; 95 % CI), the difference between 2 groups reached a statistical significance with p value = 0.019. DOSS at baseline (p=0.045) and NIHSS at baseline (p=0.049) were significantly associated with improvement on DOSS scores. Age (p=0.228) was not a good predictor for improvement based on our model analysis. Our secondary outcome included a logistic regression analysis which was based on at least 2 points improvement with DOSS. 6 out of 7 patients (86%) in tDCS group had ≥ 2 points improvement on their DOSS scores versus 3 out 7 patients (43%) in sham stimulation group (P=0.107).
Table 2.
Results of anodal tDCS versus sham stimulation in multivariate analysis.
| Anodal tDCS | Sham | P Value | |
|---|---|---|---|
| Change in DOSS scores |
2.6‡ (1.91–3.29) | 1.26‡ (0.57, 1.95) | 0.019† |
| ≥ 2 point improvement in DOSS score |
6/7 (86%) | 3/7 (43%) | 0.107* |
P value based on a general linear regression model with baseline DOSS, NIHSS, age, time-to-treatment, and stimulation group as covariates.
Least square mean and 95% confidence interval estimated from the general linear model above.
P value is based on a logistic regression model with baseline DOSS, NIHSS, age, time-to-treatment and stimulation group as covariates.
Discussion
The findings of this pilot study shows that repeated application of anodal tDCS to the unaffected swallowing cortex in combination with timed, effortful swallowing is associated with significant swallowing improvement, over sham after adjusting for the effects of baseline stroke and dysphagia severity, age and time-to-stimulation, in patients with acute-subacute unilateral hemispheric infarction. Our results also attest to the feasibility and tolerability of tDCS in this stroke sub-population during early phases of stroke recovery.
The brain-stimulation effect might be explained by an augmentation effect of the naturally occurring changes in the unaffected swallowing cortex [10, 11]. Combining the sensorimotor effects of swallowing maneuvers with simultaneous brain-stimulation of the unaffected hemisphere may have been an important component of the effect. Sensory input from the pharynx is known to increase excitability of the swallowing sensorimotor cortex through convergent afferent activity [12] and pharyngeal sensory stimulation in dysphagic stroke patients produces an increase in the excitability of the swallowing motor cortex of the unaffected hemisphere [24]. On the other hand, studies investigating induction of plasticity in the human motor cortex employing paired associative paradigm have shown that cortical stimulation, if paired with peripheral stimulation of the somatosensory afferents, leads to greater increases in cortical excitability than produced by stimulation alone and induces topographically specific plastic changes [25]. This increase in excitability was prevented by using dextromethorphan which is known to block development of Long-term-potentiation (LTP) [26]. In animal studies, motor skill learning has been shown to produce LTP and Long-term-depression (LTD) leading to changes in synaptic strength in the primary motor cortex [27]. Cortical stimulation studies in experimental stroke models have shown stronger effects when peripheral sensorimotor activities were combined with central stimulation [28]. More recently, investigators [29] have shown that training in humans or low frequency stimulation (LFS) in mouse M1 slices produces release of brain derived neurotrophic factor (BDNF) which is necessary to induce long-term synaptic plasticity from direct current stimulation. In chronic stroke patients combining peripheral nerve stimulation or peripheral sensorimotor activities with tDCS facilitates the beneficial effects of training on motor performance beyond levels reached by each intervention alone [30, 31]. Thus, data from diverse sources indicate that combining repetitive peripheral sensorimotor stimulation with non-invasive brain stimulation can potentiate relearning and consolidation of motor skills to a level unattainable by any of these interventions alone in subacute or chronic stroke patients, and appears to have benefitted our subjects.
Our statistical methods were designed to control for discrepancies of important predictors of dysphagia recovery between groups that the randomization procedures may have failed to correct in our small sample. Although there is little data published on predictors of dysphagia recovery in stroke patients, baseline NIHSS score [32], stroke lesion volume [33], and age [34] have been found to be important factors influencing functional recovery in stroke patients and were included in the analysis; since swallowing functions in our patients were expected to recover over time, time-to-stimulation was also included in our analysis. Our model shows that baseline NIHSS, DOSS scores and anodal tDCS were associated with improvement. Introduction of all these variables could have over-fitted our model and exhausted degrees of freedom for estimation with a small sample size. However, the intent of this analysis was to gain an understanding about the important covariates influencing swallowing recovery and adjust for their effects on experimental treatment and not try to build a predictive model.
It is possible that in a minority of patients, especially in those with more circumscribed lesions, the ipsilesional hemisphere may have played a role in swallowing recovery and accounted for some variability in responses to stimulation. This poses an important question whether uniform application of anodal tDCS to the uninvolved hemisphere will benefit all such patients. However, since brainstem swallowing centers have bilateral innervations with little evidence for transcallosal inhibition [35], we hypothesized that stimulation of either hemisphere would produce an increase in pharyngeal excitability. Furthermore, stimulation of the uninvolved hemisphere was less likely to be affected by neuronal loss or tissue damage and responses to be more uniform; stimulating the non-lesioned hemisphere was also expected to be safer with respect to any potential seizures risk or tissue damage in the acute stroke phase. The optimal dose for stimulating the pharyngeal motor cortex has not been established; a recent report suggests that doses higher than that used for stimulating the primary motor cortex are necessary to produce comparable responses from the swallowing cortex [19]. Our protocol predates the publication of this report and alternative doses can be tried in future studies to assess their superiority. We chose our dose based on previous study protocols that have shown that application of 2mA to the dorsolateral frontal lobes is effective and well tolerated [36]. Our decision to perform 5 sessions of stimulation was based on recent reports showing an additive effect of repeated session of tDCS [37] and taking logistical considerations such as duration of hospitalization in mind; it is possible that more sessions may have produced a stronger effect. Other study limitations include non-routine use of videofluoroscopic swallowing evaluations in all subjects which were performed based on clinical judgment of evaluating SLPs. Although DOSS enjoys excellent inter-rater reliability [20], we may have failed to account for some random variability in assigning DOSS scores by not checking for it in this study. In addition, use of a single evaluation scheme for determining swallowing functions may have been unable to capture pertinent details about changes in swallowing physiology in these subjects. In future studies, additional dysphagia assessment scoring tools should be obtained to tests the robustness of any treatment effect.
In conclusion, the results from this pilot study show a promising efficacy of anodal tDCS application to the swallowing cortex of the unaffected hemisphere combined with effortful swallowing maneuvers for improving dysphagia in stroke patients. Further studies are warranted to refine this promising intervention by exploring effects of stimulation parameters, frequency of stimulation, and timing of the intervention in improving swallowing functions in dysphagic stroke patients.
Acknowledgments
We thank Drs. Vijay Renga and Dinesh Nair for helping with the noninvasive brain stimulation of our initial patients.
Funding: Dr. Kumar receives partial salary support from the NIH (NINDS 5UO1-NS044876-03 [Site PI]) and receives research support from the Charles and Irene Goldman Neurology Research Fund [PI]. Dr. Selim receives research support from the NIH (NINDS 1R01-NS 057127-01A1 [PI], NINDS 1R01-NS 045754-01A2 [Co-I], and 5R01-HL46690-14 [Co-I]). Dr. Schlaug receives research support from the NIH (NIDCD 1RO1 DC008796 [PI], NIDCD 3R01DC008796-02S1 [PI], R01 DC009823-01 [PI], and 1R01-NS 057127 [Co-I]). This study received funding support from NIH [RO1 DC008796 and RO1 DC009823-Dr. Schlaug, PI] and CIMIT [Dr. Schlaug, PI].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Conflict of Interest: The authors do not have any conflict of interests to report.
References
- 1.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasel R. Dysphagia after stroke. Incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 2.Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989;52:236–241. doi: 10.1136/jnnp.52.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turney TM, Garraway WM, Whisnant JP. The natural history of hemispheric and brainstem infarction in Rochester Minnesota. Stroke. 1984;15:790–794. doi: 10.1161/01.str.15.5.790. [DOI] [PubMed] [Google Scholar]
- 4.Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, Loesche WJ. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 5.Dziewas R, Rittner M, Konrad C, Oelenberg S, Nabavi DG, Stogbauer F, Ringelstein EB, Ludemann P. Pneumonia in acute stroke patients fed by nasogastric tube. J Neurol Neurosurg Psychiatry. 2004;75:852–856. doi: 10.1136/jnnp.2003.019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penfield W, Boldery E. Somatic motor and sensory representation in cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 7.Martin RE, Sessle BJ. The role of cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- 8.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 9.Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes DG, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet. 1997;350:686–692. doi: 10.1016/S0140-6736(97)02068-0. [DOI] [PubMed] [Google Scholar]
- 10.Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115:1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Luo C, Yu B, Yan B, Gong Q, He C, He L, Huang X, Yao D, Lui S, Tang H, Chen Q, Zeng Y, Zhou D. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J Neurol Neurosurg Psychiatry. 2009;80:1320–1329. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- 12.Gow D, Rothwell J, Hobson A, Thompson D, Hamdy S. Induction of long-term plasticity in human swallowing cortex following repetitive cortical stimulation. Clin Neurophysiology. 2004;115:1044–1051. doi: 10.1016/j.clinph.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand. 2009;119:155–161. doi: 10.1111/j.1600-0404.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 14.Nistche MA, Paulus W. Excitability changes induced in the human cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1889–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 16.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 17.Schlaug G, Renga V, Nair D. Transcranial Direct Current Stimulation in Stroke Recovery. Arch Neurol. 2008;65:1–7. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Jefferson S, Mistry S, Singh S, Rothwell J, Hamdy S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1035–G1040. doi: 10.1152/ajpgi.00294.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neil KH, Purdy M, Falk J, Gallo L. The Dysphagia outcome and severity scale. Dysphagia. 1999;14:139–145. doi: 10.1007/PL00009595. [DOI] [PubMed] [Google Scholar]
- 21.Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad KO, Parker RA, Edelman RR, Warach S. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 22.Steinmetz H, Gunter F, Bernd-Ulrich M. Craniocerebral topography within the international 10–20 system. Electroencephalogr and Clin Neurophysiol. 1989;72:499–506. doi: 10.1016/0013-4694(89)90227-7. [DOI] [PubMed] [Google Scholar]
- 23.Miranda PC, Lomarev M, Hallet M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, Tyrell P, Hobson A, Williams S, Thompson D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;5:831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 25.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 26.Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 28.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 29.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40:1764–1771. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010 doi: 10.1212/WNL.0b013e318202013a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams HP, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MS. Baseline NIH Stroke Scale score strongly predicts outcome after stroke. Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 33.Thijs VN, Lansberg MG, Beaulieu C, Marks MP, Moseley ME, Albers GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke. 2000;31:2597–2602. doi: 10.1161/01.str.31.11.2597. [DOI] [PubMed] [Google Scholar]
- 34.Turhan N, Atalay A, Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: A special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation. 2009;24:321–326. doi: 10.3233/NRE-2009-0485. [DOI] [PubMed] [Google Scholar]
- 35.Hamdy S, Aziz Q, Rothwell JC, Hobson A, Thompson DG. Sensorimotor modulation of human cortical swallowing pathways. J Physiol. 1998;506:857–866. doi: 10.1111/j.1469-7793.1998.857bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer MB, Mattu U, Grafman J, Lomare M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 37.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Rest Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]