Abstract
Hemispheric asymmetry reduction in older adults (HAROLD) has been reported in previous imaging studies that employed not only cognitive, but also motor tasks. However, whether age-related reductions in asymmetry of hemispheric activations affect the symmetry of motor behavior in older adults remains largely untested. We now examine the effect of aging on lateralization of motor adaptation and transfer by investigating adaptation to novel visuomotor transformations in both old and young age groups. We have previously reported substantial asymmetries in interlimb transfer of learning these transformations in young adults, and attributed these asymmetries in transfer to hemispheric lateralization for motor control, as detailed by our dynamic dominance hypothesis. Based on the HAROLD model, we reasoned that older adults should recruit more symmetrical hemispheric activity, and thus show more symmetrical transfer of adaptation across the arms. Half of the subjects in each age group first adapted to a rotated visual display with the left arm, then with the right arm; and the other half in the reversed order. Naïve performance with one arm and the same-arm performance following opposite arm adaptation were compared to determine the extent of transfer in each age group. Our results showed that interlimb transfer of initial direction information only occurred from the non-dominant to dominant arm in young adults, whereas it occurred in both directions in older adults. Our findings clearly indicate substantially reduced asymmetry in visuomotor adaptation in older adults, and suggest that this reduced motor asymmetry might be related to diminished hemispheric lateralization for motor control.
Keywords: Reaching movement, Generalization, Motor learning, Motor control, Hemispheric lateralization
Introduction
Motor function of an individual declines with aging. This may result from a number of factors that involve not only physiological sources such as the loss of muscle mass (thus muscle strength) and the changes in sensory receptors due to aging (Evans 2010; Grabiner and Enoka 1995; Green 1986; Kenshalo 1977), but also neural sources such as the loss of neurons and synapses, which in turn may cause slowed nerve conductions and even some changes in neural network connectivity (Cerella 1990; Fjell and Walhovd 2010). Such changes in neural organization may include reduced hemispheric lateralization in older individuals. It has been reported that brain activations observed during cognitive tasks are more bilateral in older adults than in young adults (Bergerbest et al. 2009; Cabeza et al. 2004; Grady 2000). These findings led to the HAROLD model (hemispheric asymmetry reduction in older adults), which has been supported by a number of studies that used different types of cognitive tasks such as episodic, semantic and working memory and visual perception tasks (Cabeza et al. 2002, 2004; Dolcos et al. 2002; Grady et al. 1995; Grady 2000; Reuter-Lorenz et al. 2000; Rypma et al. 2001). Similar bilateral activations have also been observed in older adults when performing simple motor tasks (Mattay et al. 2002; Rowe et al. 2006; Ward and Frackowiak 2003), although further investigations are necessary to determine the extent to which the HAROLD model can be applied to the motor domain (Rowe et al. 2006).
It should be stressed that the aforementioned findings do not indicate that the motor system in young adults only recruits a single brain hemisphere when performing a unilateral arm and hand movements. In fact, substantial activation of ipsilateral motor cortex (in addition to contralateral cortex) during unilateral arm and hand movement has been well established (Kutas and Donchin 1974; Tanji et al. 1988; Kim et al. 1993; Urbano et al. 1996; Dassonville et al. 1997; Volkmann et al. 1998). Recruitment of both hemispheres can be understood from the perspective that each hemisphere is specialized for controlling different features of movement (Brown and Kosslyn 1993; Corballis 1991; Goodale 1990; Schaefer et al. 2007, 2009; Serrien et al. 2006; Sainburg and Wang 2002; Wang and Sainburg 2007). Our dynamic dominance hypothesis (Sainburg 2002, 2005) suggests that the two hemispheres have become functionally specialized for controlling different aspects of movement: The dominant hemisphere for controlling dynamic features of movement (e.g., limb trajectory during reaching movement) and the nondominant hemisphere for controlling steady-state limb posture (e.g., final position accuracy of reaching). Our dynamic dominance hypothesis has been supported by a number of interlimb transfer studies that examined adaptation to novel visuomotor rotations in young adults (e.g., Sainburg and Wang 2002; Wang and Sainburg 2006a). These studies showed that opposite arm training facilitated adaptation of initial movement direction for the dominant arm, and of final position accuracy for the nondominant arm. While the finding of asymmetrical transfer in initial direction adaptation has been shown to be robust across a variety of task conditions, final position transfer tends to be less robust, and depends not only on workspace location, but also on the particular nature and amplitude of the visuomotor distortion (Wang and Sainburg 2006b; Wang 2008). We, thus, focus primarily on assessing the symmetry in transfer of initial direction in the current study.
It has previously been shown that when older adults perform simple unilateral motor tasks, such as finger tapping, imaging of brain activities reveals an increased symmetry in hemispheric activation (Mattay et al. 2002; Rowe et al. 2006; Ward and Frackowiak 2003). We now ask whether aging is also associated with an increased symmetry in transfer of visuomotor adaptation. This prediction flows directly from our previous studies on visuomotor transformation transfer in younger adults. We reasoned that our findings of asymmetrical transfer in young subjects were directly related to lateralization of motor control mechanisms: Initial adaptation with the nondominant arm requires recruitment of trajectory control mechanisms that reside in ipsilateral (dominant) cortex, which leads to subsequent transfer of direction information to the dominant arm. However, initial adaptation with the dominant arm does not require nondominant hemisphere recruitment, since the dominant hemisphere is specialized for trajectory control. Thus, initial direction adaptation does not transfer from the dominant to the nondominant arm (Sainburg and Wang 2002; Wang and Sainburg 2006a). Since hemispheric recruitment becomes more symmetric with aging (Mattay et al. 2002; Rowe et al. 2006; Ward and Frackowiak 2003), it is reasonable to predict more symmetrical patterns of interlimb transfer following unilateral adaptation to visuomotor transformations. We now test this prediction by comparing the pattern of interlimb transfer following adaptation to a novel visuomotor rotation between older and young adults.
Materials and methods
Subjects
Subjects were 32 neurologically intact right-handed adults (15 Female, 17 male), aged from 18 to 38 years old (‘young adults’ group, mean age: 25 ± 5.2) and from 63 to 83 years old (‘older adults’ group, mean age: 71 ± 6.3). Subjects were paid for their participation. Informed consent was solicited prior to participation. Right handedness was assessed using the 10-item version of the Edinburgh inventory (Oldfield 1971). Handedness scores were not statistically different between young and old subject groups.
Apparatus
Our subjects sat in front of a table with the right arm supported over the tabletop positioned just below shoulder height, by a friction-less air jet system. A start circle, a target, and a cursor representing the index finger position were projected on a horizontal 52″ LCD TV positioned above the arm, which were reflected through a mirror positioned below this TV. The setup gave an illusion that the display was in the same horizontal plane as the hand. The Flock of Birds® (Ascension-Technology, Burlington, VT) magnetic 6-DOF movement recording system was used to sample the index fingertip position at 103 Hz. For more information, see Wang and Sainburg (2009).
Experimental design
Prior to movement, one of eight targets (2 cm in diameter; 13 cm away from the starting position), presented in a pseudorandom sequence, was displayed on the horizontal tabletop. Subjects were instructed to move the finger rapidly from the start circle (1.5 cm in diameter) to the target in response to an auditory “go” signal. With regard to movement speed, our preliminary data indicated that older adults could perform our visuomotor adaptation task at the speed of .4 m/s (peak tangential reaching velocity) without much difficulty. Thus, both young and older adults were asked to keep this movement speed during reaching movement. Visual feedback in the form of a horizontal speed bar was presented upon completion of each trial on top of the screen, which allowed the subjects to directly monitor their own movement speed. The length of the speed bar indicated their peak reaching velocity as compared to the required speed of .4 m/s (e.g., a speed bar that did not reach a point indicating the required speed informed the subjects that they needed to increase their speed in the following trial). During the movement, direct vision of the moving arm was blocked by a horizontal plane that was placed above the arm. Instead, a screen cursor indicating the location of the index fingertip was provided to guide the subjects’ reaching movement. At the end of each trial, knowledge of results was provided in the form of hand-path, and by points awarded for accuracy: 1 point for 2-D accuracy <3 cm, 3 points for accuracy <2 cm, and 10 points for accuracy <1 cm. Points were provided to keep the subjects motivated. In order to examine adaptation to novel visual-motor transformations, the position of the cursor was rotated 30° counterclockwise (CCW) about the start circle. The experiment consisted of two sessions: baseline (no visual rotation) and exposure (visual rotation) sessions. Subjects performed two blocks of trials in each session. Half the subjects performed with the left arm first (group LR), while the other half performed with the right arm first (group RL). Each block comprised 192 trials, divided into 24 cycles, with each cycle containing all eight of the targets. Each block of trials was separated by a 10-min break. Subjects were not informed about the CCW rotation provided during the exposure session. Table 1 shows the sequence of the experimental sessions and blocks for each subject group.
Table 1.
Experimental design
| Group | Baseline (No rotation)
|
Exposure (30° CCW rotation)
|
||
|---|---|---|---|---|
| Block 1 (192 trials) | Block 2 (192 trials) | Block 1 (192 trials) | Block 2 (192 trials) | |
| Young | ||||
| LR (n = 8) | L | R | L | R |
| RL (n = 8) | R | L | R | L |
| Old | ||||
| LR (n = 8) | L | R | L | R |
| RL (n = 8) | R | L | R | L |
Data analysis
Three measures of performance were calculated: hand-path direction error at peak tangential arm acceleration (Amax) and at peak tangential velocity (Vmax) and final position error. Direction errors were calculated as the angular difference between the vectors defined by the target and by the hand-path position at movement start and at Amax or at Vmax. Final position error was calculated as the 2-D distance between the index finger at movement termination and the center of the target.
Because the purpose of this study was to examine the effect of initial training with one arm on subsequent performance with the other arm, we were primarily interested in planned pair-wise comparisons, using independent t tests, between naïve performance and performance following opposite arm adaptation for the dominant arm blocks (right arm performances by LR and RL groups), as well as for the nondominant arm blocks (left arm performances by LR and RL groups) for each age group. An epoch is the mean of two consecutive cycles. The effect of initial training with one arm on subsequent performance with the other arm was only assessed for the first epoch (mean of cycles 1 and 2) in order to examine the extent of initial information transfer.
Results
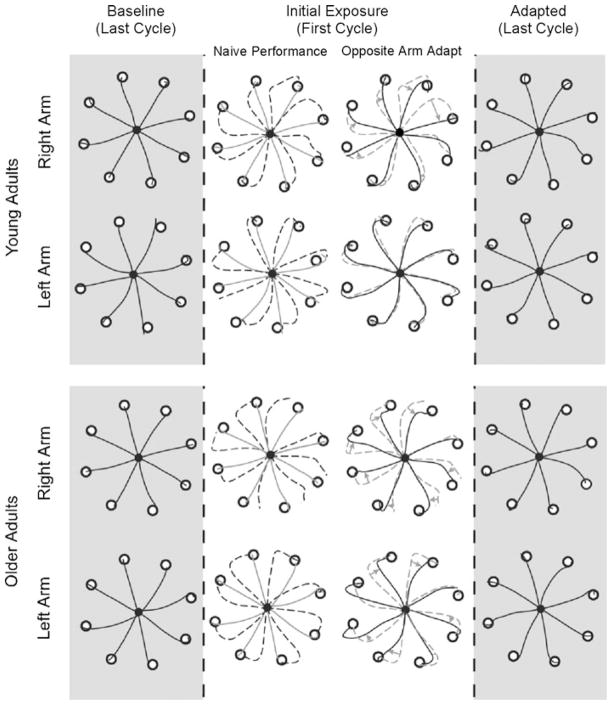
Figure 1 shows typical hand-paths of our representative subjects during the final phase of the baseline session, and during the initial and final phases of the adaptation session. Two subjects from each age group (young adults in upper, older adults in lower panel) are shown: one who adapted to the visual rotation with the right arm first (group RL), and the other who adapted to it with the left arm first (group LR). As illustrated in column 2 (broken lines), the hand-paths obtained during the first cycle (eight consecutive trials) of performance upon initial exposure to the visual rotation are initially directed approximately 20–30° CCW to the target, as expected. The effect of initial training with one arm on subsequent performance with the other arm, illustrated in column 3, appears to be different between the two subject groups: The right arm performance following initial training with the left arm appears to be substantially better than the same arm, naïve performance in both subject groups (row 1 solid vs. broken lines for young, row 3 solid vs. broken lines for old). On the other hand, the left arm performance following the right arm adaptation appears to be better than the same arm, naïve performance only in the older adults groups (row 2 solid vs. broken lines for young, row 4 solid vs. broken lines for old). Following adaptation to the visual rotation, hand-paths obtained during the performance with either arm are directed relatively straight to the targets regardless of the subject groups (column 4).
Fig. 1.
Hand-paths of representative subjects. Each column shows hand-paths of the eight consecutive trials of reaching movement made in eight different target directions. Column 2 shows naïve performance upon initial exposure to visual rotations (broken lines) as compared with baseline performance shown in column 1 (solid lines). Column 3 shows performance following opposite arm adaptation (solid lines) as compared with naïve performance (broken lines). Gray arrows shown in column 3 indicate improvements in directional control of reaching movement following opposite arm adaptation
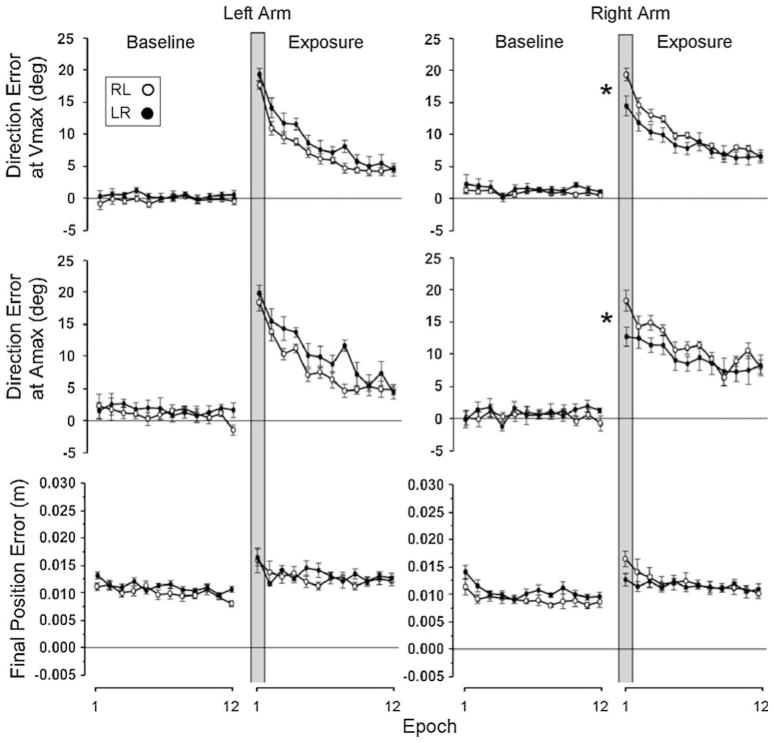
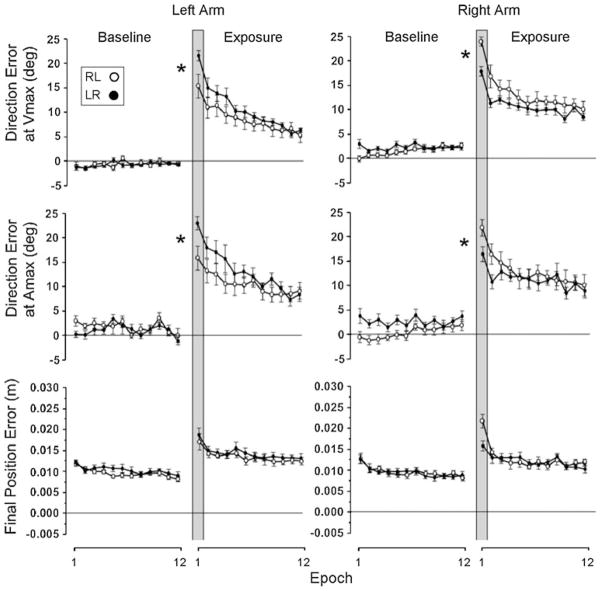
Figures 2 and 3 illustrate the changes in performance across the epochs for the young and older adults, respectively. With regard to initial direction errors, our pair-wise comparisons between naïve performance and performance following opposite arm adaptation at the first epoch indicated that the right arm performance following initial training with the left arm was significantly better than the naïve performance with the same arm in the young adults group (Fig. 2, right panel, rows 1 and 2, closed circles as compared with open circles, respectively, inside the vertical gray bar; P < .05). Similarly, the right arm performance following the opposite arm adaptation was significantly better than its naïve performance in the older adults group as well (Fig. 3, right panel, rows 1 and 2, closed circles as compared with open circles, respectively, inside the vertical gray bar; P < .05). However, the left arm performance following the right arm adaptation was significantly better than its naïve performance only in the older adults group (Fig. 3, left panel, rows 1 and 2, closed circles as compared with open circles, respectively, inside the vertical gray bar; P < .05), and not in the young adults group (Fig. 2, left panel, rows 1 and 2, closed circles as compared with open circles, respectively, inside the vertical gray bar; P = .08 and .48 for direction errors at Vmax and Amax, respectively).
Fig. 2.
Mean performance measures of direction errors and final position error obtained from young adult subjects. Every data point shown on X axis represents the average of 16 consecutive trials (epoch) across all subjects (mean ± SE). Performance measures for the subjects who performed with the right arm first (open circles) and for those who performed with the left arm first (filled circles) are shown separately. Asterisks indicates that the two values were statistically different at P < .05
Fig. 3.
Mean performance measures of direction errors and final position error obtained from older adult subjects. Every data point shown on X axis represents the average of 16 consecutive trials (epoch) across all subjects (mean ± SE). Performance measures for the subjects who performed with the right arm first (open circles) and for those who performed with the left arm first (filled circles) are shown separately. Asterisks indicates that the two values were statistically different at P < .05
It is interesting that the right arm performed somewhat differently between the naïve performance and the performance following the opposite arm adaptation, especially in terms of direction error at Amax, in the beginning of the baseline session (significantly different, P < .05, during the first three epochs). Considering that the right arm performance following left arm adaptation was poorer than the same arm naïve performance, and also that the naïve performance degraded over time, the difference might be attributed to certain factors, such as fatigue and difficulty maintaining the same level of motivation, enthusiasm and/or attention throughout the session.
With regard to final position errors, the difference between naïve performance and the performance following opposite arm adaptation at the first epoch appears to be greater for the right arm than for the left arm, as illustrated in Figs. 2 and 3 (row 3), especially in the older adults group. However, our pair-wise comparisons indicated that the differences between the two performance conditions were not statistically significant, regardless of the subject group or the arm (P > .05).
Discussion
We have previously reported substantial asymmetries in interlimb transfer of visuomotor adaptation in healthy young adults, and attributed these asymmetries in transfer to hemispheric lateralization for motor control, as detailed by our dynamic dominance hypothesis (Sainburg and Wang 2002; Wang and Sainburg 2006a). Integrating our previous findings with the HAROLD model (Cabeza 2002), we predicted that older adults should show increased symmetry in transfer of movement information across the arms, due to diminished hemispheric lateralization of motor control mechanisms. Our current results support this hypothesis with regard to performance measures that reflect limb dynamics, by demonstrating that the pattern of interlimb transfer following visuomotor adaptation is symmetrical in older adults in terms of direction errors at Amax and Vmax. Data from our young adult group of right-handers showed that performance with the right arm, following initial left arm adaptation, was substantially better compared with the same arm naïve performance at the first epoch, in terms of both direction errors at Amax and Vmax. However, such improvements following opposite arm adaptation were not observed for the left arm. This confirmed our previous findings indicating asymmetrical transfer of direction information from the nondominant to dominant arm (Sainburg and Wang 2002; Wang and Sainburg 2006a). On the other hand, our data from the older adult group indicated symmetrical transfer, in that both arms benefited from initial training with the other arm, as reflected by substantial improvement from naïve performance to that following opposite arm adaptation in terms of both our direction measures. These findings indicate increased symmetry in interlimb transfer of visuomotor adaptation in older adults, possibly due to diminished hemispheric lateralization of motor control associated with aging.
Previously, brain activations during simple motor tasks, such as button pressing and hand grip, in older adults have been shown to be more bilaterally symmetric than those observed in young adults (Mattay et al. 2002; Rowe et al. 2006; Ward and Frackowiak 2003), thus suggesting that the HAROLD model can be applied not only to the cognitive domain, but also to the motor domain. To our knowledge, the effect of this change in neural organization on the symmetry of motor behavior in older adults is not well understood. Our current findings provide initial evidence that motor adaptation mechanisms become more symmetric in older adults, as reflected by a substantial reduction in asymmetrical transfer of sensorimotor adaptation when compared with young adults. That is, while the dominant limb/hemisphere system in young adults is thought to be specialized for controlling dynamic features of reaching movements, aging may reduce this lateralization, such that both dominant and nondominant hemispheres contribute to all aspects of control. Cabeza (2002) postulated that the reduced asymmetry of hemispheric activations with aging might be related to compensation for age-related reductions in neuronal number and circuit complexity in each hemisphere. By recruiting both hemispheres, the CNS might re-establish some degree of circuit complexity and thus compensate for potential deleterious effects on behavior.
While demonstrating a lack of asymmetry in interlimb transfer, our data also showed that the older adults were able to adapt to the novel visuomotor rotation condition unilaterally to an extent similar to that observed in young adults. This is consistent with previous findings that the amount of learning obtained during visuomotor adaptation is not very different between young and older adults (Buch et al. 2003; Heuer and Hegele 2008; Anguera et al. 2011). Buch et al. (2003) studied the pattern of adaptation to a 90° rotation of the visual display in young and older adults and reported that although the final level of adaptation was somewhat reduced in older adults, the size of aftereffects was similar between the two groups. Heuer and Hegele (2008) also reported that there were no age-related changes of aftereffect in visuomotor adaptation due to aging, and further that when different age groups were matched by explicit knowledge based on which they performed the adaptation task, other age-related deficits of adaptation largely disappeared. Based on this finding, they concluded that the age-related deficits observed during visuomotor adaptation pertain primarily to cognitive aspects of the adaptation task such as strategic corrections and explicit knowledge, rather than to the acquisition of an implicit internal representation of novel visuomotor transformations. This idea is also supported by a more recent finding reported by Anguera et al. (2011), which indicated that age-related declines in spatial working memory contributes significantly to the deficits observed in older adults during visuomotor adaptation. These findings collectively indicate that older adults have intact ability to adapt to novel visuomotor conditions to the extent similar to that of young adults (as long as other cognitive factors do not cause age-related deficits), which further suggests that the effect of aging on the development of neural representation of a novel visuomotor adaptation is minimal. In fact, the fact that the adaptation process is largely intact in our older subjects may reflect the compensatory nature of the more symmetrical recruitment associated with Cabeza’s hypothesis. This would be consistent with our findings of similar levels of adaptation, but more symmetric transfer.
With respect to the transfer of positional information, our current results indicate that neither arm benefited from opposite arm adaptation in terms of final position accuracy in either subject group. We have previously demonstrated transfer of final position information from the dominant to nondominant arm in young adults (Sainburg and Wang 2002; Wang and Sainburg 2006a). In the present study, however, even young adults failed to show such transfer. In fact, a trend of transfer was observed in the opposite direction in both subject groups, although the effect was not statistically significant. This finding may be attributed to the fact that the speed of reaching movement was lower in the present study, compared to our previous studies. In our previous studies, peak tangential reaching velocity was approximately 0.9 m/s (with no significant difference between the arms), whereas in the present study both young and older adults tried to make reaching movement at 0.4 m/s to control for the potential covariate effect of speed on the pattern of interlimb transfer. This must have provided the motor system with additional time to utilize online visual feedback. We have previously suggested that initial direction control is primarily achieved by the dominant system in a feedforward fashion, whereas final position control is largely influenced by feedback-mediated control mechanisms (Wang and Sainburg 2006b). Our current findings may indicate that when enough time is available for the processing of online visual feedback, the advantage of the nondominant system over the dominant system for controlling final position accuracy becomes minimal, a hypothesis supported by preliminary findings from our laboratory (Przybyla and Sainburg 2010).
Acknowledgments
This research was supported by the National Institutes of Health, National Institute for Child Health and Human Development RO1HD39311 and 1R01HD059783 to RS, K01HD05 0245 to JW; and Career Scientist Award from the Department of Veterans Affairs to KYH.
Contributor Information
Jinsung Wang, Email: wang34@uwm.edu, Department of Human Movement Sciences, The University of Wisconsin, Milwaukee, WI 53201, USA.
Andrzej Przybyla, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA.
Kati Wuebbenhorst, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA.
Kathleen Y. Haaland, Research Service, New Mexico Veterans Affairs Healthcare System, Departments of Psychiatry and Neurology, University of New Mexico, Albuquerque, NM, USA
Robert L. Sainburg, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA
References
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2011;23:11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Gabrieli JD, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. Neuroimage. 2009;45:237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Kosslyn SM. Cerebral lateralization. Curr Opin Neurobiol. 1993;3:183–186. doi: 10.1016/0959-4388(93)90208-g. [DOI] [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cerella J. Aging and information-processing rate. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 3. Academic Press; New York: 1990. pp. 201–221. [Google Scholar]
- Corballis MC. Left brain, right brain. Science. 1991;251:575–576. doi: 10.1126/science.251.4993.575-a. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci USA. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Goodale MA. Brain asymmetries in the control of reaching. In: Goodale MA, editor. Vision and action: the control of grasping. Ablex; Norwood: 1990. pp. 14–32. [Google Scholar]
- Grabiner MD, Enoka RM. Changes in movement capabilities with aging. Exerc Sport Sci Rev. 1995;23:65–104. [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biol Psychol. 2000;54:259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Grady CL, Mcintosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Green JJ. Characteristics of aging human skeletal muscles. In: Sutton JR, Brock RM, editors. Sports medicine for the mature athlete. Benchmark Press; Indianapolis: 1986. pp. 17–26. [Google Scholar]
- Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging. 2008;23:190–202. doi: 10.1037/0882-7974.23.1.190. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR. Age changes in touch, vibration, temperature, kinesthesis, and pain sensitivity. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Van Nostrand Reinhold; New York: 1977. pp. 562–579. [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Kutas M, Donchin E. Studies of squeezing: handedness, responding hand, response force, and asymmetry of readiness potential. Science. 1974;186:545–548. doi: 10.1126/science.186.4163.545. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Przybyla A, Sainburg RL. Program no. 293.11, Neuroscience meeting planner. Society for Neuroscience; San Diego, CA: 2010. Non-dominant advantages for movement accuracy depend on task conditions. Online. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006;32:747–760. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychol Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev. 2005;33:206–213. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direct and final end position information. Exp Brain Res. 2002;145:437–447. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47:2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. Erratum in: Neuropsychologia (2010) 48:1178–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DA, Ivry RB, Swinnen SP. Dynamics of interhemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006;7:160–167. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Urbano A, Babiloni C, Onorati P, Babiloni F. Human cortical activity related to unilateral movements. A high resolution EEG study. Neuroreport. 1996;8:203–206. doi: 10.1097/00001756-199612200-00041. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Schnitzler A, Witte OW, Freund H. Handedness and asymmetry of hand representation in human motor cortex. J Neurophysiol. 1998;79:2149–2154. doi: 10.1152/jn.1998.79.4.2149. [DOI] [PubMed] [Google Scholar]
- Wang J. A dissociation between visual and motor workspace inhibits generalization of visuomotor adaptation across the limbs. Exp Brain Res. 2008;187:483–490. doi: 10.1007/s00221-008-1393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Interlimb transfer of visuomotor rotations depends on handedness. Exp Brain Res. 2006a;175:223–230. doi: 10.1007/s00221-006-0543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The symmetry of interlimb transfer depends on workspace location. Exp Brain Res. 2006b;170:464–471. doi: 10.1007/s00221-005-0230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The dominant and non-dominant arms are specialized for stabilizing different features of task performance. Exp Brain Res. 2007;178:565–570. doi: 10.1007/s00221-007-0936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Generalization of visuomotor learning between bilateral and unilateral conditions. J Neurophysiol. 2009;102:2790–2799. doi: 10.1152/jn.00444.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]