Abstract
Mammalian hibernation is characterized by profound reductions in body temperature (Tb) and metabolic, heart and respiratory rates. These reductions are characteristic of torpor, which is temporally confined to winter. Hibernators including ground squirrels are heterothermic in winter, cycling between multiday periods of torpor with low Tb and brief periods of rewarming. In contrast, ground squirrels remain homeothermic during summer, like non-hibernating mammals. The transition between the homeothermic and heterothermic phases of the circannual rhythm of hibernation is often overlooked in hibernation studies. Here, we examined the use of torpor throughout the fall transition in laboratory-housed 13-lined ground squirrels by recording core body temperature with an implanted data logger. As is typical of laboratory-based hibernation studies, animals were kept in standard housing prior to being moved into a cold, dark room to simulate natural hibernation conditions. Significantly, the vast majority of both male and female ground squirrels expressed torpor in the fall while still housed conventionally and prior to cold exposure. The expression of torpor was not predicted by body weight or age, rather it appears to be preprogrammed in a time-dependent manner that is independent of, yet enhanced by, environmental cues. The timing and duration of these torpor bouts occurring prior to cold exposure were also remarkably sporadic. Thus it is not possible to know with certainty which animals are torpor-naïve before cold exposure in the absence of continuous measurement of body temperature. We conclude that fall animals encompass variable points in the transition between summer and winter phases of the circannual cycle of hibernation, thereby confounding studies in which they are used as non-hibernating controls. Conversely, these fall transition animals offer unique opportunities to define the molecular changes that accompany and enable hibernation.
Keywords: sciurid, Ictidomys tridecemlineatus
Introduction
Small circannual hibernators divide each year into a summer season of homeothermy and a winter season of pronounced heterothermy, or hibernation (Fig. 1). The number of days spent in the winter hibernating phase vs. the summer active phase varies from animal to animal in constant conditions, but the cycle approximates one year. These hibernating species are concerned with reproduction in spring, which is followed by growth and fattening. Animals deposit large reserves of fat in preparation for winter hibernation as summer wanes. In fall, the animals become hypophagic and then disappear into sealed burrows where they remain throughout the winter in hibernation (reviewed in Carey et al. 2003). Sciurid hibernators continue to exhibit this circannual rhythm in constant conditions, for example Asian chipmunks housed in the cold and dark (Kondo et al. 2006), or woodchucks housed in the warm and light (Concannon et al. 2001), and thus are also called obligate hibernators (Drew et al. 2007).
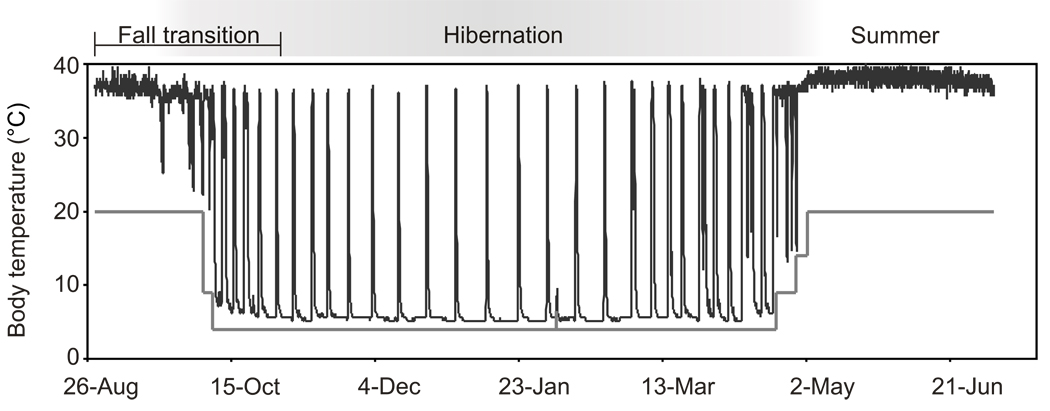
Fig. 1.
Body temperature transitions of a laboratory-housed 13-lined ground squirrel. Abdominal Tb was recorded from August 28 through the following July 6 by an implanted data logger in a 13-lined ground squirrel. The animal was housed in standard laboratory (summer housing) conditions until October 5 when it was moved into the winter housing conditions (hibernaculum) at 9°C. Ta was dropped to 4°C on October 8, where it remained until it was raised to 9°C on April 21 the following spring, and then 14°C on April 28. The animal was moved into conventional housing (20°C) on May 2. Summer homeothermy, winter heterothermy (i.e., hibernation), and the fall transition studied here, are indicated. The gray line plots ambient temperature.
Hibernation stages with their natural rhythm are recapitulated in the laboratory in a number of ground squirrel species, which provide excellent model organisms for hibernation research (Lyman et al. 1982; Wang 1988). Several attributes of natural mammalian hibernation are of particular interest for potential applications in medicine, including: an inherent resistance to ischemia-reperfusion damage; muscle, bone and intestinal preservation in spite of disuse; and reversible metabolic depression (reviewed in Andrews 2007; Carey et al. 2003; Drew et al. 2007). Therefore, there is great interest in understanding the molecular basis of this remarkable phenotype. Approaches to hibernation studies range from hypothesis driven (most recently Andrews et al. 2009; Dave et al. 2009) to discovery driven (Epperson et al. 2010; Epperson et al. 2010; Fedorov et al. 2009; Nelson et al. 2009; Shao et al. 2010) and others reviewed in (Andrews 2007; Carey et al. 2003; Drew et al. 2007; Storey 2010 ); all are plagued by the complexity of the phenotype and an inherent difficulty of defining appropriate controls. Specifically, if the goal is to identify biomolecules associated with hibernation, is it preferable to look for those that increase or decrease in torpor compared to the natural rewarming periods of heterothermy; winter torpor compared to summer homeothermic; or winter animals housed in the warm compared to those housed in the cold (Drew et al. 2007)?
The majority of biochemical studies begin with wild-trapped summer ground squirrels that are moved into standard laboratory housing with ad lib food and water, constant ambient temperature (Ta) of ~21°C, and a light:dark cycle which in some studies approximates local ambient light cycles and in others is constant 12:12 or similar. In the fall, animals are typically moved into an environmental chamber at 4°C with no or little light (the hibernaculum), and food and water are withdrawn to facilitate hibernation. Historically, torpor-arousal patterns were most often measured by checking animals daily for displacement of sawdust from their backs, although continuous electronic measurement of Tb provides more accurate information (Twente and Twente 1965) and is now often used. Torpor patterns are not typically monitored in animals housed in standard laboratory conditions before introduction to the hibernaculum, although there is anecdotal (Concannon et al. 2001; Vaughan et al. 2006 ) evidence that laboratory-housed sciurids use torpor prior to cold exposure.
The pronounced segregation of the homoeothermic and heterothermic phases of the circannual rhythm, together with predominantly seasonal changes of liver metabolites and gene products, led us to hypothesize that the expression of torpor in hibernation involves two biochemical switches: first animals must switch from summer to winter mode which then enables a second torpor-arousal switch (Serkova et al. 2007). A purely mathematical model that recapitulates the body temperature rhythm of ground squirrel hibernation is consistent with a torpor-arousal switch being imbedded within a summer-winter switch (Hampton and Andrews 2007). If the two-switch model is correct, ground squirrels undergoing the fall transition represent a particularly significant group in hibernation research. These animals could either confound our understanding of the molecular events critical for hibernation by exhibiting unappreciated and uncontrolled variability as they pass through the transition, or, alternatively, animals in the fall transition may offer a unique opportunity to dissect the molecular events that underlie the switch between the homeothermic and heterothermic states. In this study, we use implanted data loggers to continuously record Tb throughout the fall transition. The data reveal extensive use of torpor in laboratory-housed 13-lined ground squirrels in September and October as they transition from summer homeothermy to winter heterothermy prior to cold exposure.
Materials and methods
In this report, seasons of the year (summer, fall, winter and spring) are defined by the flexible biological rhythm of Tb in the hibernator (see Introduction, Fig. 1), not by the fixed astronomical calendar.
Animals
Laboratory bred 13-lined ground squirrels (Ictidomys tridecemlineatus) were purchased in the summers of 2007 and 2008 from D. Vaughan, University of Wisconsin, Oshkosh, WI. Data were obtained from 57 animals, including 17 adults and 40 juveniles (26 females, 31 males). The juveniles were born during the spring prior to the hibernation season studied, whereas adults had experienced at least one year of hibernation. Fewer adults than juveniles were used because of availability. Upon arrival in Colorado (July or early August), animals were individually housed in rodent cages containing a plastic tube (Vaughan et al. 2006). The room was maintained at 20 ± 2°C (hereafter referred to as 20°C), with light:dark cycles at 14L:10D, until late September (2008) or early October (2007). In these summer housing conditions, water, cat chow (Teklad Global Cat Diet 2060, Harlan Laboratories, Vaughan et al. 2006) and sunflower seeds were provided ad libitum, and body weights were recorded twice weekly.
On August 28, 2007, or September 3–4, 2008, 13-lined ground squirrels were surgically implanted intra-abdominally with Elvax (Minimitter, Inc.)-coated data loggers (iButton DS19222, Lawrenceburg, KY), programmed to record Tb ± 0.5°C every 20, 30 or 60 min. Ground squirrels were allowed to recover for 3–6 weeks before moving to a dark environmental chamber (hibernaculum, winter housing conditions) on either October 5, 2007, or September 29, 2008. The plastic tubes were withdrawn, and the animals were given a combination of paper towels and nestlets (Ancare) sufficient to build a nest that nearly encased them, such that only a small patch of their back remained visible when torpid in hibernation. Twelve animals were euthanized before moving into the hibernaculum. The temperature in the chamber was lowered in a stepwise fashion over 1–2 weeks until reaching 4°C where it remained for the remainder of the season (Fig. 1). Once in the hibernaculum, food and water were removed as animals displayed torpor and disturbance was minimized. Thus, animals were weighed on the day they moved into the hibernaculum and the day the implanted data loggers were recovered. Animal use was approved by the University of Colorado Denver Institutional Animal Care and Use Committee.
Data Analysis
The data loggers were retrieved at various times between September and the following July when animals were euthanized for tissue collection for use in other biochemical studies. The data obtained from one male and one female, both juveniles, were excluded from further analysis; these two animals could not be classified into either the group that expressed torpor prior to introduction to the hibernaculum or those that did not because they remained homeothermic until their dataloggers were recovered on September 26 while still housed at Ta = 20°C. Therefore, we were unable to determine if or when these two animals would have expressed torpor had they not been euthanized. All Tb data were analyzed using a program written in-house that extracted torpor bout information from the logged time and temperature recordings. The program captured the timepoints at which Tb fell below 32°C and the time at which Tb recovered to above 32°C, and then calculated the elapsed time with Tb above (euthermic interval) and below (torpor bout length) 32°C. We defined a torpor bout as the time with at least two continuous Tb data points below 32°C. Torpor bouts of less than 24 hrs in length were categorized as daily torpor, and those with decreased temperature lasting more than 24hrs were categorized as multiday torpor.
The date of first torpor was calculated by determining the time and date an entrance into torpor occurred. In order to merge the data collected in 2007 and 2008, the dates were transformed from calendar date to the number of days relative to the date of transfer into the hibernaculum, which was defined as day zero. Therefore, days prior to cold exposure are negative in value and those following are positive. The weight on the first date of torpor was the measurement made closest to that day if the torpor bout occurred while the animals were housed at 20°C. The reported weight for animals moved into the hibernaculum was as determined at the time of transfer.
The Fisher’s Exact Test (http://www.danielsoper.com/statcalc/calc29.aspx) was used to determine significance between males and females, juveniles and adults, and between 2007 and 2008. The chi-square log-rank test (R statistical survival package) was used to evaluate differences between males and females in the timing of their first use of torpor. Regression lines were fit to data showing weight at first torpor, torpor bout length, and euthermic interval and evaluated for correlation in R. Circadian rhythmicity of torpor bouts occurring at Ta = 20°C was evaluated using the circular statistical package in R (Circular Statistics version 0.3–8). Data were evaluated by the Rayleigh test for circular uniformity, with p <0.05 indicating that the entrance times were not evenly distributed (Zar 1996). A modified clock-plot function was used to plot the mean vector direction (i.e., preferred time of torpor entrance) and mean vector length, or concentration (i.e., r ~1 indicates the observed entrance times cluster at the mean time of torpor entrance, whereas as r ~0 indicates entrance times approach random). Values for torpor entrances were determined for < 1d and multiday torpor bouts separately.
Results
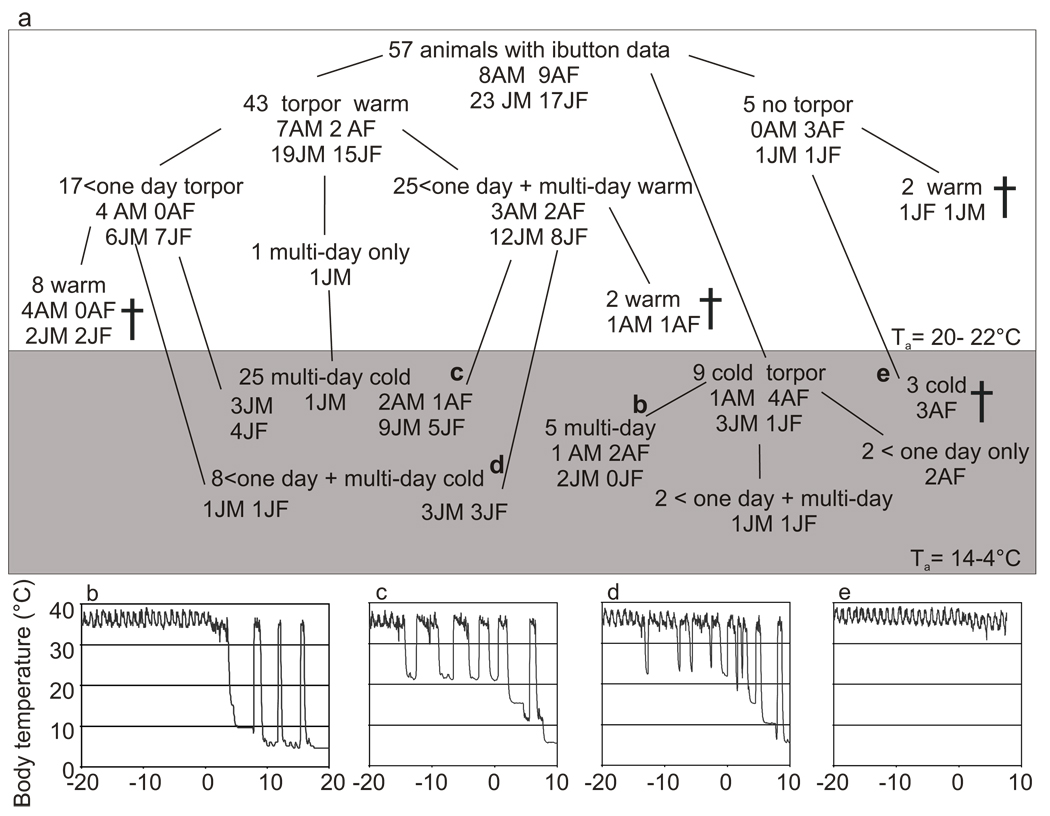
The goal of this study was to examine the onset of heterothermy in laboratory-housed 13-lined ground squirrels as they transition from summer to winter. This transition is a critical time in the circannual cycle, but one that is often not considered in hibernation studies. Body temperature data from implanted data loggers were recovered from 57 animals and analyzed for torpor expression based upon time with Tb < 32°C from early September through October. The pattern was recorded from the time of data logger implantation to, in most cases, establishment of regular multiday torpor bouts at Ta = 4°C. For the purpose of this analysis, torpor was defined as two or more consecutive data points with Tb < 32°C. Animals were subdivided into groups depending upon their Tb data. Initially groups were based upon whether the animal had expressed torpor prior to cold exposure or not, or, for five individuals, whether torpor was expressed before euthanasia (Fig. 2a). These groups were then further subdivided based on the specific patterns of torpor, i.e., whether the torpor were ≤ 1d, multiple days, or both. The data from two animals were excluded from further analysis (see Methods and Fig. 2a). The majority of 13-lined ground squirrels, 43 of 55 (78%), expressed torpor while still housed in Ta = 20°C compared to 9 of 55 (16%) that entered torpor for the first time only after exposure to the cold and dark upon transfer into the hibernaculum. The final three animals in this study remained euthermic throughout the data collection period even after spending 7–21 days in the cold and dark. Representative Tb traces from four individuals representing four of the various groups in Fig. 2a are shown in Fig. 2b–e.
Fig. 2.
Complex patterns of torpor use by 13-lined ground squirrels in fall. a) Summary of individual torpor patterns, based on length of time with Tb < 32°C, for 57 animals during September and October. Groups are subdivided based upon details of torpor expression, with lines leading to the various patterns observed within each grouping. † indicates data logger recovery (AM= adult male, AF= adult female, JM= juvenile male, and JF= juvenile female), b–e are individual Tb traces illustrating the indicated groups; b) no torpor at Ta = 20°C and only multiday torpor at Ta = 4°C; c) <1d and multiday torpor at Ta = 20°C, only multiday at Ta = 4°C; d) <1d and multiday torpor at Ta = 20°C and Ta = 4°C; e) no torpor in either Ta = 20°C or Ta = 4°C. Day zero in panels b–e indicates the time of transfer into the hibernaculum; the step-wise lowering of Ta in the hibernaculum is reflected in Tb in panels b–d.
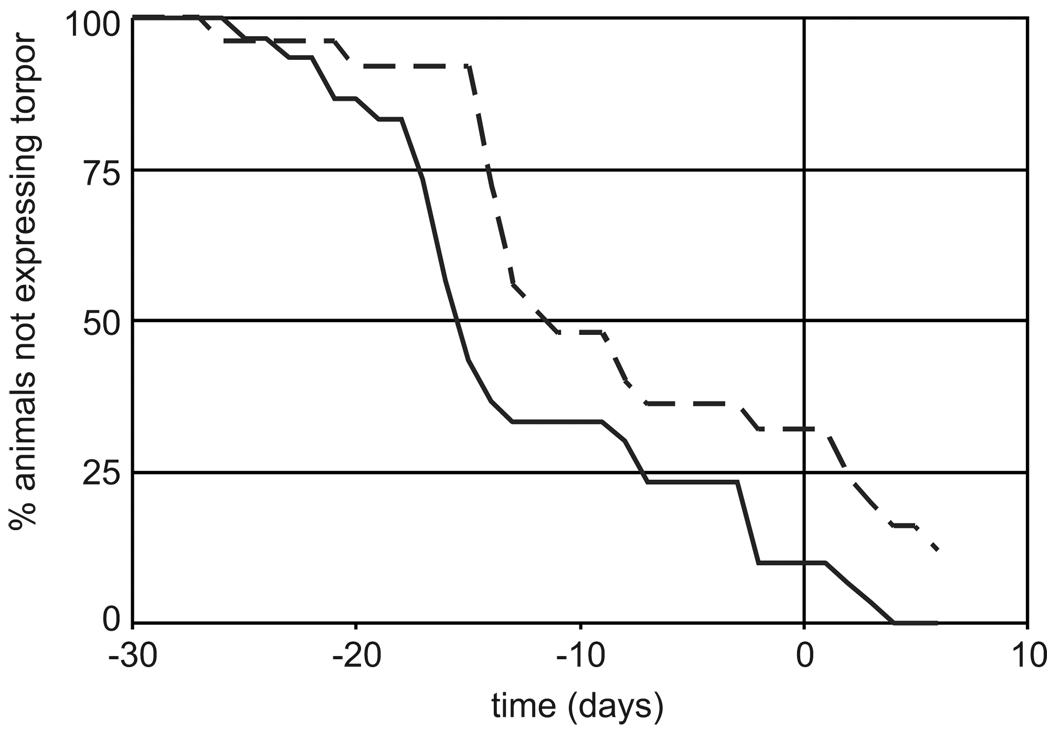
The 55 animals of this study included a mixture of males and females, juveniles and adults. Data were collected over two years; because torpor expression did not differ (Fisher’s Exact Test p = 0.96), the data from these two years were combined for all further analyses. 17 of 25 females (68%) and 26 of 29 males (90%) entered torpor at Ta = 20°C; thus sex did not predict whether torpor was used prior to cold exposure (p=0.09, Fisher’s Exact Test). Males were, however, more likely to use torpor earlier in the season than females (Fig. 3, Chi-square=6.7, p=0.01). Age was also considered as a possible factor for predicting the use of torpor prior to transfer to the hibernaculum; juveniles in this study were significantly more likely than adults to become torpid at Ta = 20°C (34 of 38, compared to 9 of 17, respectively, p=0.005, Fisher’s Exact Test).
Fig. 3.
Fall torpor prior to cold-exposure in both male and female 13-lined ground squirrels. Plot of percent males (solid line) compared to females (dashed line) that have used torpor vs. time before (negative numbers) and after (day zero) moving into the hibernaculum.
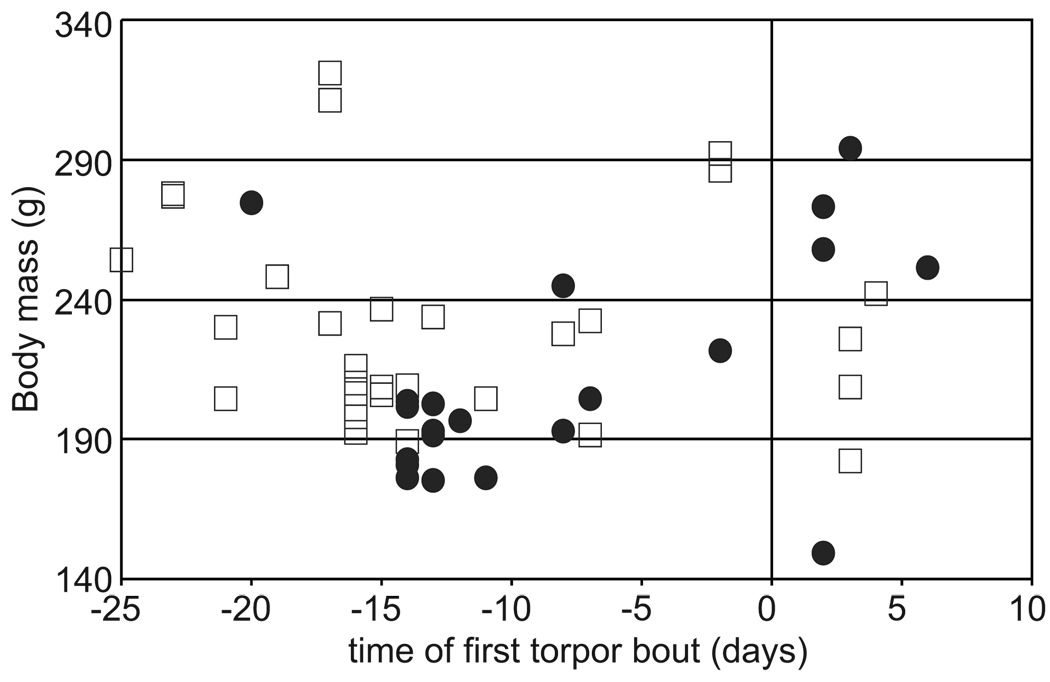
The data were also examined for the effect of body mass on the expression of torpor. Absolute body mass of 13-lined ground squirrels does not predict the use of torpor at Ta = 20°C (Fig. 4). The body mass of animals on the date of first torpor varied widely, as did the date of first torpor of the heaviest animals (e.g., those >250g, Fig. 4). However, males were generally heavier than females in the fall and entered torpor earlier on average, as shown in Fig. 3. Body mass for each individual fluctuated during this time period, typically decreasing immediately following surgery, and then increasing slowly and steadily before torpor bouts ensued. The heaviest animal was male but he was not the first to enter torpor. The lightest animal studied, an adult female, had not exhibited torpor before being euthanized after three weeks at Ta = 4°C.
Fig. 4.
Body mass at time of first torpor. Females are shown as circles, males as open squares. Time zero is the day of transfer to the hibernaculum. Body masses of 52 animals at first use of torpor are plotted, R2 = 0.0003, p = 0.90 (see Methods).
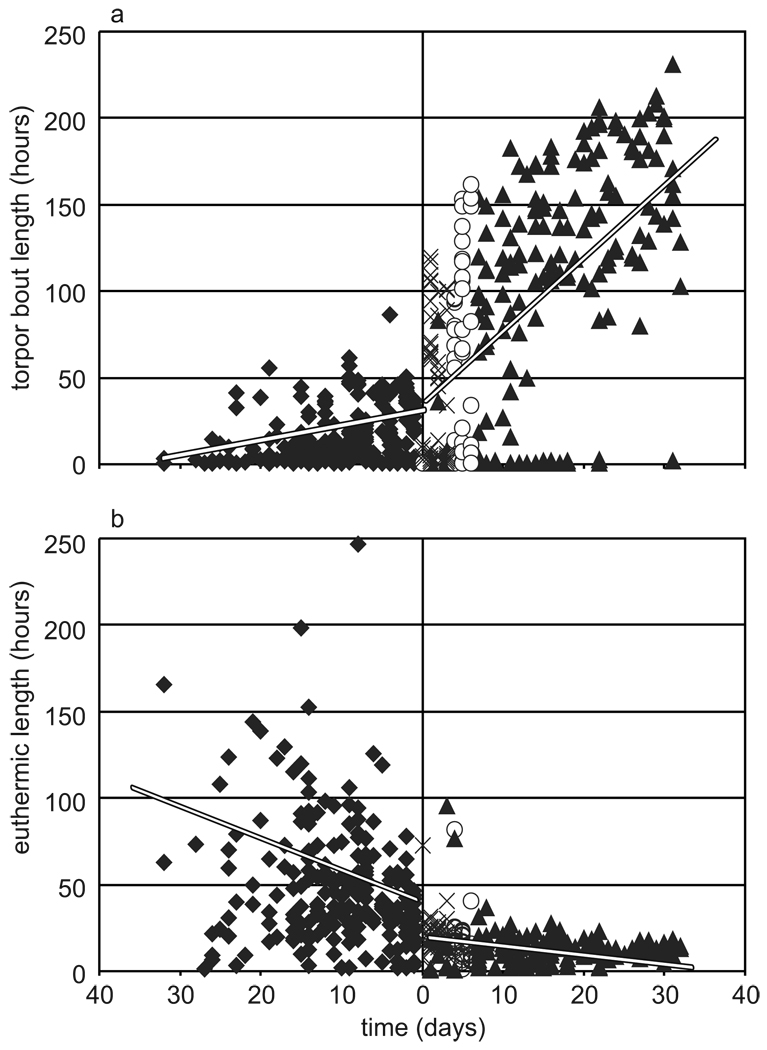
The torpor patterns displayed by these 55 animals were highly variable. Torpor bouts ranged from less than 1 hr to 86 hrs when animals were using torpor at Ta = 20°C, and from less than 1 hr to more than 220 hrs in the cold and dark. In spite of this variability, the length of time spent torpid steadily increased as the season progressed. This trend of increasing bout length over time occurred regardless of Ta, however, the increase was greater when animals were housed in the hibernaculum than when they were housed in standard conditions (compare the slopes of the two regression lines in Fig 5a). Although less dramatic, the inverse relationship was observed in the time spent euthermic between torpor bouts; the euthermic intervals decreased as fall progressed (Fig. 5b). Considering individual animals, the most common pattern of torpor (25 of 43 animals) at Ta = 20°C included a mixture of short (<24 hrs) and multiday torpor bouts, with typically, but not exclusively, short bouts preceding multiday bouts. An additional 17 animals only used short bouts of torpor at Ta = 20°C, but most of these switched immediately to long bouts when exposed to Ta = 4°C. Just one of the 43 animals that expressed torpor in Ta = 20°C bypassed the use of short bouts altogether.
Fig. 5.
Increase in use of torpor throughout the fall transition. Events occurring at Ta 20, 14, 9 or 4°C are marked by diamonds, x, open circles, and triangles, respectively. Day 0 was date of transfer into the hibernaculum. a) Plot of torpor bout length over time. Torpor bouts decrease in length as fall progresses at Ta = 20°C (R2 = 0.10, p = 1.7 × 10−6) and Ta = 4°C (R2 = 0.36, p = 2.2 × 10−16) although the decrease is greater after transfer to the hibernaculum. b) Plot of euthermic interval over time. As animals progressed through the fall transition, the time spent euthermic decreased (R2 = 0.07, p = 1.1 × 10−4). Euthermic intervals further decreased upon transfer to the hibernaculum (R2 = 0.06, p = 4.9 × 10−5).
The Tb traces from a minority of animals (12, 22%) in this study indicated that torpor was not used prior to moving into the hibernaculum (Fig. 2a,e). Nine of these 12 animals (3 JM, 1 AM, 1 JF and 4 AF) became torpid 1–7 days after moving into winter housing conditions, five (2 JM, 1 AM and 2 AF) of which completely bypassed the use of short bouts of torpor and immediately entered multiday torpor. The other four animals in this group (1 JM, 1 JF and 2 AF) exhibited at least one short bout of torpor (<1 day) prior to any multiday torpor bouts. Three additional adult females did not enter torpor, even after 1–3 weeks in the hibernaculum.
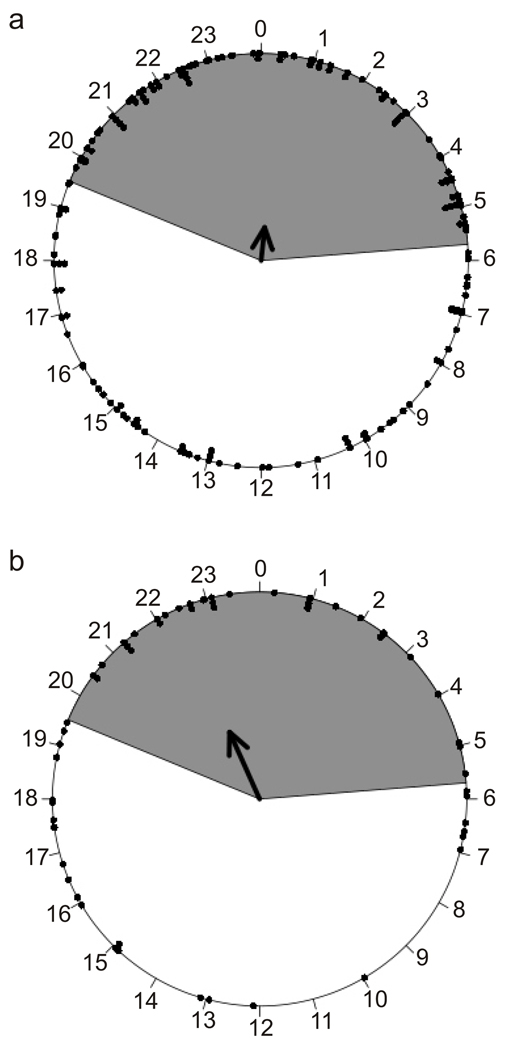
The timing of Tb reductions of animals using torpor at Ta = 20°C were modestly correlated to the 14L:10D photoperiod. Fig. 6 reveals that both < 1d (Fig. 6a) and multiday (Fig. 6b) bouts of torpor were preferentially initiated during the dark hours, between 19:30 and 5:45. Among the 167 torpor bouts lasting <1d, there were just four examples of a pattern similar to that of daily torpor, i.e., Tb fell to ≤32°C within 2.5 hrs of lights off, reached ~ Ta , and then recovered to Tb ≥ 32°C within 2.5 hrs of lights on (Fig. 7).
Fig. 6.
Distribution of entrance times on a 24hr clock for torpor bouts occurring at Ta = 20°C. a) Torpor bouts <1day, the entrance times are not uniform (Rayleigh uniformity p-value=0.01), with a preferred mean entrance time of 00:24 (r=0.1611, S2=0.8389, Rayleigh preferred mean p-value=0.002, n=167). b) Multiday torpor bouts, the entrance times are not uniform (Rayleigh uniformity p-value=0.0007), with a preferred mean entrance time of 23:24 (r=0.3544, S2=0.6456, Rayleigh preferred mean p-value=0.0001, n=58). The arrows define the mean preferred entrance time, and the length of the arrow indicates the concentration (r). Each dot represents the time at which Tb dropped to ≤32°C; the dark phase of the L:D cycle is indicated by gray shading.
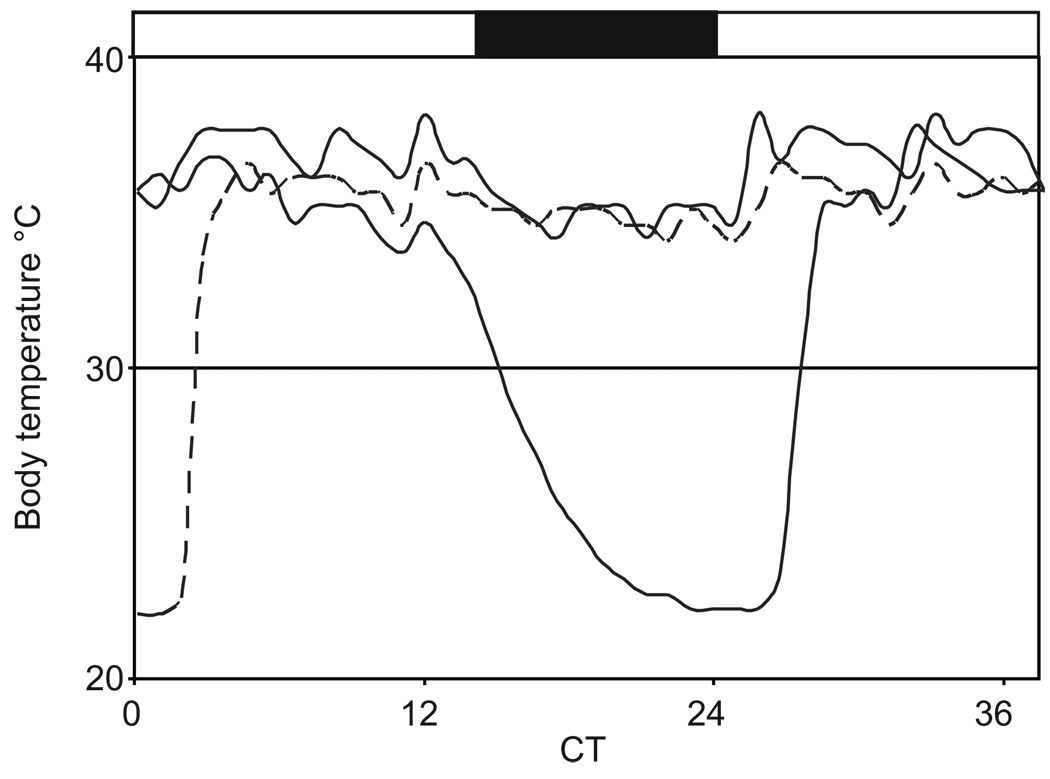
Fig. 7.
Individual Tb traces during the fall transition in 13-lined ground squirrels prior to cold exposure. The trace covers ~36 hrs with time zero at lights on (CT, circadian time). The filled bar coincides with the dark phase. Three individuals are shown for the same time period to illustrate the daily nocturnal Tb depression which is enhanced to a daily torpor-like event in one of the animals.
Discussion
Three features of the Tb traces throughout the fall transition are noteworthy: first, and most surprisingly, the majority of the ground squirrels, 78%, used torpor while still housed in summer conditions. Second, the individual patterns of torpor were highly variable, particularly prior to transfer to the hibernaculum, hence the large number of groups in Fig. 2a. Third, no readily measurable parameter, e.g., sex, age or body mass, predicted which 13-lined ground squirrels used torpor at Ta = 20°C and which remained homeothermic prior to cold exposure. Although others have noted the apparent use of torpor in laboratory-housed 13-lined ground squirrels (Vaughan et al. 2006) and other sciurid hibernators (Concannon et al. 2001; Gur et al. 2009; Kondo et al. 2006) prior to, or without, exposure to low Ta, to the best of our knowledge this is the first quantitative evaluation of this behavior. The extent, variability, and unpredictability of torpor utilization under standard laboratory housing conditions in the fall were unexpected, not only based on the existing hibernation literature, but also our daily visual observation of the animals while still housed at Ta = 20°C. The extensive use of torpor by 13-lined ground squirrels during the fall transition and prior to cold exposure has substantial ramifications for the design of hibernation studies.
The view that hibernation in ground squirrels and other sciurid rodents is induced by moving the animal into a cold, dark environment in the fall has permeated the hibernation literature for decades (Andrews 2007; Arendt et al. 2003; D'Alecy et al. 1990; Kalia et al. 1983; Klain 1976). Rather than inducing hibernation, however, the data in Fig. 5 indicate that moving the animals into the cold and dark facilitates the expression of torpor in the pattern characteristic of hibernation: multiday periods with Tb near Ta interrupted by fewer and shorter periods of arousal to euthermic Tb. The belief that hibernation is induced by transfer to the cold and dark may explain why many studies have used fall or winter animals housed in standard laboratory animal environmental conditions as non-hibernating controls. In almost all cases, the history of torpor use in these animals is not known because Tb is not monitored continuously. Based on our data, however, conventionally-housed animals in the fall, and most likely winter as well, are not suitable non-hibernating controls because these animals are frequently heterothermic (Fig. 5a). The findings reported here clearly demonstrate the need for continuous Tb recording (Drew et al. 2007) to document the non-hibernating status of fall or winter ground squirrels that are used as non-hibernating controls.
Based on the results of a liver metabolomics screen, we proposed that the circannual hibernation cycle in 13-lined ground squirrels comprises two biochemical switches, the first, from summer to winter, enables the expression of heterothermy, and the second controls torpor and arousal. The model further predicts that the torpor-arousal switch is only enabled when the summer-winter switch is in winter mode (Serkova et al. 2007). It is likely that animals using torpor at Ta = 20°C have already begun or even completed critical alterations in the summer to winter switch. Therefore, comparisons between conventionally- and hibernacula-housed winter animals will be unable to reveal the important components and features of physiological and biochemical alterations that enable heterothermy.
Three patterns of heterothermy have been reported in mammals: daily torpor, hibernation and estivation. The edible dormouse (Glis glis) has been documented to use all three (Wilz and Heldmaier 2000). In contrast to hibernation, estivation is characterized by multiday periods of torpor in hot, dry conditions at higher Tb, and daily torpor is characterized by Tb <32°C for <1d. Based on common physiological parameters among these three patterns of torpor utilization, Wilz and Heldmaier concluded that all three are based on the same physiological mechanisms of thermal and metabolic regulation (Wilz and Heldmaier 2000). In contrast, Geiser argues that the shorter periods of torpor in hibernators should not be considered equivalent to daily torpor because the torpid metabolic rate of the hibernators is below that of animals in daily torpor (2004). We did not assess metabolic rates in this study, however, the Tb records from these fall transition 13-lined ground squirrels reveal several quantitatively-distinct patterns of torpor utilization. A small percentage of the <1d bouts of torpor at Ta = 20°C are reminiscent of daily torpor, because they coincide with the environmental light:dark cycle (e.g., Fig. 7). There are also numerous examples of Tb traces showing multiday torpor prior to transfer into the hibernaculum, where Tb is held just above Ta near 20°C, thereby exhibiting similarity to estivation. In the case of 13-lined ground squirrels preparing for hibernation in the fall, it may not be meaningful to distinguish among these different temporal patterns of torpor utilization. Rather, the changing torpor patterns, which converge towards maximizing the time spent in torpor by increasing torpor bout duration while simultaneously decreasing the time spent euthermic between bouts of torpor, suggest that the animals readjust their biochemical status to enhance expression of torpor as they progress through the fall transition. Interestingly, there is a great deal of individual variation in the ability to remain torpid, with some animals maximizing time spent in torpor more readily than others.
The results of this study demonstrate that 13-lined ground squirrels use torpor extensively yet unpredictably in the fall, before exposure to the cold and dark. Because hibernators are typically not assessed for torpor utilization prior to placement into a special cold environment, or hibernaculum, this behavior is largely unexplored. Nevertheless, expression of torpor in conventionally-housed model hibernators during the fall transition has significant consequences for the design of hibernation studies, particularly those studies that claim to use fall animals as non-hibernating controls for biochemical work. Such animals should be continuously monitored and may offer unique opportunities to identify molecular pathways involved in the orchestration of torpor.
Acknowledgements
We thank K. Grabek for helpful discussion. This work was supported in part by Hiberna, Inc, and by a grant from the National Institutes of Health, HL089049.
References
- Andrews MT. Advances in molecular biology of hibernation in mammals. Bioessays. 2007;29:431–440. doi: 10.1002/bies.20560. [DOI] [PubMed] [Google Scholar]
- Andrews MT, Russeth KP, Drewes LR, Henry PG. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R383–R393. doi: 10.1152/ajpregu.90795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rudiger J, Van der Zee EA, Harkany T, Holzer M, Hartig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Concannon P, Levac K, Rawson R, Tennant B, Bensadoun A. Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am J Physiol Regul Integr Comp Physiol. 2001;281:R951–R959. doi: 10.1152/ajpregu.2001.281.3.R951. [DOI] [PubMed] [Google Scholar]
- D'Alecy LG, Lundy EF, Kluger MJ, Harker CT, LeMay DR, Shlafer M. Beta-hydroxybutyrate and response to hypoxia in the ground squirrel, Spermophilus tridecimlineatus. Comp Biochem Physiol B Biochem Mol Biol. 1990;96:189–193. doi: 10.1016/0305-0491(90)90361-v. [DOI] [PubMed] [Google Scholar]
- Dave KR, DeFazio RA, Raval A, Dashkin O, Saul I, Iceman KE, Perez-Pinzon MA, Drew KL. Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. Journal of Neurochemistry. 2009;110:1170–1179. doi: 10.1111/j.1471-4159.2009.06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson LE, Rose JC, Carey HV, Martin SL. Seasonal proteomic changes reveal molecular adaptations to preserve and replenish liver proteins during ground squirrel hibernation. Am. J. Physiol. Regul Integr Comp Physiol. 2010;298:R329–R340. doi: 10.1152/ajpregu.00416.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson LE, Rose JC, Russell RL, Nikrad MP, Carey HV, Martin SL. Seasonal protein changes support rapid energy production in hibernator brainstem. J Comp Physiol B. 2010;180:599–617. doi: 10.1007/s00360-009-0422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VB, Goropashnaya AV, Toien O, Stewart NC, Gracey AY, Chang C, Qin S, Pertea G, Quackenbush J, Showe LC, Showe MK, Boyer BB, Barnes BM. Elevated expression of protein biosynthesis genes in liver and muscle of hibernating black bears (Ursus americanus) Physiol Genomics. 2009;37:108–118. doi: 10.1152/physiolgenomics.90398.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- Gur MK, Refinetti R, Gur H. Daily rhythmicity and hibernation in the Anatolian ground squirrel under natural and laboratory conditions. J Comp Physiol B. 2009;179:155–164. doi: 10.1007/s00360-008-0298-0. [DOI] [PubMed] [Google Scholar]
- Hampton M, Andrews MT. A simple molecular mathematical model of mammalian hibernation. J Theor Biol. 2007;247:297–302. doi: 10.1016/j.jtbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Deshpande SS, Ducker T, Albuquerque EX. Evaluation of regeneration of nerve and reinnervation of skeletal muscle in the hibernating ground squirrel. Experimental Neurology. 1983;81:407–425. doi: 10.1016/0014-4886(83)90272-8. [DOI] [PubMed] [Google Scholar]
- Klain GJ. Hepatic utilization of glutamate during the hibernation cycle. Life Sciences. 1976;19:591–596. doi: 10.1016/0024-3205(76)90241-1. [DOI] [PubMed] [Google Scholar]
- Kondo N, Sekijima T, Kondo J, Takamatsu N, Tohya K, Ohtsu T. Circannual Control of Hibernation by HP Complex in the Brain. Cell. 2006;125:161–172. doi: 10.1016/j.cell.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and Torpor in Mammals and Birds. New York: Academic Press; 1982. [Google Scholar]
- Nelson CJ, Otis JP, Martin SL, Carey HV. Analysis of the Hibernation Cycle Using LC-MS Based Metabolomics in Ground Squirrel Liver. Physiol Genomics. 2009;37:43–51. doi: 10.1152/physiolgenomics.90323.2008. [DOI] [PubMed] [Google Scholar]
- Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics. 2007;31:15–24. doi: 10.1152/physiolgenomics.00028.2007. [DOI] [PubMed] [Google Scholar]
- Shao C, Liu Y, Ruan H, Li Y, Wang H, Kohl F, Goropashnaya AV, Fedorov VB, Zeng R, Barnes BM, Yan J. Shotgun proteomic analysis of hibernating arctic ground squirrels. Mol Cell Proteomics. 2010;9:313–326. doi: 10.1074/mcp.M900260-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB. Out Cold: Biochemical Regulation of Mammalian Hibernation - A Mini-Review. Gerontology. 2010;56:220–230. doi: 10.1159/000228829. [DOI] [PubMed] [Google Scholar]
- Twente JW, Twente JA. Effects of core temperature upon duration of hibernation Citellus lateralis. J Appl Physiol. 1965;20:411–416. doi: 10.1152/jappl.1965.20.3.411. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Gruber AR, Michalski ML, Seidling J, Schlink S. Capture, care, and captive breeding of 13-lined ground squirrels, Spermophilus tridecemlineatus. Lab Anim (NY) 2006;35:33–40. doi: 10.1038/laban0406-33. [DOI] [PubMed] [Google Scholar]
- Wang LCH. Mammalian Hibernation: An Escape from the Cold. Advances in Comparative and Environmental Physiology. 1988;2:1–45. [Google Scholar]
- Wilz M, Heldmaier G. Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. J Comp Physiol [B] 2000;170:511–521. doi: 10.1007/s003600000129. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. London: Prentice-Hall International; 1996. [Google Scholar]