Abstract
Background
Hundreds of hypermethylated genes have been described in breast cancer, yet the nature and contribution of these genes in their methylated state to overall risk and prognosis is under-characterized in non-sporadic breast cancers. We therefore compared associations of DNA methylation with tumor stage, hormone/growth receptor status and clinical outcomes in a familial breast cancer cohort. Because few previous methylation studies have considered the oncogenic or tumor suppressor properties of their gene sets, this functional status was included as part of our correlative analysis.
Results
We found methylation of oncogenes was associated with better prognostic indicators, whereas tumor suppressor gene methylation was associated with a more severe phenotype in women that were either HER2+ or lymph node positive at diagnosis, and/or tended to recur or develop distant metastases. For example, the methylation of the tumor suppressor gene APC was strongly associated with a specific subset of tumors that were both ER+ and HER2+, while methylation of the TWIST oncogene was associated with breast cancers that did not metastasize.
Methods
This was a retrospective, hospital-based study of n = 99 archival breast tumors derived from women with a germline genetic BRCA1 or BRCA2 mutation and/or familial breast cancer history. DNA methylation was quantified from formalin fixed, paraffin embedded tumors using the established protocol of quantitative multiplex-methylation specific PCR (QM-MSP). Non-parametric statistics were used to analyze candidate gene methylation in association with clinical outcomes.
Conclusion
We report several novel, positive associations between percent methylation of the APC, RASSF1A, TWIST, ERα, CDH1 and Cyclin D2 genes and key variables such as tumor stage, hormone and growth receptor status, and a history of recurrent or metastatic disease. Our data suggest the potential utility of parsing gene methylation by functional status and breast tumor subtype.
Key words: DNA methylation, breast cancer, familial, BRCA1, BRCA2, epigenetic, protective, risk, QM-MSP, methylation specific PCR
Introduction
We compared associations of DNA methylation with tumor features and clinical variables in n = 99 women with breast cancer. Hundreds of hypermethylated genes have been described in breast cancer, yet the nature and contribution of these genes in their methylated state to overall risk and prognosis is under-characterized in non-sporadic breast cancers. The inherent heterogeneity of breast cancer and its myriad subtypes pose a challenge in any research undertaking. In an effort to limit heterogeneity, we narrowed the inclusion criteria for this study to an at risk cohort of women with the rationale that distinct epigenetic factors common to those with familial breast cancers and/or known BRCA1 or BRCA2 genetic mutations, are at differing frequencies than in sporadic breast cancers.1–4 Recent studies suggest that specific gene methylation patterns are associated with distinct histopathological categories, raising the possibility that such patterns may have prognostic value.4–6 This study sought to further refine these categories by testing associations of candidate gene methylation with tumor features and clinical outcomes such as recurrence and metastasis.
How complex epigenetic processes occur, evolve and interact with each other at the DNA, RNA and chromatin level are vastly complex and not well understood. Although there is debate about the nature of these epigenetic phenomena and whether they occur in a random or targeted manner,7–11 there is nevertheless evidence that global DNA methylation pathways may “overshoot their biological targets,”11 and cause some gene methylation to occur secondary to upstream effects. Therefore, this may partly explain the occurrence of gene hypermethylation in the absence of changes in gene expression, but also underscores the potential utility of secondarily methylated genes as “surrogate” biomarkers when their presence is consistently predictive of specific clinical outcomes.
Using quantitative multiplex methylation specific PCR (QM-MSP) on a real-time platform, we quantified DNA methylation for ten genes (APC, RASSF1A, TWIST, ESR1, CDH1, Cyclin D2, BRCA1, RARβ, BRCA2 and HIN1), in n = 99 tumors derived from women with a family history of breast cancer with and without deleterious BRCA1 or BRCA2 genetic mutations. The ten genes were chosen because they were either strong biological candidate genes for breast carcinogenesis, their known or suspected involvement in various aspects of cellular regulation and development appears to be suppressed upon methylation, or because previous studies showed they were methylated in breast tumor cell lines or breast tissues. We report associations of percent methylation for six genes with distinct tumor characteristics and clinical outcomes that, depending on their tumor suppressor or oncogenic properties, may confer either a protective role or an increased risk for recurrence or metastasis.
Results
Frequency distributions of demographic and clinical variables.
As expected in this cohort, there was an earlier average age of breast cancer onset of 44.8 years as compared to the reported mean (54 years) and median (61 years) age of onset in the general population.12,13 Hormone receptor IHC tumor staining and lymph node status of the n = 99 breast cancer cases were similar to the general population; slightly over two thirds were estrogen/progesterone receptor positive, and approximately two thirds were lymph node and HER2 negative at diagnosis (Table 2). These tumor type distributions by histology have been shown to correlate highly with breast cancer subtypes by gene expression profiling.14 Frequency distributions for the remaining variables used in the statistical analyses are provided in Table 2. Table 3 shows the number of tumors of n = 99 total with methylation equal to or above the 5% methylation threshold. Additionally, the average percent methylation per tumor as well as the range of percent methylation values are provided in Table 3.
Table 2.
Frequency distribution of clinical and tumor features for N = 99 cases
| Breast diagnosis | N (%) | Percent × 100 |
| CIS | 11 | 11 |
| DCIS-mINV | 15 | 15 |
| Invasive carcinoma | 73 | 73 |
| Tumor stage | ||
| TIS | 11 | 11 |
| T1 | 53 | 53 |
| T2 | 30 | 30 |
| T3 | 5 | 5 |
| Tumor grade | ||
| I | 10 | 10 |
| II | 71 | 70 |
| III | 15 | 15 |
| LCIS | 3 | 3 |
| Bilateral breast Dx | ||
| Yes | 19 | 19 |
| No | 80 | 80 |
| Metastatic disease | ||
| Yes | 17 | 17 |
| No | 82 | 82 |
| Recurrence | ||
| Yes | 24 | 24 |
| No | 75 | 75 |
| Age of cancer onset | ||
| <30 | 4 | 4 |
| 30–39 | 19 | 19 |
| 40–49 | 43 | 43 |
| 50–59 | 28 | 28 |
| 60–69 | 4 | 4 |
| 70–79 | 1 | 1 |
| Lymph node status | ||
| Positive | 27 | 27 |
| Negative | 72 | 72 |
| ER IHC status | ||
| Positive | 60 | 60 |
| Negative | 28 | 28 |
| ND | 11 | 11 |
| PR IHC status | ||
| Positive | 63 | 63 |
| Negative | 24 | 24 |
| ND | 12 | 12 |
| HER2 IHC status | ||
| Positive | 21 | 21 |
| Negative | 36 | 36 |
| ND | 42 | 42 |
| Deceased | ||
| Yes | 13 | 13 |
| No | 86 | 86 |
| BRCA mutation status | ||
| BRCA+ | 11 | 11 |
| UNK | 7 | 7 |
| VUS | 6 | 6 |
| NEG | 26 | 26 |
| NT | 49 | 49 |
Breast diagnosis: CIS, carcinoma in situ; DCIS-mINV, case diagnosed with a <1.0 cm micro-invasive carcinoma, but for which only DCIS remained on the tissue block; IC, invasive carcinoma. Lymph node status: indicates if lymph nodes were positive or negative for tumor cells at initial diagnosis of breast cancer. ER, PR and HER2 status: IHC tumor staining with antibodies to the estrogen and progesterone receptors, and HER2 growth factor receptor, respectively. BRCA mutation status: genetic mutation testing of BRCA1 and BRCA2 genes; BRCA+, carrier of a known deleterious BRCA1 or BRCA2 mutation; UNK, test results unknown. By report, the individual underwent BRCA mutation testing, but the test result was not reported in any of the three Johns Hopkins clinical databases abstracted in the study. VUS, variant of unknown significance; e.g., a BRCA gene mutation was found, but no population data as yet exists to determine if the mutation is deleterious; NEG, negative, the patient did not carry a BRCA1 or BRCA2 gene mutation. NT, not tested.
Table 3.
Methylation in n = 99 Breast tumors exceeding 5.0% methylation threshold
| No. of tumors | Average percent (% ×100) | Range of percent (% ×100) | |
| Gene name | Methylated* | Tumor Methylation | Tumor Methylation |
| ERα | 26 | 32 | 6–84 |
| TWIST | 37 | 38 | 6–93 |
| Cyclin D2 | 24 | 44 | 7–93 |
| CDH1 | 2 | 19 | 17–21 |
| APC | 44 | 41 | 8–91 |
| RASSF1A | 62 | 38 | 6–96 |
| HIN1 | 28 | 62 | 5–95 |
| RARb | 40 | 37 | 6–93 |
| BRCA1 | 23 | 47 | 7–84 |
| BRCA2 | 7 | 33 | 7–89 |
Numer of tumors methylated: Total number of tumors that exceeded a methylation threshold of 5.0 percent for each of ten genes. Range of percent tumor methylation and percent tumor methylation: Range of percent methylation and mean methylation values, respectively for those genes that exceeded a 5.0% methylation threshold.
Associations of percent methylation with clinical variables.
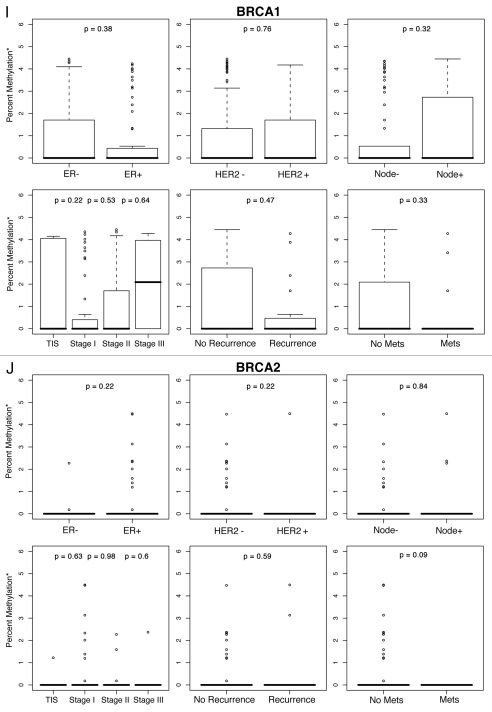
To examine the contribution of % methylation for each gene to a selected set of histological and clinical variables, box plots of median percent methylation, based on the log of percent methylation + 1 or log (% M + 1), were constructed by tumor stage, recurrent, metastatic or bilateral breast disease, age at diagnosis, BRCA mutation status, and by ER, HER2 and lymph node status (Fig. 1A–J). Percent methylation varies widely between genes and often has a non-normal distribution, with many values skewed toward zero for multiple genes in a particular assay. The standard statistical correction for such skewed distribution is to log transform the data. Accordingly, we log transformed methylation values for each gene in our study with a zero correction factor of log (% M + 1). Because log of zero is undefined, adding 1 was necessary to obtain finite values.
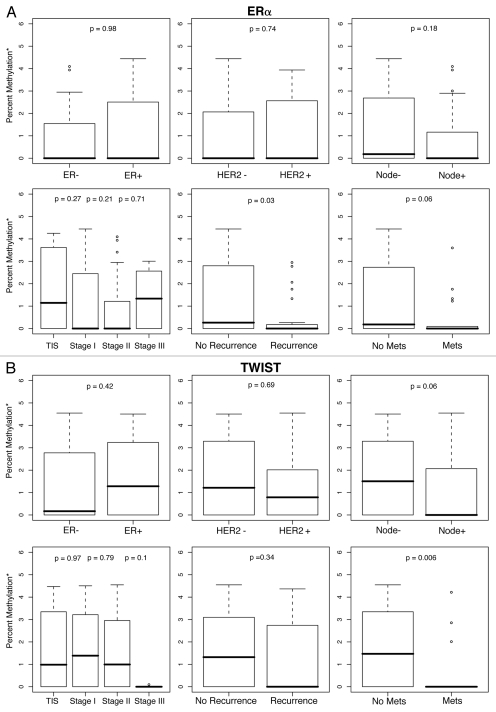
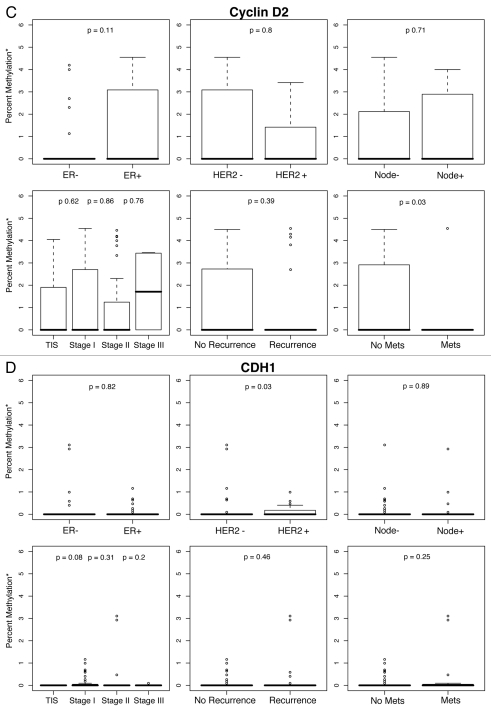
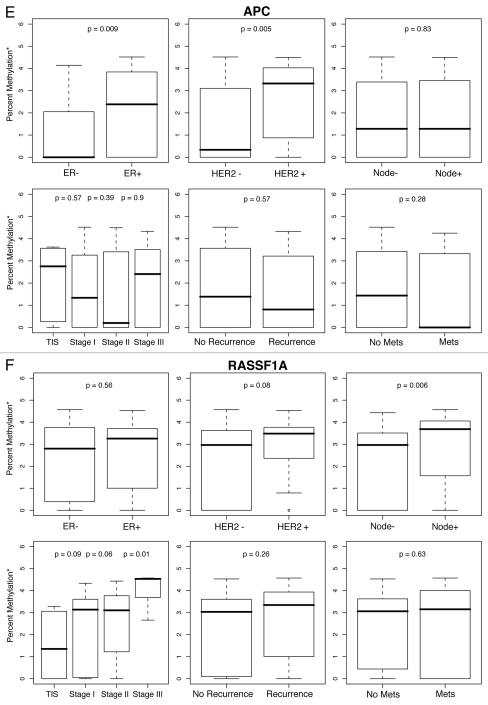
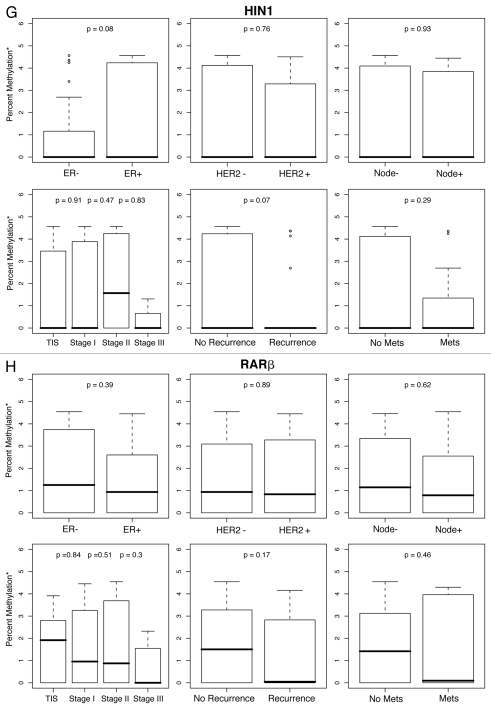
Figure 1.
Percent methylation* by ER, HER2, lymph node status, tumor stage and history of recurrent or metastatic disease. *Percent methylation is based on the log of percent methylation + 1 or log (% M + 1). Percent ERα (A), TWIST (B), CyclinD2 (C), CDH1 (D), AP C (E), RASS F1A (F), HIN1 (G), RARβ (H), BRCA1 (I), BRCA2 (J) methylation by ER, HER2, lymph node status, tumor stage and history of recurrent or metastatic disease.
For each gene in the study, differences in percent methylation (as measured by the median log (% M + 1), between these variables were tested using the Wilcoxon rank sum test (Fig. 1A–J). The following variables were excluded from the analysis as none of the ten genes were significantly methylated by age (<45, ≥45 yo), bilateral breast disease (yes, no), BRCA1 or BRCA2 germ-line mutation status (positive/negative: unknown test results and variants of unknown significance were not included in the model), or progesterone receptor (PR) status. In summary, Figures 1A–J are box plot comparisons of ER, HER2, lymph node status, tumor stage and recurrent or metastatic disease variables by percent methylation for the following ten genes: ERα, TWIST, Cyclin D2, CDH1, APC, RASSF1A, HIN1, RARβ, BRCA1 and BRCA2, respectively.
Primer selection for quantitative methylation assays.
QM-MSP was carried out on DNA extracted from formalin fixed breast tumors using the primer and probe sequences listed in Table 1. Given the phenomenon of field cancerization effects reported in histologically “normal” tissues adjacent to tumors, and the observation that even microdissected tumors will have some percentage of normal cells embedded therein,15,16 these primer and probe sets were previously tested in normal breast parenchyma derived from healthy women without a breast cancer history and showed very low to no methylation.17–19
Table 1.
DNA primer and probe sequences used in steps 1 + 2 of QM-MSP
| Primer | Primer | Amplimer | |||
| Gene | abbr. | Forward primer (5′ → 3′) | abbr. | Reverse primer (3′ a 5′) | size (bp) |
| APC | Ext: | AAA ACC CTA TAC CCC ACT AC | Ext: | GGT TGT ATT AAT ATA GTT ATA TGT | 163 |
| APC | FM: | AAT ACG AAC CAA AAC GCT CCC | RM: | TAT GTC GGT TAC GTG CGT TTA TAT | 78 |
| APC | FU: | TAA ATA CAA ACC AAA ACA CTC CC | RU: | GTT ATA TGT TGG TTA TGT GTG TTT | 79 |
| APC | MP: | CCC GTC GAA AAC CCG CCG ATT A | UP: | TTC CCA TCA AAA ACC CAC CAA TTA AC | |
| BRCA1 | Ext: | TAT TTT GAG AGG TTG TTG TTT AG | Ext: | AAA CAT CAC TTA AAC CCC CTA T | 241 |
| BRCA1 | FM: | TTT CGT GGT AAC GGA AAA GCG | RM: | CCG TCC AAA AAA TCT CAA CGA A | 86 |
| BRCA1 | FU: | TGG TAA TGG AAA AGT GTG GGA A | RU: | CCC ATC CAA AAA ATC TCA ACA AA | 73 |
| BRCA1 | MP: | CTC ACG CCG CGC AAT CGC AAT TT | UP: | CTC ACA CCA CAC AAT CAC AAT TTT AAT | |
| BRCA2 | Ext: | GTT GGG ATG TTT GAT AA G GAA T | Ext: | ATC ACA AAT CTA TCC CCT CAC | 262 |
| BRCA2 | FM: | TTT GAT TTT CGG GTG GTG CGT | RM: | TCA AAA ACT CGC GCC ACA AAC | 82 |
| BRCA2 | FU: | ATT TTT GGG TGG TGT GTG TGT T | RU: | TCA AAA ACT CAC ACC ACA AAC C | 78 |
| BRCA2 | MP: | AAC CAC GTA ACG CCG TAA CGC GA | UP: | AAC CAC ATA ACA CCA TAA CAC AAC AC | |
| CDH1 | Ext: | TAA TTT TAG GTT AGA GGG TTA T | Ext: | AAC TCA CAA ATA CTT TAC AAT TC | 214 |
| CDH1 | FM: | AGG GTT ATC GCG TTT ATG CGA G | RM: | ACA ACC AAT CAA CAA CGC GAA C | 82 |
| CDH1 | FU: | GAG GGT TAT TGT GTT TAT GTG AG | RU: | CAC AAC CAA TCA ACA ACA CAA AC | 84 |
| CDH1 | MP: | CCC AAA ACG AAA CTA ACG ACC CGC | UP: | CTC CCC AAA ACA AAA CTA ACA ACC CAC | |
| CyclinD2 | Ext: | TAT TTT TTG TAA AGA TAG TTT TGA T | Ext: | TAC AAC TTT CTA AAA AAT AAC CC | 286 |
| CyclinD2 | FM: | TTT GAT TTA AGG ATG CGT TAG AGT ACG | RM: | ACT TTC TCC CTA AAA ACC GAC TAC G | 83 |
| CyclinD2 | FU: | TTA AGG ATG TGT TAG AGT ATG TG | RU: | AAA CTT TCT CCC TAA AAA CCA ACT ACA AT | 78 |
| CyclinD2 | MP: | AAT CCG CCA ACA CGA TCG ACC CTA | UP: | AAT CCA CCA ACA CAA TCA ACC CTA AC | |
| ERα | Ext: | TTG GAG GTT TGG GAG TTT AG | Ext: | ACA ATA AAA CCA TCC CAA ATA C | 298 |
| ERα | FM: | AGG GCG TTC GTT TTG GGA TTG | RM: | CGC CGA CAC GCG AAC TCT AA | 82 |
| ERα | FU: | TGG TGG AGG GTG TTT GTT TTG | RU: | ATC CCA CCA ACA CAC AAA CTC | 93 |
| ERα | MP: | CGA TAA AAC CGA ACA ACC CGA CGA | UP: | CCC AAT AAA ACC AAA CAA CCC AAC AA | |
| HIN1 | Ext: | GTT TGT TAA GAG GAA GTT TT | Ext: | CAC CGA AAC ATA CAA AAC AAA ACC AC | 286 |
| HIN1 | FM: | TAG GGA AGG GGG TAC GGG TTT | RM: | CGC TCA CGA CCG TAC CCT AA | 74 |
| HIN1 | FU: | AA G TTT TTG AGG TTT GGG TAG GGA | RU: | ACC AAC CTC ACC CAC ACT CCT A | 123 |
| HIN1 | MP: | ACT TCC TAC TAC GAC CGA CGA ACC | UP: | CAA CTT CCT ACT ACA ACC AAC AAA CC | |
| RARβ | Ext: | GTA GGA GGG TTT ATT TTT TGT T | Ext: | AAT TAC ATT TTC CAA ACT TAC TC | 239 |
| RARβ | FM: | AGA ACG CGA GCG ATT CGA GTA G | RM: | TAC AAA AAA CCT TCC GAA TAC GTT | 82 |
| RARβ | FU: | TTG AGA ATG TGA GTG ATT TGA GTA G | RU: | TTA CAA AAA ACC TTC CAA ATA CAT TC | 83 |
| RARβ | MP: | ATC CTA CCC CGA CGA TAC CCA AAC | UP: | AAA TCC TAC CCC AAC AAT ACC CAA AC | |
| RASSF1A | Ext: | GTT TTA TAG TTT TTG TAT TTA GG | Ext: | AAC TCA ATA AAC TCA AAC TCC C | 198 |
| RASSF1A | FM: | GCG TTG AAG TCG GGG TTC | RM: | CCC GTA CTT CGC TAA CTT TAA ACG | 74 |
| RASSF1A | FU: | GGT GTT GAA GTT GGG GTT TG | RU: | CCC ATA CTT CAC TAA CTT TAA AC | 75 |
| RASSF1A | MP: | ACA AAC GCG AAC CGA ACG AAA CCA | UP: | CTA ACA AAC ACA AAC CAA ACA AAA CCA | |
| TWIST | Ext: | GAG ATG AGA TAT TAT TTA TTG TG | Ext: | CCT CCC AAA CCA TTC AAA AAC | 273 |
| TWIST | FM: | GTT AGG GTT CGG GGG CGT TGT T | RM: | CCG TCG CCT TCC TCC GAC GAA | 78 |
| TWIST | FU: | GGT TTG GGG GTG TTG TTT GTA TG | RU: | CCC ACC TCC TAA CCA CCC TCC | 104 |
| TWIST | MP: | AAA CGA TTT CCT TCC CCG CCG AAA | UP: | AAA CAA TTT CCT TCC CCA CCA AAA CA |
QM-MSP, quantitative multiplex-methylation specific PCR. Primer abbreviations: Ext, external methylation independent primers used in the first step of QM-MSP; FM, forward methylated primers; RM, reverse methylated primers; FU, forward unmethylated primers; MP, methylated probe sequence labeled with VIC fluorophore; UP, unmethylated probe sequence labeled with FAM fluorophore.
The classical approach in DNA methylation studies has been to consider gene hypermethylation when linked to loss of gene expression as a modified version of Knudson's Two Hit Hypothesis.20 However, not all promoter gene methylation is associated with silenced or decreased gene expression.21–23 Moreover, gene hypermethylation in the absence of changes in expression, may nevertheless be useful as a biomarker if consistently found to be predictive of clinical outcomes such recurrence, metastasis and survival. For example, only two studies have examined the relationship between TWIST promoter hypermethylation and TWIST RNA and protein, and did not find any alteration in gene expression.24,25 Although the limited archival tumor tissues in our sample set did not allow gene expression to be assayed in tandem with methylation, with the exception of TWIST, methylation has consistently been associated with transcriptional silencing of the remaining gene loci covered by our primers.24,26–35
Discussion
The main purpose of this study was to explore the predictive contributions of DNA methylation of selected genes, together with clinical and tumor features, on breast cancer recurrence and progression. Of the percent methylation for the ten genes studied in tumor tissues, six genes; ERα, TWIST, Cyclin D2, CDH1, APC and RASSF1A (Fig. 1A–F) were preferentially methylated for at least one of the histopathological variables tested. No significant associations were found for the remaining four genes: HIN1, RARβ, BRCA1 or BRCA2 (Fig. 1G–J).
Depending on multiple factors including epigenetic regulation, downstream signaling and tissue type, these genes may possess oncogenic, tumor suppressor properties or both. For example, ERα (estrogen receptor alpha, ESR1) is a transcription factor that can act as both an oncogene and a tumor suppressor gene by its role in modifying expression of a variety of genes in a tissue specific manner as a consequence of the binding of estrogens and estrogen receptor modulators.36,37 ERα expression is silenced through methylation in a variety of tissues including lung, colon and breast and its methylation predicts hormone receptor status of breast tissues.6 Therefore, it follows that if the tumorigenic functions of ERα are modified via epigenetic pathways, ERα methylation would manifest as a protective factor. Supporting this hypothesis was our finding that ERα methylation was associated with less severe histological features. It was significantly more methylated in those breast cancers which did not recur (p = 0.03) and trended toward tumors that did not metastasize (p = 0.06). These results complement the work of Kim et al. (2004) who also found ERα methylation was not associated with more severe histological indicators such as node positive or high grade tumors.38
Similarly, the bHLH transcription factor TWIST is considered an oncogene due to its role in inhibiting apoptosis, blocking expression of other tumor control genes in response to DNA damage and promoting epithelial-mesenchymal transition.39–41 Also supporting the protective hypothesis for oncogene methylation, was our finding that TWIST was preferentially methylated in lymph node negative tumors (p = 0.06), and in breast cancer cases which did not metastasize (p = 0.006), with a trend (p = 0.1) towards very low to no methylation in high grade stage III tumors. Likewise, Cyclin D2 (CCND2), is most often considered an oncogene that acts as a crucial cell cycle regulator involved in cell growth and malignant processes.42–44 Several previous studies have shown Cyclin D2 to be methylated in invasive breast carcinoma,42–44 and we found Cyclin D2 methylation was associated (p = 0.03) with non-metastatic breast cancers. As with the other genes in our set with the exception of TWIST, hypermethylation of Cyclin D2 has consistently been linked to decreased expression.
Because CDH1 (E-cadherin), mediates normal cell to cell adhesion in epithelial cells,45 its loss of expression, either though epigenetic or other mechanisms, has been associated with more severe clinical outcomes such as lymph node invasion and metastasis.46–48 Interestingly, neither this study nor a study of sporadic breast cancer,49 found CDH1 methylation in association with lymph node metastases. Although we found CDH1 methylation was associated with HER2+ (p = 0.03) tumors, only two tumors in this cohort had methylation values that exceeded the 5% threshold (Table 3) and therefore this result must be viewed with caution.
As opposed to oncogene methylation, we found methylation of the tumor suppressor genes, APC and RASSF1A, was associated with more prognostically severe clinical outcomes. For example, the tumor suppressor APC (adenomatous polyposis coli), can be lost through genetic and epigenetic mechanisms, but in breast cancer, methylation frequency increases with tumor stage and size, and has been associated with a poor prognosis.50–52 Likewise, we found APC methylation was associated with ER+ tumors (p = 0.009), generally considered to have a better prognosis than ER-tumors, but also with more aggressive HER2+ tumors (p = 0.005), suggesting that APC methylation merits further study as a possible marker to identify a subclass of ER+ positive tumors that behave more aggressively and have poorer clinical outcomes.
The tumor suppressor RASSF1A (human RAS effector homolog), has been found to be epigenetically inactivated in lung, ovary, bladder, kidney and breast tumor tissue,53 and is methylated in approximately 60–70% of breast cancers.34,54 Complementing previous studies which show RASSF1A methylation confers a poorer prognosis,55–57 was our finding methylation in 62 of n = 99 tumors (Table 3), and significantly higher methylation with increasing tumor stage from TIS to stage III (p = 0.09, p = 0.06 and p = 0.01, respectively), with a trend (p = 0.08) towards HER2+ tumors and in women who were lymph node positive at diagnosis (p = 0.006). These findings replicate a previous study finding of higher RASSF1A methylation in lymph node and HER2+ tumors.46
Methylation of the remaining genes (HIN1, RARb, BRCA1, BRCA2), were all negative for associations with clinical and tumor features. Negative findings for the tumor suppressor genes BRCA1 and BRCA2 were not surprising given the characteristically low frequencies of BRCA2 tumor methylation in our study (Table 3) and others.58,59 Moreover, data have shown lower percent BRCA1 methylation or varied methylation levels of other candidate genes in women with BRCA germline genetic mutations,3,60 as well as varied gene methylation levels in familial breast “BRCAx” cancers negative for deleterious BRCA1 or BRCA2 genetic mutations.1,61
Both tumor heterogeneity and varied methylation findings in BRCAx or familial breast cancers have been previously documented.1,62–64 Indeed, even in our familial breast cancer cohort, there was heterogeneity by tumor type, age of onset and BRCA mutation status. For example, the majority of women (56%), had not been tested for heritable mutations in the BRCA1 or BRCA2 genes. Of the women that were tested, 22% were negative for a BRCA1 or BRCA2 genetic mutation, 12% were either BRCA1 or BRCA2 positive, 5% had a mutation or variant of unknown significance (VUS) and 5% were tested, but their BRCA test result was not reported in any of the three clinical databases abstracted for this study (Table 2). Also, 30% of the women with a confirmed deleterious BRCA genetic mutation did not have a strong family history, but rather only had either one first or second degree relative with breast cancer (data not shown).
This retrospective study was conducted using archival breast tumors and medical records abstraction dating from 1986–2004. The amount of missing data (Table 2) for HER2 IHC status reflects those tumors that were archived prior to the discovery, development or common use of the HER2 antibody in clinical pathology practice. Therefore, both negative and positive findings for associations between gene methylation and HER status must be considered in this context. Due to the finite amount of nucleic acids obtained from limited archival specimens, we were not able to perform gene expression profiling in tandem with methylation analyses to determine intrinsic (e.g., luminal A, luminal B, HER2-enriched, basal-like and normal-like),65 breast cancer subtypes. However, the study strengths include our selection of a non-sporadic breast cancer cohort and the highly quantitative QM-MSP method that allowed us to detect and set thresholds for low levels of methylation that might otherwise test differently on qualitative gel-based approaches.
In summary, although our methods include the classical approach of considering gene hypermethylation linked to loss of gene expression, we also support the merits of evaluating hypermethylated biomarkers in the context of purported oncogene or tumor suppressor function, regardless of their effects on gene expression. Also, the parsing of analyses by consideration of oncogene or tumor suppressor function, allows the exploration of a protective or risk factor role when analyzed in the context of important clinical outcomes such as recurrence or metastasis.
Taken as a whole, these findings illustrate the existence of specific patterns of gene methylation in association with distinct combinations of histopathological features. Such results emphasize the potential utility of characterizing methylation patterns in order to better define breast tumors such as the luminal, HER2, normal like and basal like subtypes characterized by gene expression profiling. Future studies of DNA methylation and breast cancer will assay gene expression in tandem with other clinical correlates and outcomes.
Materials and Methods
Population and sample procurement.
This archival, retrospective hospital-based study tested associations of tumor DNA methylation with clinical variables and tumor features in a cohort of n = 99 women with breast cancer. Inclusion criteria for at risk status were having a known deleterious BRCA1 or BRCA2 germline gene mutation, early age of breast cancer onset of 40 years or younger and/or a moderate to strong family history of breast cancer depending on the number of first (FDR), second (SDR) and third (TDR) degree relatives with breast cancer and their respective ages of cancer onset. Approximately one half of the cases had a strong family history ranging from three to eight members with breast cancer. Cohort demographics included 61% White, 20% Ashkenazi, 15% Black, 3% Middle-eastern and 1% Hispanic women. Archival, formalin fixed, paraffin embedded tumors (FFPEs) and clinical data for all women in the study were collected subsequent to HIPAA and IRB approval.
H&E slides from archival FFPE tissue blocks for each case were reviewed by a diagnostic breast pathologist (R. Vang, MD), who provided the breast cancer diagnosis, tumor type and grade. During slide review, the percentage of tumor epithelium relative to stroma/adjacent histologically normal tissue was estimated, with values of tumor epithelium achieving as much as 85% per sample. Once the presence of tumor was confirmed by H&E, a 5 µm section was cut from each tumor block and used for DNA extraction. Due to the unavoidable and variable admixture of each tumor with adjacent histologically “normal” epithelium and stroma, we previously assayed methylation in normal breast tissues as a comparison, and found very low to no methylation for the 10 loci in this study.17–19
DNA extraction from tumors, cell lines and human sperm.
FFPE sections were de-paraffinized in xylene for 20 minutes, scraped from the slide and extracted in 30 µl TNES (10 mM Tris, pH 8.0, 150 mM NaCl, 2 mM EDTA, 0.5% SDS) containing 40 µg of proteinase K for 5 h at 52°C. After Proteinase K treatment, cells were heat inactivated at 99°C for 10 min and centrifuged at 16,000x g for 10 min. 13.5 of the 30 µl supernatant was used directly as a source of DNA for sodium bisulfite (NaBi) treatment, performed according to Fackler et al. (2004).56 NaBi treatment converts non-methylated cytosine residues to uracil (later replicated as thymidine during PCR), whereas methylated cytosines remain unchanged. Such conversion of the original sequence allows distinct primer sets to be made that are specific to unmethylated and methylated CG sequences respectively for each sample. DNA was extracted from cell lines with phenol-chloroform,66 and from human semen using the PUREGENE DNA Purification Kit (Gentra Systems, Minneapolis, MN), according to the manufacturer's instructions and stored at −20°C.
Positive and negative methylation controls.
Breast carcinoma cell line MDA-MB231 was obtained from American Type Culture Collection (www.atcc.org, Manassas, VA) and cultured as directed. Human sperm was obtained from a healthy volunteer. Many genes are highly methylated in MDA-MB231 cells and therefore this cell line is used as a positive methylation control both before and after treatment with a DNA methyltransferase. Human sperm DNA (HSD) has been shown to have a lower degree of genomic methylation as compared to other somatic cells,67 and has therefore been used as a negative, unmethylated reference control in methylation studies of tumor control genes.68 HSD treated with SssI methyltransferase (New England Biolabs Inc., Beverly, MA) was also used as 100% methylation positive control for the 10 candidate genes assayed in this study. Positive methylation controls: 2 µg of either MDA-MB231 or HSD was incubated with S-adenosyl-L-methionine and 1 unit of SssI methyltransferase (New England Biolabs, Beverly, MA) for 1.5 h at 37°C according to manufacturers instructions prior to sodium bisulfite treatment.
Quantitative multiplex—methylation specific polymerase chain reaction.
Quantitative methylation analysis was performed on sodium bisulfite treated tumor DNAs and controls with the QM-MSP real-time method using “protocol C” as described in Swift-Scanlan et al. (2006).19 Both methylated and unmethylated targets for a single gene were amplified simultaneously in the same well using FAM and VIC probes, respectively on the ABI 7900 real-time platform (Applied Biosystems, Foster City, CA).19 The following controls were included on each 96 well real-time plate: (1) a 100% methylation control using MDA-MB-231 DNA or human sperm DNA treated with SssI methyltransferase; (2) human sperm DNA methylation negative controls, (3) nontemplate negative reaction controls using water as a template in the multiplex reaction, (4) internal sample controls of multiplex reactions (randomly chosen from cases and controls), repeated on a different day and on a different reaction plate and (5) internal standard controls in the form of an 80,000 methylated copy number and 1% methylation control standard run on each 96 well real-time reaction plate. Primer and probe sequences are listed in Table 1.
Statistical analysis.
We employed non-parametric analysis to examine differences in percent methylation (% M) for each tumor by clinical outcomes and tumor features. Box plots of percent methylation (as measured by median log [% M + 1]), were constructed by estrogen receptor (ER) and HER2 immunohistochemical (IHC) tumor stain results, nodal status at diagnosis, tumor stage (TNM system), and history of breast cancer recurrence or metastatic disease. A boxplot is a graphical display of the how the data are distributed and includes the median center, spread and skewness of the methylation values. The vertical height of the box extends from the 25–75th percentile methylation values (interquartile range or IQR), with the median (50th percentile) denoted by the horizontal line within the box. The whiskers of the plot extend to the data points that are between −1.5 and 1.5*IQR. Individual points beyond the whiskers are data points that are considered outliers. Differences in percent methylation between variables were tested with Wilcoxon rank sum tests. It was observed prior to statistical testing that % M, and cumulative % M values were skewed toward zero. Therefore, these data were log transformed prior to analysis. Genetic, epigenetic and histopathological variables are described in Table 2. Percent methylation for each gene was calculated as the total (methylated copies of gene/[unmethylated + methylated copies of gene] × 100).
Acknowledgments
We gratefully acknowledge the support of a National Institutes of Health/National Cancer Institute (NIH/NCI) Specialized Program of Research Excellence (SPORE) in Breast Cancer P50CA88843 to S.S. and an NIH/NINR F31 NR008311-01A1 and American Cancer Society (ACS) DSCN-04-162-01 to T.S.S. We are also thankful for the current support to T.S.S. of a Susan G. Komen Foundation KG090180 (Swift-Scanlan), an NIH/NCRR 1KL2RR025746-01, and an NIH/NCI Breast SPORE CA058823 (Earp).
Abbreviations
- QM-MSP
quantitative multiplex methylation specific PCR
- IHC stain
immunohistochemical stain
- % M
percent methylation
References
- 1.Flanagan JM, Cocciardi S, Waddell N, Johnstone CN, Marsh A, Henderson S, et al. DNA methylome of familial breast cancer identifies distinct profiles defined by mutation status. Am J Hum Genet. 2010;86:420–433. doi: 10.1016/j.ajhg.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin W, Chen L, Chen Y, Xu SG, Di GH, Yin WJ, et al. UHRF1 is associated with epigenetic silencing of BRCA1 in sporadic breast cancer. Breast Cancer Res Treat. 2010;123:359–373. doi: 10.1007/s10549-009-0652-2. [DOI] [PubMed] [Google Scholar]
- 3.Suijkerbuijk KP, Fackler MJ, Sukumar S, van Gils CH, van Laar T, van der Wall E, et al. Methylation is less abundant in BRCA1-associated compared with sporadic breast cancer. Ann Oncol. 2008;19:1870–1874. doi: 10.1093/annonc/mdn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei M, Xu J, Dignam J, Nanda R, Sveen L, Fackenthal J, et al. Estrogen receptor alpha, BRCA1 and FANCF promoter methylation occur in distinct subsets of sporadic breast cancers. Breast Cancer Res Treat. 2008;111:113–120. doi: 10.1007/s10549-007-9766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fackler MJ, Malone K, Zhang Z, Schilling E, Garrett-Mayer E, Swift-Scanlan T, et al. Quantitative multiplex methylation-specific PCR analysis doubles detection of tumor cells in breast ductal fluid. Clin Cancer Res. 2006;12:3306–3310. doi: 10.1158/1078-0432.CCR-05-2733. [DOI] [PubMed] [Google Scholar]
- 6.Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, et al. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 7.Narayan A, Ji W, Zhang XY, Marrogi A, Graff JR, Baylin SB, et al. Hypomethylation of pericentromeric DNA in breast adenocarcinomas. Int J Cancer. 1998;77:833–838. doi: 10.1002/(sici)1097-0215(19980911)77:6<833::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci USA. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich M. Cancer-linked DNA hypomethylation and its relationship to hypermethylation. Curr Top Microbiol Immunol. 2006;310:251–274. doi: 10.1007/3-540-31181-5_12. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veeck J, Esteller M. Breast cancer epigenetics: from DNA methylation to microRNAs. J Mammary Gland Biol Neoplasia. 2010;15:5–17. doi: 10.1007/s10911-010-9165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barba M, McCann SE, Nie J, Vito D, Stranges S, Fuhrman B, et al. Perinatal exposures and breast cancer risk in the western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control. 2006;17:395–401. doi: 10.1007/s10552-005-0481-5. [DOI] [PubMed] [Google Scholar]
- 13.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 14.Sorlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40:2667–2675. doi: 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Trujillo KA, Heaphy CM, Mai M, Vargas KM, Jones AC, Vo P, et al. Markers of fibrosis and epithelial to mesenchymal transition demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2010 doi: 10.1002/ijc.25788. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balogh GA, Heulings R, Mailo D, Wang R, Li YS, Hardy R, et al. Methodological approach to study the genomic profile of the human breast. Int J Oncol. 2007;31:253–260. [PubMed] [Google Scholar]
- 17.Fackler MJ, Rivers A, Teo WW, Mangat A, Taylor E, Zhang Z, et al. Hypermethylated genes as biomarkers of cancer in women with pathologic nipple discharge. Clin Cancer Res. 2009;15:3802–3811. doi: 10.1158/1078-0432.CCR-08-1981. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Fackler MJ, Teo WW, Lee JH, Choi C, Park MH, et al. Quantitative promoter hypermethylation profiles of ductal carcinoma in situ in North American and Korean women: Potential applications for diagnosis. Cancer Biol Ther. 2008;7:1398–1406. doi: 10.4161/cbt.7.9.6425. [DOI] [PubMed] [Google Scholar]
- 19.Swift-Scanlan T, Blackford A, Argani P, Sukumar S, Fackler MJ. Two-color quantitative multiplex methylation-specific PCR. Biotechniques. 2006;40:210–219. doi: 10.2144/000112097. [DOI] [PubMed] [Google Scholar]
- 20.Jones PA, Laird PW. Cancer epigenetics comes of age. NatGenet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 21.Zinn RL, Pruitt K, Eguchi S, Baylin SB, Herman JG. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67:194–201. doi: 10.1158/0008-5472.CAN-06-3396. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen C, Liang G, Nguyen TT, Tsao-Wei D, Groshen S, Lubbert M, et al. Susceptibility of nonpromoter CpG islands to de novo methylation in normal and neoplastic cells. J Natl Cancer Inst. 2001;93:1465–1472. doi: 10.1093/jnci/93.19.1465. [DOI] [PubMed] [Google Scholar]
- 23.Morey Kinney SR, Smiraglia DJ, James SR, Moser MT, Foster BA, Karpf AR. Stage-specific alterations of DNA methyltransferase expression, DNA hypermethylation and DNA hypomethylation during prostate cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Res. 2008;6:1365–1374. doi: 10.1158/1541-7786.MCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gort EH, Suijkerbuijk KP, Roothaan SM, Raman V, Vooijs M, van der Wall E, et al. Methylation of the TWIST1 promoter, TWIST1 mRNA levels and immunohistochemical expression of TWIST1 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3325–3330. doi: 10.1158/1055-9965.EPI-08-0472. [DOI] [PubMed] [Google Scholar]
- 25.Okada T, Suehiro Y, Ueno K, Mitomori S, Kaneko S, Nishioka M, et al. TWIST1 hypermethylation is observed frequently in colorectal tumors and its overexpression is associated with unfavorable outcomes in patients with colorectal cancer. Genes Chromosomes Cancer. 2010;49:452–462. doi: 10.1002/gcc.20755. [DOI] [PubMed] [Google Scholar]
- 26.Henrique R, Costa VL, Cerveira N, Carvalho AL, Hoque MO, Ribeiro FR, et al. Hypermethylation of Cyclin D2 is associated with loss of mRNA expression and tumor development in prostate cancer. J Mol Med. 2006;84:911–918. doi: 10.1007/s00109-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 27.Evron E, Umbricht CB, Korz D, Raman V, Loeb DM, Niranjan B, et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res. 2001;61:2782–2787. [PubMed] [Google Scholar]
- 28.Tapia T, Smalley SV, Kohen P, Munoz A, Solis LM, Corvalan A, et al. Promoter hypermethylation of BRCA1 correlates with absence of expression in hereditary breast cancer tumors. Epigenetics. 2008;3:157–163. doi: 10.4161/epi.3.3.6387. [DOI] [PubMed] [Google Scholar]
- 29.Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer. 2000;36:2294–2300. doi: 10.1016/s0959-8049(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 30.Lee MN, Tseng RC, Hsu HS, Chen JY, Tzao C, Ho WL, et al. Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2 and XRCC5 in non-small cell lung cancer. Clin Cancer Res. 2007;13:832–838. doi: 10.1158/1078-0432.CCR-05-2694. [DOI] [PubMed] [Google Scholar]
- 31.Sirchia SM, Ren M, Pili R, Sironi E, Somenzi G, Ghidoni R, et al. Endogenous reactivation of the RARbeta2 tumor suppressor gene epigenetically silenced in breast cancer. Cancer Res. 2002;62:2455–2461. [PubMed] [Google Scholar]
- 32.Shigematsu H, Suzuki M, Takahashi T, Miyajima K, Toyooka S, Shivapurkar N, et al. Aberrant methylation of HIN-1 (high in normal-1) is a frequent event in many human malignancies. Int J Cancer. 2005;113:600–604. doi: 10.1002/ijc.20622. [DOI] [PubMed] [Google Scholar]
- 33.Mirza S, Sharma G, Prasad CP, Parshad R, Srivastava A, Gupta SD, et al. Promoter hypermethylation of TMS1, BRCA1, ERalpha and PRB in serum and tumor DNA of invasive ductal breast carcinoma patients. Life Sci. 2007;81:280–287. doi: 10.1016/j.lfs.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Dammann R, Yang G, Pfeifer GP. Hypermethylation of the cpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001;61:3105–3109. [PubMed] [Google Scholar]
- 35.Lombaerts M, van WT, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–671. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez BA, Cheng AS, Yan PS, Potter D, Agosto-Perez FJ, Shapiro CL, et al. Epigenetic repression of the estrogen-regulated Homeobox B13 gene in breast cancer. Carcinogenesis. 2008;29:1459–1465. doi: 10.1093/carcin/bgn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24:4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SJ, Kim TW, Lee SY, Park SJ, Kim HS, Chung KW, et al. CpG methylation of the ERalpha and ERbeta genes in breast cancer. Int J Mol Med. 2004;14:289–293. [PubMed] [Google Scholar]
- 39.Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, et al. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207–2217. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MS, Lowe GN, Strong DD, Wergedal JE, Glackin CA. TWIST, a basic helix-loop-helix transcription factor, can regulate the human osteogenic lineage. J Cell Biochem. 1999;75:566–577. doi: 10.1002/(sici)1097-4644(19991215)75:4<566::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Fackler MJ, McVeigh M, Evron E, Garrett E, Mehrotra J, Polyak K, et al. DNA methylation of RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107:970–975. doi: 10.1002/ijc.11508. [DOI] [PubMed] [Google Scholar]
- 43.Pu RT, Laitala LE, Alli PM, Fackler MJ, Sukumar S, Clark DP. Methylation profiling of benign and malignant breast lesions and its application to cytopathology. Mod Pathol. 2003;16:1095–1101. doi: 10.1097/01.MP.0000095782.79895.E2. [DOI] [PubMed] [Google Scholar]
- 44.Sweeney KJ, Sarcevic B, Sutherland RL, Musgrove EA. Cyclin D2 activates Cdk2 in preference to Cdk4 in human breast epithelial cells. Oncogene. 1997;14:1329–1340. doi: 10.1038/sj.onc.1200951. [DOI] [PubMed] [Google Scholar]
- 45.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 46.Shinozaki M, Hoon DS, Giuliano AE, Hansen NM, Wang HJ, Turner R, et al. Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clin Cancer Res. 2005;11:2156–2162. doi: 10.1158/1078-0432.CCR-04-1810. [DOI] [PubMed] [Google Scholar]
- 47.Caldeira JR, Prando EC, Quevedo FC, Neto FA, Rainho CA, Rogatto SR. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer. 2006;6:48. doi: 10.1186/1471-2407-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, et al. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. doi: 10.1158/0008-5472.CAN-05-2508. [DOI] [PubMed] [Google Scholar]
- 49.Fabianowska-Majewska K, Kordek R, Krawczyk B. Studies on the methylation status of CpG sequences located in promoters of selected tumour suppressor genes in breast cancer cells. Nucleosides Nucleotides Nucleic Acids. 2006;25:1025–1028. doi: 10.1080/15257770600890640. [DOI] [PubMed] [Google Scholar]
- 50.Virmani AK, Rathi A, Sathyanarayana UG, Padar A, Huang CX, Cunnigham HT, et al. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter 1A in breast and lung carcinomas. Clin Cancer Res. 2001;7:1998–2004. [PubMed] [Google Scholar]
- 51.Sarrio D, Moreno-Bueno G, Hardisson D, Sanchez-Estevez C, Guo M, Herman JG, et al. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer. 2003;106:208–215. doi: 10.1002/ijc.11197. [DOI] [PubMed] [Google Scholar]
- 52.Muller HM, Widschwendter M. Methylated DNA as a possible screening marker for neoplastic disease in several body fluids. Expert Rev Mol Diagn. 2003;3:443–458. doi: 10.1586/14737159.3.4.443. [DOI] [PubMed] [Google Scholar]
- 53.Agathanggelou A, Honorio S, Macartney DP, Martinez A, Dallol A, Rader J, et al. Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene. 2001;20:1509–1518. doi: 10.1038/sj.onc.1204175. [DOI] [PubMed] [Google Scholar]
- 54.Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005;65:3497–3508. doi: 10.1158/0008-5472.CAN-04-4088. [DOI] [PubMed] [Google Scholar]
- 55.Lewis CM, Cler LR, Bu DW, Zochbauer-Muller S, Milchgrub S, Naftalis EZ, et al. Promoter hypermethylation in benign breast epithelium in relation to predicted breast cancer risk. Clin Cancer Res. 2005;11:166–172. [PubMed] [Google Scholar]
- 56.Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, Lapides A, et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64:4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- 57.Yan PS, Venkataramu C, Ibrahim A, Liu JC, Shen RZ, Diaz NM, et al. Mapping geographic zones of cancer risk with epigenetic biomarkers in normal breast tissue. Clin Cancer Res. 2006;12:6626–6636. doi: 10.1158/1078-0432.CCR-06-0467. [DOI] [PubMed] [Google Scholar]
- 58.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kontorovich T, Cohen Y, Nir U, Friedman E. Promoter methylation patterns of ATM ATR, BRCA1, BRCA2 and p53 as putative cancer risk modifiers in Jewish BRCA1/BRCA2 mutation carriers. Breast Cancer Res Treat. 2009;116:195–200. doi: 10.1007/s10549-008-0121-3. [DOI] [PubMed] [Google Scholar]
- 60.Locke I, Kote-Jarai Z, Fackler MJ, Bancroft E, Osin P, Nerurkar A, et al. Gene promoter hypermethylation in ductal lavage fluid from healthy BRCA gene mutation carriers and mutation-negative controls. Breast Cancer Res. 2007;9:20. doi: 10.1186/bcr1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez S, Diaz-Uriarte R, Osorio A, Barroso A, Melchor L, Paz MF, et al. A predictor based on the somatic genomic changes of the BRCA1/BRCA2 breast cancer tumors identifies the non-BRCA1/BRCA2 tumors with BRCA1 promoter hypermethylation. Clin Cancer Res. 2005;11:1146–1153. [PubMed] [Google Scholar]
- 62.Loughrey M, Provan PJ, Byth K, Balleine RL. Histopathological features of ‘BRCAX’ familial breast cancers in the kConFab resource. Pathology. 2008;40:352–358. doi: 10.1080/00313020802035899. [DOI] [PubMed] [Google Scholar]
- 63.Honrado E, Osorio A, Milne RL, Paz MF, Melchor L, Cascon A, et al. Immunohistochemical classification of non-BRCA1/2 tumors identifies different groups that demonstrate the heterogeneity of BRCAX families. Mod Pathol. 2007;20:1298–1306. doi: 10.1038/modpathol.3800969. [DOI] [PubMed] [Google Scholar]
- 64.Hedenfalk I, Ringner M, Ben-Dor A, Yakhini Z, Chen Y, Chebil G, et al. Molecular classification of familial non-BRCA1/BRCA2 breast cancer. Proc Natl Acad Sci USA. 2003;100:2532–2537. doi: 10.1073/pnas.0533805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sambrook J, Fritsch EF, Mantias T, editors. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 67.Martorell MR, Navarro J, Marquez C, Egozcue J, Benet J. Hypomethylation of human sperm pronuclear chromosomes. Cytogenet Cell Genet. 1997;76:123–127. doi: 10.1159/000134529. [DOI] [PubMed] [Google Scholar]
- 68.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]