Abstract
Purpose
The goal of this study was to describe a novel pattern of genitourinary (GU) anomalies in VACTERL association, which involves congenital anomalies affecting the vertebrae, anus, heart, trachea and esophagus, kidneys, and limbs.
Procedures
We collected clinical data on 105 patients diagnosed with VACTERL association and analyzed a subset of 89 patients who met more stringent inclusion criteria.
Findings
Twenty-one percent of patients have GU anomalies, which are more severe (but not more frequent) in females. Anomalies were noted in patients without malformations affecting the renal, lower vertebral, or lower gastrointestinal systems.
Conclusions
There should be a high index of suspicion for the presence of GU anomalies even in patient who do not have spatially similar malformations.
Keywords: VACTERL, VACTERL association, VATER, VATER association, genitourinary anomalies
Introduction
VACTERL association, sometimes termed VATER association depending on the criteria used for diagnosis, is estimated to occur in 0.3 to 2.1 per 10,000 live births (de Jong et al., 2008) and is a relatively poorly understood condition that includes at least several of the following defining component features: Vertebral anomalies, Anal atresia, Cardiac malformations, Tracheo-Esophageal fistula, Renal anomalies, and Limb abnormalities (Quan & Smith, 1973; Czeizel & Ludányi, 1985). The presence of many overlapping conditions makes accurate diagnosis challenging for both clinicians and researchers, and the exact criteria for diagnosis, in terms of the number and nature of anomalies included, are controversial (Rittler et al., 1996; Källén et al., 2001). Overlapping conditions include deletion 22q11.2 syndrome, Fanconi anemia, Feingold syndrome, oculo-auriculo-vertebral defect spectrum, Pallister-Hall syndrome, Townes-Brocks syndrome, as well as MURCS association, which specifically describes patients with Mullerian duct aplasia, renal aplasia, and cervicothoracic somite dysplasia (Duncan et al., 1979; Shaw-Smith, 2006). Though there is some evidence for inheritance of component features, the condition frequently occurs sporadically, and the causes remain elusive in all but a very small fraction of patients (Weaver et al., 1986; Brown et al., 1999; Solomon et al., 2010).
Although genitourinary (GU) anomalies are not considered one of the core component features, these malformations have been reported often in patients with VACTERL association (Weaver et al., 1986; Rittler et al., 1996; Botto et al., 1997; Stoll et al., 2007). GU anomalies have typically been described in patients who were also affected by malformations of the lower intestinal and renal systems. Ascertainment of such anomalies can be critical, as they may lead to significant morbidity and mortality (Rich et al., 1988).
Consideration of the shared developmental biology of the hindgut and the GU system reveals that anomalies affecting both the lower gastrointestinal (GI) and GU systems may be expected; however, recent findings regarding this developmental process reveal that the primordial structures giving rise to these systems may be less linked than previously thought, raising the theoretical possibility that malformations in one system may often also occur independently of the others (this is further explored in the Discussion section).
We present data on over one hundred affected patients ascertained through our National Human Genome Research Institute (NHGRI)/National Institutes of Health (NIH) research protocol on VACTERL association, and describe the spectrum of GU anomalies in these patients. We show clinical evidence that GU anomalies can occur in patients who do not have malformations involving proximally located systems such as anorectal, renal anomalies, or lower vertebral anomalies.
Materials and Methods
We collected patient information on 105 individuals affected by VACTERL association through our National Human Genome Research Institute IRB-approved protocol on VACTERL association. Patients had been diagnosed with VACTERL association or VATER association prior to inclusion in our study. For this specific research study, in addition to previous consideration of a diagnosis of VACTERL association, inclusion criteria had to be met through one of the following conditions: at least three component features of VACTERL association; at least two component features of VACTERL association in which one feature was either anal atresia or tracheo-esophageal fistula. Patients were excluded if at any time they were felt to better meet criteria for an overlapping (but distinct) condition, either clinically or through genetic testing. Of the 105 patients, 16 patients were excluded for analysis because of incomplete information or because alternate diagnoses were made. Though the genetic work-up differed between patients, no patient had an identified genetic etiology or other known cause for VACTERL association. We saw 17 probands along with their families at the NIH Clinical Center for a comprehensive clinical analysis. For patients who did not come to the NIH, we reviewed available medical records, with medical histories provided directly from patients, relatives, and referring clinicians (available details, as reflected in Table 1, differed among patients). Because of the hypothetical developmental origin of the GU anomalies described here (as detailed in the Discussion section), patients were considered to have GU anomalies if the anomaly affected the genitourinary system either through an apparent primary anomaly of GU organ development (such as hypospadias) or if there was structural disturbance of a GU organ even if the primary anomaly anatomically appears to be part of the anorectal system (such as a rectovestibular or rectourethral fistula); often, anomalies could be thought to meet both criteria (such as a cloaca).
Table 1.
Patients with genitourinary (GU) anomalies in addition to VACTERL association. Details of urogenital anomalies are given as reported by the referring clinician, and available specifics varied between patients.
| Patient | Gendera | GU Anomalies | V | A | C | TE | R | L | Other |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Rectovestibular fistula | + | Imperforate anus | - | - | Right renal agenesis | - | - |
| 2 | F | Cloaca with common vaginal, anal and urethral meatus | + | Imperforate anus | + | + | Multicystic horseshoe kidney | + | - |
| 3 | F | Cloaca with common urogenital sinus, duplex vagina with midline septum, and superficial rectum connected to urogenital sinus | + | See GU anomaly | + | + | Right renal agenesis, left hydronephrosis | - | - |
| 4 | F | Absent anal, genital, and urinary orifices, persistent cloaca, ambiguous genitalia, bicornuate uteri | - | See GU anomalies | + | + | Bilateral renal agenesis and atretic ureters | - | Duodenal atresia, asplenia |
| 5b | F | Cloacal anomaly with rectovestibular sinus | + | Anteriorly- placed anus | + | - | Left pyelectasis and caliectasis | - | - |
| 6 | F | Perineal fistula | + | - | + | + | VUR | + | |
| 7 | F | Rectovestibular fistula | + | Low imperforate anus | + | - | Ectopic ureter | + | - |
| 8 | F | Uterus didelphys | + | Imperforate anus | - | - | - | - | - |
| 9 | F | Cloaca | - | See GU | + | - | + | - | - |
| 10b | M | Rectourethral fistula | + | Imperforate anus | - | - | Dysplastic left kidney | - | - |
| 11 | M | Hypospadias, ambiguous genitalia | + | Imperforate anus | - | + | Left kidney agenesis | - | Unilateral hearing loss |
| 12 | M | Hypospadias and “urogenital anomalies”c | + | Imperforate anus | - | - | Left kidney agenesis | + | - |
| 13 | M | Rectocystic fistula | + | Imperforate anus | - | - | Right renal agenesis, duplicate left kidney | + | - |
| 14 | M | Hydrocele, hypospadias | - | - | + | + | - | - | Cystic hygroma |
| 15b | M | Hypospadias, rectourethral fistula | + | Imperforate anus | - | - | Horseshoe kidney | - | - |
| 16 | M | Hypospadias | + | - | + | + | Small kidneys but normal fx | - | - |
| 17b | M | Cryptorchidism, rectourethral fistula | + | Imperforate anus | + | - | - | + | Hydrocephalus |
| 18 | M | Micropenis, hypospadias | + | Imperforate anus | - | - | Grade III VUR, but normal appearance/function | + | CL/P, unilateral microtia, micrognathia |
| 19 | M | Left cryptorchidism | + | Imperforate anus | - | + | High urine output, history of UTI, but normal appearance | - | - |
Gender determined by karyotype analysis
Examined in person
Further details not available
M: Male; F: Female; V: Vertebral anomalies; A: Anal atresia; C: Cardiac malformations; TE: Tracheo-Esophageal fistula; R:Renal anomalies; L: Limb abnormalities; CL/P: Cleft lip/palate; GU: genitourinary; UTI: urinary tract infection; VUR: Vesicoureteral reflux
Results
Nineteen of the 89 patients in our cohort (21.3%) had evidence of anomalies affecting the GU system (Table 1). The presence of GU anomalies in our cohort was statistically significantly lower than that of each of the other core features; however, when corrected for multiple comparisons, there was not a significantly lower prevalence of GU vs. limb anomalies (Table 2). Nine of 43 female patients (20.9%) and 10 of 46 male patients (21.7%) had GU anomalies (with gender defined by chromosome analysis). There was not a statistically significant difference between the prevalence of GU anomalies by gender (χ2(1) = 0.009, p= 0.926). However, the extent of genitourinary anomalies appears to be qualitatively more severe in females. Fifty-six percent of females (5/9) with GU anomalies had cloacal anomalies requiring extensive surgical correction, 33% (3/9) had GU fistulae, and 11% (1/9) had a didelphys uterus. Of males with GU anomalies, 20% (2/10) had hypsospadias without any structural anomalies (one of these two had micropenis), 20% (2/10) had GU fistulae only and 10% (1/10) each had: ambiguous genitalia (with hypospadias); hypsopadias as well as more severe anomalies (not specified); cryptorchidism only; cryptorchidism and a GU fistula; hypospadias and a GU fistula; hypospadias and hydrocele.
Table 2.
Presence of the major component features of VACTERL association and genitourinary (GU) anomalies. GU anomalies were significantly less prevalent than the other core features (although when corrected for multiple comparisons, there was not a significantly lower rate of GU vs. limb anomalies).
| Feature | Prevalence | p-value (compared to presence of GU anomalies)a |
|---|---|---|
| V | 71/89 (79.7%) | <0.0001 |
| A | 50/89 (56.2%) | <0.0001 |
| C | 65/89 (73.0%) | <0.0001 |
| TE | 45/89 (50.6%) | <0.0001 |
| R | 57/89 (64.0%) | <0.0001 |
| L | 36/89 (40.4%) | 0.0091 |
| GU | 19/89 (21.3%) | N/A |
Two-tailed p-values calculated by Fisher’s exact test.
V: Vertebral anomalies; A: Anal atresia; C: Cardiac malformations; TE: Tracheo-Esophageal fistula; R: Renal anomalies; L: Limb abnormalities; GU: genitourinary anomalies
While 16/19 patients (84.2%) had GU anomalies in conjunction with imperforate anus and renal anomalies, three patients (patients 6, 14, and 16) presented with GU anomalies, but no imperforate anus, and only minor renal anomalies. Specifically, patient 6 was a female with a perineal fistula, but no imperforate anus, and vesicoureteral reflux but no other renal anomalies; patient 14 was a male with hypospadias and a hydrocele, but neither imperforate anus nor renal anomalies, and patient 16 was a male with hypospadias, but no imperforate anus, and no renal anomalies, except that his kidney size was slightly below normal limits for age. Patients 6 and 16, but not patient 14, had lower vertebral anomalies.
Discussion
The intertwined development of the hindgut and the GU system indicates that GU malformations in patients with hindgut malformations, as described by previous groups, may be expected. At the caudal end of the embryo, as early as four weeks of gestation, the primitive urogenital sinus, which ultimately gives rise to the bladder, urethra, and, in females, the vaginal vestibule, forms in the ventral cloaca; the anorectum forms in the dorsal cloaca (Paidas et al., 1999; Lau and Caty, 2006). During embryonic growth, folding of extraembryonic mesoderm from the latter two structures results in the formation of the urorectal septum (Paidas et al., 1999). While the urorectal septum approaches the cloacal membrane, it does not fuse with the membrane; instead, the cells of the cloacal membrane undergo apoptosis, exposing the external orifices of the urogenital system and the anorectum (Nievelstein et al., 1998; Paidas et al., 1999). The precise positions and sizes of the urogenital and anorectal structures are thought to be determined by a process termed “transformation”, wherein the caudal end of the embryo expands and rotates, and the cloacal structures are incorporated into the contour of the embryo (Paidas et al., 1999) (Figure 1). Because the spinal cord and the musculoskeletal system also take part in the process of transformation, it is conceivable that anomalies affecting the vertebral, anal, and GU systems may occur concurrently if transformation is dysregulated (Paidas et al., 1999). (This is hypothetical, as the normal developmental biology is still being clarified and concordance has not been achieved between human and animal studies (Qi et al., 2000), and it is not completely clear how dysregulation of this process leads to malformations.)
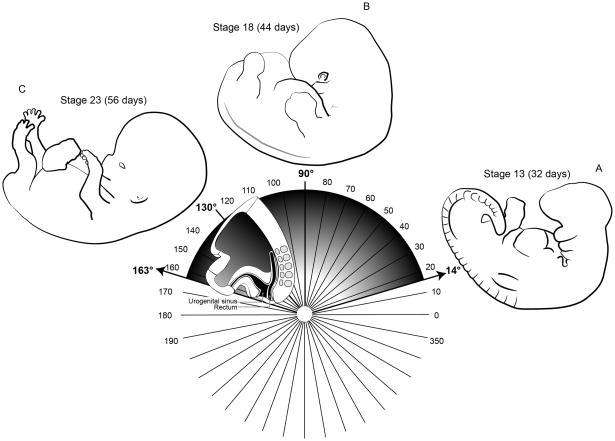
Figure 1.
Depiction of the process of “transformation” as described by Paidas et al. (1999), which includes expansion of the caudal portion of the embryo and counterclockwise rotation around the Y-axis (here depicted as the vertical line at 90°, though late in gestation, the Y-axis would extend through the upper cervical vertebrae). As the anorectal and GU systems, as well as the spinal cord and the musculoskeletal system, all take part in transformation, anomalies affecting the vertebral, anal, and GU systems may co-occur due to dysrgegulation of transformation. Depictions of the overall morphology of the embryo are shown starting at Carnegie stage 13 (~32 days) through stage 23 (~56 days), though the process of rotation/transformation continues in later fetal life. As depicted, the caudal morphology changes considerably (while the counterclockwise rotation occurs) such that the caudal end of the embryo expands and rotates; the cloacal structures become incorporated into the contour of the embryo (Paidas et al., 1999).
At the same time, examination of the development of the cloacal structures shows that defects within the urogenital and anorectal structures may also occur independently. The realization that the urorectal septum does not fuse with the cloacal membrane occurred fairly recently (Lau and Caty, 2006), and until that time, malformations such as so-called fistulae between the urogenital and anorectal structures were thought to result from failed fusion of the urorectal septum and the cloacal membrane (Nievelstein et al., 1998). Such “fistulae” are now thought to represent ectopic anal orifices that result from malformation and shortening of the dorsal cloaca alone, rather than a defect of a structure common to or bounding both the urogenital and anorectal systems (Kluth et al., 1995; Nievelstein et al., 1998). Moreover, the fact that the primitive urogenital sinus and the anorectum form independently by four weeks of gestation suggests that defects outside of the shared transformation process may not necessarily compromise both structures. For instance, anorectal malformations occurring with the anus located in the normal position may represent failure of the anorectum to recanalize following secondary occlusion of the canal: a late embryonic defect not involving the urogenital sinus (Nievelstein et al., 1998).
A complex and interacting network of signaling pathways are involved in GU development. Despite the paucity of knowledge involving genetic causes of VACTERL association, implicated genes in a very small number of patients echo embryologic observations. For example, a mutation in HOXD13 (Hox genes determine vertebrate craniocaudal segmentation and are involved in both GU and gastrointestinal (GI) development) has been reported in a patient with VACTERL association, though without reported GU anomalies (Garcia-Barceló et al., 2008). Mutations in HOXD13 have also been reported in male patients with genital anomalies (as well as the more classic association with limb anomalies) but without VACTERL association (Tüzel et al., 2007). The fact that GU anomalies are not seen in females with HOXD13 mutations is in contrast to the patients described in our cohort. Sonic Hedgehog (SHH), which regulates HOXD13 (among a wide host of other signaling targets), also plays key roles in GI and GU development (Parkin and Ingham, 2008; Lin et al., 2009). SHH has been implicated in VACTERL pathogenesis from animal models (Kim et al., 2001). While there is no evidence that mutations in the SHH gene itself result in VACTERL association in humans, many different perturbations of the SHH signaling pathway could hypothetically cause VACTERL association in humans (Aguinaga et al., 2010).
A number of previous studies have examined the presence of GU anomalies in patients with VACTERL association. For example, there is statistical evidence that ambiguous genitalia should be considered one of the core component features of VACTERL association (Rittler et al., 1996). Botto et al. (1997) noted that GU anomalies were more frequently common in patients with VACTERL with anorectal atresia and renal anomalies, and conversely less common in patients with esophageal atresia and preaxial limb anomalies (Botto et al., 1997). However, ascertainment biases make comparisons between studies very difficult. For example, the GU anomalies we describe here may be overall milder than those in other study cohorts that are based on, for examples, patients in congenital malformation registries (Rittler et al., 1996; Botto et al., 1997; Källén et al., 2001; Stoll et al., 2007).
Several additional limitations to this study must be noted. These limitations include the retrospective nature of the study and the heterogeneous ascertainment of patients, which includes the fact that only a minority of patients were seen in person at the NIH. Additionally, the relatively small sample size renders attempts at generalizations problematic. Finally, the current lack of knowledge regarding genetic or other etiologies in the patients described here can not be overlooked. Because genetic or other causes of VACTERL association have not yet been identified in our cohort of patients, comparing findings such as the type and severity of GU malformations among males vs. females is admittedly quite speculative at this point.
In summary, the presence of GU anomalies in patients with specific features of VACTERL association, such as imperforate anus and renal anomalies, has been well documented (Botto et al., 1997; Stoll et al., 2007). Data from our cohort of patients with VACTERL association supports previous studies in that the prevalence of GU anomalies is relatively high in patients with VACTERL association. Some of the patients described here may also fit under the rubric of “MURCS association”, and overlap between the two conditions is to be expected. However, we describe patients with GU anomalies who did not have anorectal or renal anomalies; consideration of the developmental origin of these systems demonstrates that while there are processes common to the development of all these structures that may concurrently compromise multiple systems, independent formation of the primordia of these structures also admits the possibility that individual systems may be compromised independently. Given the findings in this study and in previous studies, clinicians should have a high index of suspicion for GU anomalies in patients with VACTERL association, even if they do not have anorectal or renal anomalies.
Acknowledgments
This research was supported by the Division of Intramural Research, National Human Genome Research Institute, National Institutes of Health and Human Services, United States of America. The authors would like to thank Dr. Maximilian Muenke for his support and mentorship, and would like to extend their deepest gratitude to all participating patients and families.
References
- Aguinaga M, Zenteno JC, Pérez-Cano H, Morán V. Sonic hedgehog mutation analysis in patients with VACTERL association. Am J Med Genet A. 2010;152A:781–783. doi: 10.1002/ajmg.a.33293. [DOI] [PubMed] [Google Scholar]
- Botto LD, Khoury MJ, Mastroiacovo P, et al. The spectrum of congenital anomalies of the VATER association: an international study. Am J Med Genet. 1997;71:8–15. doi: 10.1002/(sici)1096-8628(19970711)71:1<8::aid-ajmg2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Brown AK, Roddam AW, Spitz L, Ward SJ. Oesophageal atresia, related malformations, and medical problems: a family study. Am J Med Genet. 1999;85:31–37. doi: 10.1002/(sici)1096-8628(19990702)85:1<31::aid-ajmg7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Ludányi I. An aetiological study of the VACTERL-association. Eur J Pediatr. 1985;144:331–337. doi: 10.1007/BF00441773. [DOI] [PubMed] [Google Scholar]
- Duncan PA, Shapiro LR, Stangel JJ, Klein RM, Addonizio JC. The MURCS association: Müllerian duct aplasia, renal aplasia, and cervicothoracic somite dysplasia. J Pediatr. 1979;95:399–402. doi: 10.1016/s0022-3476(79)80514-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Barceló MM, Wong KK, Lui VC, et al. Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A. 2008;146A:3181–3185. doi: 10.1002/ajmg.a.32426. [DOI] [PubMed] [Google Scholar]
- de Jong EM, Felix JF, Deurloo JA, et al. Non-VACTERL-type anomalies are frequent in patients with esophageal atresia/tracheo-esophageal fistula with full or partial VACTERL association. Birth Defects Res A. 2008;82:92–97. doi: 10.1002/bdra.20437. [DOI] [PubMed] [Google Scholar]
- Källén K, Mastroiacovo P, Castilla EE, Robert E, Källén B. VATER non-random association of congenital malformations: study based on data from four malformation registers. Am J Med Genet. 2001;101:26–32. doi: 10.1002/ajmg.1201. [DOI] [PubMed] [Google Scholar]
- Kim PC, Mo R, Hui CcC. Murine models of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J Pediatr Surg. 2001;36:381–384. doi: 10.1053/jpsu.2001.20722. [DOI] [PubMed] [Google Scholar]
- Kluth D, Hillen M, Lambrecht W. The principles of normal and abnormal hindgut development. J Pediatr Surg. 1995;30:1143–1147. doi: 10.1016/0022-3468(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Lau ST, Caty MG. Hindgut abnormalities. Surg Clin N Am. 2006;86:301–316. doi: 10.1016/j.suc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136:3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievelstein RAJ, van der Werff JFA, Verbeek FJ, Valk J, Vermeij-Keers C. Normal and abnormal embryonic development of the anorectum in human embryos. Teratology. 1998;57:70–78. doi: 10.1002/(SICI)1096-9926(199802)57:2<70::AID-TERA5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Paidas CN, Morreale RF, Holoski KM, Lund RE, Hutchins GM. Septation and differentiation of the embryonic human cloaca. J Pediatr Surg. 1999;34:877–884. doi: 10.1016/s0022-3468(99)90391-3. [DOI] [PubMed] [Google Scholar]
- Parkin CA, Ingham PW. The adventures of Sonic Hedgehog in development and repair. I. Hedgehog signaling in gastrointestinal development and disease. Am J Physiol Gastrointest Liver Physiol. 2008;294:G363–367. doi: 10.1152/ajpgi.00457.2007. [DOI] [PubMed] [Google Scholar]
- Qi BQ, Beasley SW, Williams AK, Frizelle F. Does the urorectal septum fuse with the cloacal membrane? J Urology. 2000;164:2070–2072. doi: 10.1016/s0022-5347(05)66969-8. [DOI] [PubMed] [Google Scholar]
- Quan L, Smith DW. The VATER association. Vertebral defects, Anal atresia, T-E fistula with esophageal atresia, Radial and Renal dysplasia: a spectrum of associated defects. J Pediatr. 1973;82:104–107. doi: 10.1016/s0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- Rich MA, Brock WA, Peña A. Spectrum of genitourinary malformations in patients with imperforate anus. Pediatr Surg Int. 1988;3:110–113. [Google Scholar]
- Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–536. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C. Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet. 2006;43:545–554. doi: 10.1136/jmg.2005.038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Pineda-Alvarez DE, Raam MS, Cummings DAT. Evidence for inheritance in patients with VACTERL association. Hum Genet. 2010;127:731–733. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B, Roth MP. Associated malformations in patients with anorectal anomalies. Eur J Med Genet. 2007;50:281–290. doi: 10.1016/j.ejmg.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Tüzel E, Samli H, Kuru I, Türkmen S, Demir Y, Maralcan G, Güler C. Association of hypospadias with hypoplastic synpolydactyly and role of HOXD13 gene mutations. Urology. 2007;70:161–164. doi: 10.1016/j.urology.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Weaver DD, Mapstone CL, Yu PL. The VATER association. Analysis of 46 patients. Am J Dis Child. 1986;140:225–229. doi: 10.1001/archpedi.1986.02140170051027. [DOI] [PubMed] [Google Scholar]