Abstract
Extracorporeal photopheresis (ECP) and the purine analog pentostatin exert potent immunomodulatory effects but have not been evaluated for their ability to enhance engraftment of hematopoietic stem cells. We evaluated in a canine model of dog leukocyte antigen (DLA)-identical hematopoietic cell transplantation (HCT) whether ECP in combination with pentostatin could enhance engraftment using a nonmyeloablative regimen consisting of 100 cGy total body irradiation (TBI) and postgrafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP). We have shown previously that with 100 cGy TBI alone as conditioning, all of six dogs rejected their graft 2–12 weeks after HCT. With the addition of pentostatin to 100 cGy TBI, 6 of 10 dogs rejected their graft. We now tested the additional use of ECP alone (n=2) or ECP plus 3–6 doses of pentostatin (n=7) before 100 cGy TBI and HCT. Eight out of 9 dogs rejected their grafts within 6–11 weeks after HCT. Compared to data without ECP, we failed to demonstrate a positive impact of the use of either ECP or pentostatin for prevention of rejection.
Keywords: Photopheresis, hematopoietic cell transplantation, pentostatin, engraftment
INTRODUCTION
Nonmyeloablative hematopoietic cell transplantation (HCT) relies on the immunosuppressive effect of the conditioning for successful engraftment in addition to postgrafting immunosuppression and the graft-versus-recipient hematopoiesis effect. Extensive dose escalation studies in the canine model have established 200 cGy total body irradiation (TBI) as the lowest TBI dose necessary for successful sustained donor engraftment.1 This model has been successfully translated into the clinic by a subsequent series of clinical trials.2 For the treatment of patients with non-malignant disorders or with serious comorbidities, further reduction of radiation dose below 200 cGy TBI is of interest. Previous studies demonstrated that dose de-escalation to 100 cGy TBI, even with the addition of pentostatin, resulted in insufficient rates of sustained engraftment.1,3 The addition of various regimens of postgrafting immunosuppression did not improve sustained engraftment rates using 100 cGy TBI.4,5 New strategies to allow further dose reduction in radiation dose for nonmyeloablative HCT are therefore warranted.
Extracorporeal photopheresis (ECP) was initially used to successfully treat patients with cutaneous T-cell lymphoma.6 In these patients, immunomodulatory effects were observed which triggered evaluation of the immunosuppressive effects of ECP in animal models.7 The immunosuppressive effects of ECP have now been used in patients with autoimmune disorders, solid organ rejection, and GVHD.8–13 We speculated whether these effects of ECP may be exploited to induce host tolerance against the incoming donor graft.
Pentostatin is a purine analog that induces T-cell apoptosis through adenosine deaminase inhibition. Pentostatin has been used particularly for the treatment of hairy cell leukemia, achieving complete remissions in 33 to 92% of patients, but also demonstrated significant activity in patients with chronic lymphocytic leukemia, prolymphocytic leukemia, adult T cell leukemia/lymphoma and cutaneous T cell lymphoma refractory to conventional chemotherapy.14 Used as part of the conditioning regimen in HCT, pentostatin can produce prolonged host T-cell depletion, thereby preventing graft rejection.15–17 The combined use of pentostatin and ECP in the conditioning regimen has been reported to result in low rates of GVHD and stable engraftment if used with 600 cGy TBI.18
In order to elucidate the potential role of ECP and pentostatin in reducing the incidence of graft rejection, we report on our results using a well established dog model of dog leukocyte antigen (DLA)-identical marrow grafts.
MATERIALS AND METHODS
Dogs and DLA typing
Litters of beagles, harriers, walker hounds, and crossbred dogs were used in this study. Dogs weighed from 12.5 to 26 (median, 14.4) kg and were 7 to 21 (median, 9) months old. Details of the dogs are also described in the supplements to this article. DLA-identical littermates were selected on the basis of identity for highly polymorphic MHC class I and class II microsatellite markers and identity for DLA-DRBI alleles as determined by direct sequencing.19–21
Marrow transplantation
Dogs in group 1 received ECP alone administered on days −2 and −1, dogs in group 2 received ECP on days −6 and −5 in combination with 3 doses of pentostatin IV (kindly provided by Supergen, Incorporated) at a dose of 4 mg/m2/day on days −4 to −2, and dogs in group 3 received ECP combined with 6 doses of pentostatin IV at a dose of 4 mg/m2/day on days −14 to −12, then −4 to −2 (Table 1). Timing of ECP was different between group 1 and group 2+3 to allow administration of pentostatin in the same schedule as reported previously1 in our canine model to allow further comparisons.
Table 1.
DLA-identical marrow transplantation after ECP± pentostatin and 100cGy TBI with postgrafting MMF and CSP1
| Recipient Dog # | Marrow TNCs × 108/kg | Neutrophil Increase5 | % Donor MNC Chimerism (Max-final) | Rejection | GVHD | Duration of Chimerism (weeks) | |
|---|---|---|---|---|---|---|---|
| Group 12 | G347 | 5.5 | Yes | 37-0 | Yes | No | 8 |
| G406 | 2.1 | Yes | 40-0 | Yes | No | 11 | |
| Group 23 | G417 | 2.1 | Yes | 0-0 | Yes | No | 0 |
| G331 | 4.7 | Yes | 0-0 | Yes | No | 0 | |
| G442 | 4.8 | Yes | 67-30 | No | No | 31 | |
| Group 34 | G449 | 3.2 | Yes | 34-0 | Yes | No | 10 |
| G472 | 2.6 | Yes | 22-0 | Yes | No | 6 | |
| G342 | 5.9 | Yes | 0-0 | Yes | No | 0 | |
| G5256 | 3.1 | Yes | 29-0 | Yes | No | 7 |
Dog leukocyte antigen (DLA), total body irradiation (TBI) extracorporeal photophoresis (ECP), mycophenolate mofetil (MMF), and cyclosporine (CSP)
Dogs in Group 1 received ECP product on days -2 and -1; 100 cGy TBI on day 0; MMF (10 mg/kg twice daily subcutaneously) day 0 to day 27, and CSP (15 mg/kg twice daily orally on day -1 to day 35).
Dogs in Group 2 received ECP product on days -6 and -5; 4 mg/m2 pentostatin i.v. on days -4, -3, and -2; 100 cGy TBI on day 0; and same regimen of MMF and CSP as in group 1.
Dogs in Group 3 received ECP product on days -6 and -5; 4 mg/m2 pentostatin i.v. on days -14, -13, -12, -4, -3, and -2; 100 cGy TBI on day 0; and same regimen of MMF and CSP as in group 1.
Absolute neutrophil counts increase greater than 500/μL
G525 received ECP product on day -6 only due to machine malfunction.
On day 0, all recipient dogs were conditioned for transplantation with 100 cGy TBI at 7 cGy/minute using a linear accelerator (Varian CLINAC 4, Palo Alto, CA).22 Donor marrow cells from DLA-identical littermates were collected under general anesthesia.22 The recipient dogs received the marrow graft IV with a median cell dose of 3.2 (range, 2.1–5.9) total nucleated cells (TNC)/kg. As postgrafting immunosuppression, MMF, 10 mg/kg s.c. twice daily, on days 0 to 27 and CSP, 15 mg/kg twice a day p.o., on days −1 to 35, were administered.1
Hematopoietic engraftment was assessed by sustained increases in granulocyte and platelet counts after the post-irradiation nadir, by documentation of donor cells with microsatellite marker studies in peripheral blood and marrow, or by marrow histology. Peripheral blood samples were obtained from dogs on day −7, day 0, then daily until full hematologic recovery, then monthly until end of study. Chimerism was evaluated weekly after transplantation until end of study. Dogs were euthanized or adopted after completion of the study.
ECP
ECP was performed using the XTS® Photopheresis System (kindly provided by Therakos, Incorporated, Exton, PA, USA) according to standard procedures in the manufacturer's guidelines. The dogs were placed under general anesthesia and a central vein catheter was used. During the photopheresis procedure, whole blood was collected from the dog while on the machine and separated by centrifugation over several cycles to obtain the leukocyte enriched fraction. Red blood cells and plasma were returned to the dog without being treated. The collection of ECP product for ultraviolet (UVA) exposure was done in 3 cycles/day for 2 consecutive days using the 125-mL bowl. Liquid 8-methoxypsoralen (UVADEX® or 8-MOP, Therakos, Exton, PA) was injected into the recirculation bag after collection of buffy coat, but prior to the photoactivation process. A dosage of 8-MOP was calculated based on the volume of buffy coat collected using the following formula: treatment volume collected × 0.017 = mL of 8-MOP (20 mcg /mL). After completion of photoactivation the product was reinfused back into the animal.
MLC
Mixed leukocyte cultures were used to assess the dogs' cellular immune function before and after ECP as described previously.23 Details are described in Supplemental Data: Materials and Methods.
NK cell cytoxicity assay
To evaluate NK cell activity before and after ECP, chromium release assays were performed as described previously.24 Details are described in Supplemental Data: Materials and Methods.
Chimerism analysis
Donor and host cell chimerism levels were evaluated using a polymerase chain reaction (PCR) based assay of polymorphic (CA)n dinucleotide repeats with primers specific for informative microsatellite markers.25 Details are described in Supplemental Data: Materials and Methods.
Flow cytometry
Flow cytometry was used to quantify the leukocyte subsets. Antibodies used are described in Supplemental Data: Materials and Methods. Ten μg/mL of the respective fluorescein conjugated mAb were added to 50 μL of whole blood and incubated at 4°C for 30 minutes. Subsequently, red blood cells were lysed with a hemolytic buffer containing EDTA, then cells were washed twice with HBSS/2% HS and once with HBSS alone. After resuspension in 1% paraformaldehyde, the cells were analyzed on a FACScan Flow Cytometer (Becton Dickinson, San Jose, CA).
Detection of apoptosis by Annexin V (Ax)/PI staining
Apoptosis of cells exposed to ECP was assessed by flow cytometry with the use of Annexin V binding, which allows detection of phosphatidylserine on the cell surface of apoptotic cells.26 Details are described in the supplements.
Statistical methods
Durations of chimerism were compared between groups using the log-rank test. The two-sample t-test was used to compare the average blood count nadirs between groups as well as the mean of the average blood count values between days 7 and 16. Pre- and post-ECP apoptosis levels were compared using the one-sample t-test.
RESULTS
ECP products
In 9 dogs a total of 17 ECP procedures were performed. A median of 254 mL (range 236–298) of ECP product was collected on each day with a median white blood count of 12,880/μL (range 7300–25,800), representing a median of 3.4 (range 2.2 –6.4) × 109 total nucleated cells. The percentages of different cell populations in the dogs' peripheral blood and ECP products were similar (data not shown). All dogs tolerated the extracorporeal volume of blood automatically determined by ECP machine. No immediate toxic effects were observed during the ECP procedure.
DLA-identical marrow grafts
Nine dogs were studied, two dogs in group 1 (ECP alone), three dogs in group 2 (ECP + 3 doses pentostatin), and four dogs in group 3 (ECP + 6 doses pentostatin). Table 1 summarizes the results of allogeneic marrow transplants in groups 1–3.
Group 1 (ECP alone) (n=2)
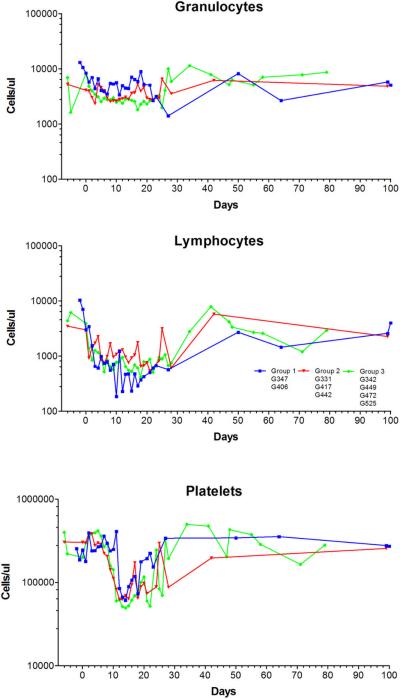
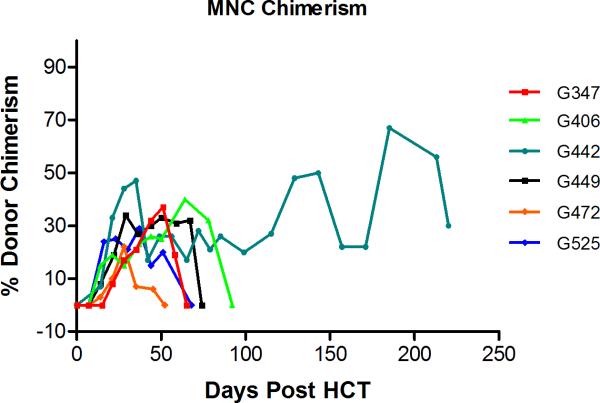
In neither dog, neutropenia (<500 granulocytes/μL) was not observed after HCT. Also thrombocytopenia with <20,000/μL platelets was not observed, and no platelet transfusions were required. Both dogs had complete hematopoietic recovery (Figure 1). After initial engraftment with mixed hematopoietic chimerism in PBMC starting 2–3 weeks after HCT and sustained until weeks 8 and 11, respectively, both dogs rejected their graft with autologous hematopoietic recovery (Figure 2). No GVHD occurred throughout the study.
Figure 1.
Peripheral blood granulocyte (A), lymphocyte (B), and platelet counts (C) after DLA-identical dog marrow transplantation conditioning with 100 cGy TBI and postgrafting MMF and CSP (median and range). Dogs in group 1 received extracorporeal photopheresis (ECP) product only; dogs in group 2 received ECP product and 3 doses of pentostatin; dogs in group 3 received ECP product and 6 doses of pentostatin.
Figure 2.
Percent donor chimerism in peripheral blood mononuclear cells (MNC) after DLA-identical dog marrow transplantation (Dogs G331, G417, and G342 rejected their graft).
Group 2 (ECP + 3 doses of pentostatin) (n=3)
None of the dogs had neutropenia <500 granulocytes/μL or thrombocytopenia <20,000/μL platelets after HCT. No transfusions were required. Median nadir of neutrophils was 1807 (range, 1653–4140) /μL and that of platelets 42,000 (range, 53,000–204,000)/μL which occurred on days +13 and +15 after HCT, respectively. All three dogs had complete hematopoietic recovery (Figure 1). Only one of the three dogs had sustained engraftment with mixed hematopoietic chimerism in PBMC sustained for the duration of observation (week 31). The other two dogs rejected their grafts and showed autologous hematopoietic recovery (Figure 2). No GVHD occurred throughout the study.
Group 3 (ECP + 6 doses of pentostatin) (n=4)
None of the dogs had neutropenia <500 granulocytes/μL or thrombocytopenia <20,000/μL platelets after HCT. No transfusions were required. Median nadir of neutrophils was 1639 (range, 1092–2380)/μL and that of platelets was 42,000 (range, 11,000 – 97,000)/μL which occurred on days +3 and +15 after HCT, respectively. All four dogs had complete hematopoietic recovery (Figure 1). Three of four dogs showed signs of donor engraftment with mixed hematopoietic chimerism in PBMC up to 34% and sustained up to weeks 6–10. One dog rejected his graft immediately with autologous hematopoietic recovery (Figure 2). No GVHD occurred throughout the study.
The duration of chimerism in the 7 dogs that received ECP + pentostatin (3 or 6 doses) was statistically significantly shorter than that among the 10 dogs that received pentostatin (3 or 6 doses) alone (p=.02) but not statistically significantly different than that among the 6 control dogs that received 100 cGy TBI (p=.57).
Comparison of hematologic recovery
The mean nadir of neutrophils in 8 dogs that received 100 cGy TBI combined with 6 doses of pentostatin without ECP was 1985 (range, 1736 – 3922), and the mean nadir of neutrophils in 6 control dogs that received 100 cGy TBI was 2731 (range, 1584 – 3640). Difference of hematologic recovery of dogs treated with 100 cGy TBI alone versus 100 cGy TBI in addition to 6 doses of pentostatin and ECP was not significant for ANC and platelet nadir with p=0.12 and p=0.63, respectively. Difference in hematologic recovery between dogs treated with 100 cGy in addition to 6 doses of pentostatin and dogs treated with 100 cGy TBI combined with 6 doses pentostatin and ECP was also non significant (ANC, p=0.19; platelets, p=0.49).
In vitro characteristics of ECP PBMC
For all in vitro studies described below, PBMCs from recipients were collected and analyzed at 4 different time points during ECP treatment: 1) peripheral blood pre ECP treatment (Pre-ECP blood); 2) peripheral blood immediately after ECP product infusion (Post-ECP blood); 3) Buffy coat (BC) cells from the ECP product before UVA light activation (Pre-ECP BC); and 4) BC from the ECP product immediately after UVA light activation (Post-ECP BC) (Table 2).
Table 2.
In vitro characteristics of ECP PBMC:
| In vitro study | Pre-ECP PBMC | Post-ECP PBMC | Pre-ECP BC | Post-ECP BC |
|---|---|---|---|---|
| Mixed leukocyte culture: | ||||
| % Alloreactivity, median, (range) | 100% | 92.1(59.32–178.2) | 48.7(21.3–375) | 3.5(0.08–19.6) |
| NK-cell function: | ||||
| % specific lysis, median, (range) | 53.8 (14–57.3) | 32 (28–57) | 38.7 (16.2–50.4) | 16.6 (8.7–33.2) |
| Apoptosis-early: | ||||
| % Annexin-V FITC+/Pl− Mean, (standard deviation) | 14.4 (5.2–23.6) | 16.9 (5.3–29.1) | 25 (15.4–40) | 34.5 (10.6–5.3) |
| Apoptosis-late: | ||||
| % Annexin-V FITC+/Pl− Mean, (standard deviation) | 16.2 (6.5–27.7) | 19.1 (7.9–32.7) | 31.2 (15.4–40) | 65.2 (45.8–81.3) |
Mixed leukocyte cultures (MLCs) of PBMCs from the ECP product after UVA light activation (Post- ECP BC) in 6 dogs showed a median decrease of 96 (range 80–99) % in alloreactivity against DLA-mismatched unrelated stimulator cells when compared to PBMCs from the product before UVA light activation (Pre-ECP BC). In contrast, there was no decrease in alloreactivity against DLA-mismatched unrelated stimulator cells of the Post-ECP blood when compared to Pre-ECP BC. NK cell function was tested in 5 dogs. PBMC from ECP product after UVA light activation (Post-ECP BC) showed a median decrease of 31% (range, 58–62%) in NK-function when compared with PBMCs from the ECP product before UVA light activation (Pre-ECP BC). In contrast, there was no decrease in NK-function of the PBMCs from the dog after ECP product infusion (Post-ECP blood) when compared to the ECP product before UVA light activation (Pre-ECP BC). Levels of apoptosis induction before and after ECP were compared in 6 of 7 dogs studied. Evaluating early apoptosis, percent Annexin-V FITC+/PI− cells in blood drawn before ECP ranged from 5.2 to 23.6 (mean, 14.4 ± 6.3) compared to 5.3 to 29.1 (mean, 16.9 ± 7.9) in blood drawn after ECP (mean difference, 1.5 ± 5.7, p=0.33). Percent Annexin-V FITC+/PI− of buffy coat cells before ECP ranged from 15.4 to 40 (mean, 25 ± 7.8) and buffy coat cells after ECP ranged from 10.6 to 5.3 (mean, 34.5 ± 15) (mean difference, 9.5 ± 14.3, p=0.16). Evaluating late apoptosis, percent Annexin-V FITC+/PI+ of blood drawn before ECP range from 6.5 to 27.7 (mean, 16.2 ± 7.6) compared to 7.9 to 32.7 (mean, 19.1 ± 8.7) of blood drawn after ECP (mean difference, 2.9 ± 7.0, p=0.35). The percent Annexin-V FITC+/PI+ of buffy coat cells before ECP ranged from 15.7 to 42 (mean, 31.2 ± 8.8) and after ECP range from 45.8 to 81.3 (mean, 65.2 ± 13.7). Post-ECP buffy coat cells showed a significant level of apoptosis as determined by annexin-V+/PI+ expression, when compared with the pre buffy coat cells (mean difference, 34.0 ± 8.2, p=0.0002).
DISCUSSION
ECP and pentostatin can both exert potent immunomodulatory effects but their impact on engraftment if used as part of a nonmyeloablative conditioning regimen has not been studied in a large animial model. We have shown previously that after conditioning with 100 cGy TBI alone, six of six dogs rejected their grafts within 2–12 weeks after HCT.1 With the addition of 3–6 doses of 4 mg/m2 pentostatin to 100 cGy TBI, 4 of 10 dogs accepted their DLA-identical sibling allografts while 6 rejected with a median duration of donor chimerism of 12 weeks.3 Combining this approach with ECP did not lead to a significant higher engraftment rate or longer duration of mixed chimerism, as 8 of 9 dogs rejected their grafts and the median duration of mixed chimerism was 7 weeks. Compared to our previous experience with 100 cGy TBI with or without pentostatin we observed no significant benefit of the addition of ECP.
The immunosuppressive effects observed with ECP clinically in the treatment of GVHD and solid organ rejection triggered the interest in the evaluation of this treatment modality as part of the conditioning regimen for allogeneic HCT in order to prevent GVHD or graft rejection. Miller et al. reported a low incidence of acute (grade II to IV of 9%) and chronic GVHD (43%) using a conditioning regimen combining ECP, pentostatin and 600 cGy TBI for human leukocyte antigen (HLA)-identical and non-identical (5/6 HLA match) allogeneic HCT. These rates were lower compared to the reported incidence with a similar regimen without ECP and pentostatin.18,27 The positive effects seen with ECP in the treatment of renal and cardiac allograft rejection10,11 lead to the evaluation of ECP in our study in order to prevent graft rejection or enhance engraftment.
What were the potential reasons for the negative result of our study? We used a similar treatment protocol, drug doses and treatment volumes as applied in the clinical setting of ECP for human patients. ECP of the dogs treated in our studies was effective in inducing apoptosis of T lymphocytes, similar to other published studies.28,29 The absence of apoptotic cells in peripheral blood of dogs immediately after ECP could be the result either of reticuloendothelial system uptake or simply may be due to dilution, as the estimated amount of PBMC collected in our dogs was only around 5–10% of total circulating PBMC.29 Although both NK and lymphocyte functions were decreased in the ECP product, no difference was shown in the PBMCs collected from the dogs before and after ECP nor on day zero. Our negative results might be explained by the mechanism and potency of the immunosuppressive effect of ECP. ECP has been successfully used to treat patients with acute and chronic GVHD in HCT.13,18,30–32 The mechanism of action of ECP in the setting of GVHD is based on its effect on dendritic cell (DC) integrity and T-cell function.9,33 ECP leads to a shift in the DC1/DC2 ratio favoring DC1.9,34 In vitro studies have shown that ECP treatment led to a shift from an activated monocytoid dendritic cell (DC)/T-cell state to a tolerogenic state.33 DC function was not assessed in this study. However, the combination of TBI and additional immunosuppressive agents may have impaired or even completely abrogated DC function or numbers, explaining in part, a decreased effect of ECP. In addition, an inverted CD4-to-CD8 ratio and overall increase in the number of CD3−/CD56+ natural killer (NK) cells, and an attenuation of the capacity of dendritic cells to stimulate the proliferation of allogeneic T-cells in mixed lymphocyte assays was observed.33,35 ECP treatment was also associated with a shift in the cytokine profile of circulating T cells from a predominantly inflammatory or Th1 (interleukin-2 [IL-2], interferon-γ) profile to a Th2 (IL-4, IL-10) profile. It was subsequently demonstrated that ECP induced a population of CD4+ CD25+ T cells, which functioned as suppressors in mixed lymphocyte reactions, consistent with regulatory T cells.36 Furthermore it was postulated that the ingestion of apoptotic T cells initiates the process of activation and cytokine secretion by antigen-presenting cells, which subsequently leads to generation of tolerogenic DCs and thus a regulatory T-cell response.37 To achieve such immunomodulatory effects and clinical responses patients with GVHD usually required several treatment courses of ECP for weeks to months.13,30–32 Translated into our model, it is possible that many cycles of ECP would be required to prevent rejection, not only before but also after bone marrow infusion.
Sustained engraftment versus eventual graft rejection in this model depends on a delicate balance between donor and host T-cells. We previously showed that sustained engraftment was achieved after 100 cGy TBI when marrow was supplemented with PBSC.38 However late graft rejection was the rule when the PBSC graft was depleted of CD3+ T-cells. In other studies, engraftment was facilitated by inducing host versus donor hyporesponsiveness through blockade via CD28 or CD154.39,40 Further we documented less profound T-cell depletion in lymph nodes of dogs given 100 cGy compared to 200 cGy TBI; the same was seen with antibody formation to a T-dependant antigen, sheep red blood cells.5 ECP, while affecting peripheral blood lymphocytes, might not have the same effect on the central lymphoid tissues as 100 cGy and, therefore, was unable to shift the T-cell balance in favor of the grafts.
In conclusion, our data demonstrates that the use of short course ECP alone or in combination with pentostatin was not potent enough to prevent graft rejection in our nonmyeloablative protocol using only 100cGy TBI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Michele Spector, DVM for providing veterinary care along with the Investigators who participated in the weekend treatments and the technicians in the canine facilities of the Fred Hutchinson Cancer Research Center. We are grateful to Roche for kindly providing MMF. We also would like to thank Stacy Zellmer for coordination of dog use and DLA-typing. We are also grateful for the assistance of Helen Crawford, Bonnie Larson, and Sue Carbonneau in manuscript preparation.
Grant Support: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grants P01HL036444, P01CA078902, and P30CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. In addition, this work was support by a grant from Therakos, Incorporated and Supergen, Incorporated. WAB was supported by a fellowship from Deutsche Krebshilfe, Dr. Mildred-Scheel-Stiftung für Krebsforschung. FRK was supported by a grant from FAPESP/Brazil and an award from the Oncology Research Faculty Development Program from the Department of Health and Human Services, NIH, Bethesda, MD.
Footnotes
CONFLICT OF INTEREST The authors have no competing financial interests in relation to the work described.
REFERENCES
- 1.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 2.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 3.Panse JP, Storb R, Storer B, Santos EB, Wentzel C, Sandmaier BM. Prolonged allogeneic marrow engraftment following nonmyeloablative conditioning using 100 cGy total body irradiation and pentostatin before and pharmocological immunosuppression after transplantation. Transplantation. 2005;80:1518–1521. doi: 10.1097/01.tp.0000181168.42461.0b. [DOI] [PubMed] [Google Scholar]
- 4.Hogan WJ, Little M-T, Zellmer E, Friedetzky A, Diaconescu R, Gisburne S, et al. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9:489–495. doi: 10.1016/s1083-8791(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 5.Sorror ML, Leisenring W, Mielcarek M, Baron F, Diaconescu R, Hogan WJ, et al. Intensified postgrafting immunosuppression failed to assure long-term engraftment of dog leukocyte antigen-identical canine marrow grafts after 1 gray total body irradiation. Transplantation. 2008;85:1023–1029. doi: 10.1097/TP.0b013e318169be24. [DOI] [PubMed] [Google Scholar]
- 6.Zic JA, Stricklin GP, Greer JP, Kinney MC, Shyr Y, Wilson DC, et al. Long-term follow-up of patients with cutaneous T-cell lymphoma treated with extracorporeal photochemotherapy. J Am Acad Dermatol. 1996;35:935–945. doi: 10.1016/s0190-9622(96)90118-8. [DOI] [PubMed] [Google Scholar]
- 7.Berger CL. Experimental murine and primate models for dissection of the immunosuppressive potential of photochemotherapy in autoimmune disease and transplantation (Review) Yale Journal of Biology & Medicine. 1989;62:611–620. [PMC free article] [PubMed] [Google Scholar]
- 8.Dall'Amico R, Rossetti F, Zulian F, Montini G, Murer L, Andreetta B, et al. Photopheresis in paediatric patients with drug-resistant chronic graft-versus-host disease. Br J Haematol. 1997;97:848–854. doi: 10.1046/j.1365-2141.1997.1092927.x. [DOI] [PubMed] [Google Scholar]
- 9.Foss FM, Gorgun G, Miller KB. Extracorporeal photopheresis in chronic graft-versus-host disease (Review) Bone Marrow Transplant. 2002;29:719–725. doi: 10.1038/sj.bmt.1703529. [DOI] [PubMed] [Google Scholar]
- 10.Giunti G, Schurfeld K, Maccherini M, Tanganelli P, Rubegni P, Alfani D, et al. Photopheresis for recurrent acute rejection in cardiac transplantation. Transplant Proc. 1999;31:128–129. doi: 10.1016/s0041-1345(98)01471-7. [DOI] [PubMed] [Google Scholar]
- 11.Horina JH, Mullegger RR, Horn S, Holzer H, Halwachs G, Kerl H, et al. Photopheresis for renal allograft rejection. Lancet. 1995;346:61. doi: 10.1016/s0140-6736(95)92696-8. [DOI] [PubMed] [Google Scholar]
- 12.Oliven A, Shechter Y. Extracorporeal photopheresis: a review (Review) Blood Rev. 2001;15:103–108. doi: 10.1054/blre.2001.0155. [DOI] [PubMed] [Google Scholar]
- 13.Greinix HT, Volc-Platzer B, Rabitsch W, Gmeinhart B, Guevara-Pineda C, Kalhs P, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–3104. [PubMed] [Google Scholar]
- 14.Brogden RN, Sorkin EM. Pentostatin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in lymphoproliferative disorders. Drugs. 1993;46:652–677. doi: 10.2165/00003495-199346040-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 16.Chan GW, Foss FM, Klein AK, Sprague K, Miller KB. Reduced-intensity transplantation for patients with myelodysplastic syndrome achieves durable remission with less graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:753–759. doi: 10.1016/j.bbmt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Pavletic SZ, Bociek RG, Foran JM, Rubocki RJ, Kuszynski CA, Wisecarver JL, et al. Lymphodepleting effects and safety of pentostatin for nonmyeloablative allogeneic stem-cell transplantation. Transplantation. 2003;76:877–881. doi: 10.1097/01.TP.0000084869.08639.A0. [DOI] [PubMed] [Google Scholar]
- 18.Miller KB, Roberts TF, Chan G, Schenkein DP, Lawrence D, Sprague K, et al. A novel reduced intensity regimen for allogeneic hematopoietic stem cell transplantation associated with a reduced incidence of graft-versus-host disease. Bone Marrow Transplant. 2004;33:881–889. doi: 10.1038/sj.bmt.1704454. [DOI] [PubMed] [Google Scholar]
- 19.Wagner JL, Burnett RC, Works JD, Storb R. Molecular analysis of DLA-DRBB1 polymorphism. Tissue Antigens. 1996;48:554–561. doi: 10.1111/j.1399-0039.1996.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 20.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 22.Ladiges WC, Storb R, Graham T, Thomas ED. Experimental techniques used to study the immune system of dogs and other large animals. In: Gay WI, Heavener JE, editors. Methods of Animal Experimentation. Academic Press; New York, NY: 1989. pp. 103–133. [Google Scholar]
- 23.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- 24.Loughran TP, Jr., Deeg HJ, Storb R. Morphologic and phenotypic analysis of canine natural killer cells: Evidence for T-cell lineage. Cell Immunol. 1985;95:207–217. doi: 10.1016/0008-8749(85)90309-0. [DOI] [PubMed] [Google Scholar]
- 25.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 26.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 27.Foss FM. The role of purine analogues in low-intensity regimens with allogeneic hematopoietic stem cell transplantation (Review) Semin Hematol. 2006;43:S35–S43. doi: 10.1053/j.seminhematol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction of ultraviolet light A and photochemotherapy in cutaneous T-cell Lymphoma: relevance to mechanism of therapeutic action. J Invest Dermatol. 1996;107:235–242. doi: 10.1111/1523-1747.ep12329711. [DOI] [PubMed] [Google Scholar]
- 29.Bladon J, Taylor PC. Extracorporeal photopheresis induces apoptosis in the lymphocytes of cutaneous T-cell lymphoma and graft-versus-host disease patients. Br J Haematol. 1999;107:707–711. doi: 10.1046/j.1365-2141.1999.01773.x. [DOI] [PubMed] [Google Scholar]
- 30.Owsianowski M, Gollnick H, Siegert W, Schwerdtfeger R, Orfanos CE. Successful treatment of chronic graft-versus-host disease with extracorporeal photopheresis. Bone Marrow Transplant. 1994;14:845–848. [PubMed] [Google Scholar]
- 31.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 32.Perfetti P, Carlier P, Strada P, Gualandi F, Occhini D, van Lint MT, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42:609–617. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 33.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 34.Tambur AR, Ortegel JW, Morales A, Klingemann H, Gebel HM, Tharp MD. Extracorporeal photopheresis induces lymphocyte but not monocyte apoptosis. Transplant Proc. 2000;32:747–748. doi: 10.1016/s0041-1345(00)00966-0. [DOI] [PubMed] [Google Scholar]
- 35.Alcindor T, Gorgun G, Miller KB, Roberts TF, Sprague K, Schenkein DP, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- 36.Biagi E, Di B, I, Leoni V, Gaipa G, Rossi V, Bugarin C, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 37.Di Renzo M, Sbano P, De Aloe G, Pasqui AL, Rubegni P, Ghezzi A, et al. Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients. Clin Exp Immunol. 2008;151:407–413. doi: 10.1111/j.1365-2249.2007.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaucha JM, Zellmer E, Georges G, Little M-T, Storb R, Storer B, et al. G-CSF-mobilized peripheral blood mononuclear cells added to marrow facilitates engraftment in nonmyeloablated canine recipients: CD3 cells are required. Biol Blood Marrow Transplant. 2001;7:613–619. doi: 10.1053/bbmt.2001.v7.pm11760149. [DOI] [PubMed] [Google Scholar]
- 39.Storb R, Yu C, Zaucha JM, Deeg HJ, Georges G, Kiem H-P, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 40.Jochum C, Beste M, Zellmer E, Graves SS, Storb R. CD154 blockade and donor-specific transfusions in DLA-identical marrow transplantation in dogs conditioned with 1-Gy total body irradiation. Biol Blood Marrow Transplant. 2007;13:164–171. doi: 10.1016/j.bbmt.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francisco LV, Langston AA, Mellersh CS, Neal CL, Ostrander EA. A class of highly polymorphic tetranucleotide repeats for canine genetic mapping. Mamm Genome. 1996;7:359–362. doi: 10.1007/s003359900104. [DOI] [PubMed] [Google Scholar]
- 42.Reichert WL, Stein JE, French B, Goodwin P, Varanasi U. Storage phosphor imaging technique for detection and quantitation of DNA adducts measured by the 32P-postlabeling assay. Carcinogenesis. 1992;13:1475–1479. doi: 10.1093/carcin/13.8.1475. [DOI] [PubMed] [Google Scholar]
- 43.Moore PF, Rossitto PV, Danilenko DM, Wielenga JJ, Raff RF, Severns E. Monoclonal antibodies specific for canine CD4 and CD8 define functional T-lymphocyte subsets and high density expression of CD4 by canine neutrophils. Tissue Antigens. 1992;40:75–85. doi: 10.1111/j.1399-0039.1992.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 44.Sandmaier BM, Storb R, Appelbaum FR, Gallatin WM. An antibody that facilitates hematopoietic engraftment recognizes CD44. Blood. 1990;76:630–635. [PubMed] [Google Scholar]
- 45.Sandmaier BM, Schuening FG, Bianco JA, Rosenman SJ, Bernstein I, Goehle S, et al. Biochemical characterization of a unique canine myeloid antigen. Leukemia. 1991;5:125–130. [PubMed] [Google Scholar]
- 46.Denkers E, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. J Immunol. 1985;135:2183–2186. [PubMed] [Google Scholar]
- 47.Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Veterinary Immunology & Immunopathology. 1993;39:461–466. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.