Abstract
Interregional correlations between blood oxygen level dependent (BOLD) magnetic resonance imaging (fMRI) signals in the resting state have been interpreted as measures of connectivity across the brain. Here we investigate whether such connectivity in the working memory and default mode networks is modulated by changes in cognitive load. Functional connectivity was measured in a steady‐state verbal identity N‐back task for three different conditions (N = 1, 2, and 3) as well as in the resting state. We found that as cognitive load increases, the functional connectivity within both the working memory the default mode network increases. To test whether functional connectivity between the working memory and the default mode networks changed, we constructed maps of functional connectivity to the working memory network as a whole and found that increasingly negative correlations emerged in a dorsal region of the posterior cingulate cortex. These results provide further evidence that low frequency fluctuations in BOLD signals reflect variations in neural activity and suggests interaction between the default mode network and other cognitive networks. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, functional connectivity, working memory, default mode
INTRODUCTION
Steady‐state correlations in low frequency blood oxygen level dependent (BOLD) magnetic resonance imaging (MRI) signals have been interpreted as revealing functional connectivity between regions within the human brain [Biswal et al.,1995,1997; Hampson et al.,2001; Lowe et al.,2000]. Most studies have examined interregional correlations in the resting state in which no task or stimulus effects are specifically encoded into the temporal variance of BOLD signals [Cordes et al.,2001; Lowe et al.,1998; Prohovnik et al.,1980; Rogers et al.,2007; van de Ven et al.,2004], though functional connectivity can also be measured during steady state tasks providing a method for studying the effects of task load on functional connectivity [Hampson et al.,2001; Newton et al.,2007].
Previous reports have shown that functional connectivity can be modulated by cognitive load in some networks. For example, in previous studies of the motor system, we have shown that measures of functional connectivity scale with the rate of finger tapping in some regions of the motor network [Newton et al.,2007]. We and others have established that connectivity measured during performance of steady state tasks may be greater than at rest [Fox et al.,2005; Hampson et al.,2004; Hampson et al.,2001,2006b; Lowe et al.,2000; Newton et al.,2007]. In the working memory system specifically, path analysis has shown that effective connectivity increases under increased cognitive loads [Honey et al.,2002]. Correlation analysis of the trial‐by‐trial variance in event related working memory responses (as a different measure of steady state functional connectivity) has shown decreasing functional connectivity during delay periods between the hippocampus, the fusiform face area, and the inferior frontal gyrus [Rissman et al.,2004; Rissman et al.,2008]. Principal component analysis (PCA) has also been used to examine working memory systems, showing different networks with load dependent connectivity for the encoding and maintenance periods of an event related task [Woodward et al.,2006]. However, none of these studies specifically addressed how measurements of functional connectivity are modulated across a range of steady‐state working memory task loads.
Steady state fMRI data have also been used to identify a network comprised of brain regions that are more active during resting periods, absent of directed attention demanding tasks. Some of these regions have become known as the default mode network [Fox et al.,2005; Greicius et al.,2003; Raichle et al.,2001], and can be identified in cognitive steady states in most subjects [Arfanakis et al.,2000; Beckmann et al.,2005; Meyer‐Baese et al.,2004; van de Ven et al.,2004]. It has been recently shown that these default mode regions are functionally connected not only in conscious awake states but during light stages of sleep [Horovitz et al.,2008], and under light anesthesia [Greicius et al.,2008]. However, the specific role played by these regions in the resting state remains a topic of debate, as does their relationship to those networks recruited for specific cognitive tasks. While changes in functional connectivity within a network have been measured between the resting state (when the default‐mode network may be most active) and during task performance [Esposito et al.,2006; Hampson et al.,2004; Hampson et al.,2006b], few studies have looked closely at changes in functional connectivity between the working memory and default mode networks. One recent study that looked at this effect [Fransson,2006] described a change in correlations between a recruited cognitive network and the default mode network, in response to a particular task. However, it remains unclear whether the connectivity between working memory regions and those in the default mode network scales over a range of cognitive loads.
In light of this background, the current study focused on two specific aims. The first aim was to quantify the degree to which functional connectivity (rather than just activation level) within the working memory network is modulated by cognitive load. Second, we aimed to identify whether the functional connectivity of the default mode network (either within itself or between it and the working memory network) changes as a function of cognitive load. We hypothesized that if BOLD fluctuations reflect mental processes, then interregional correlations within the working memory and the default mode networks should vary with increasing cognitive loads. Further, we postulated that connectivity between the working memory network and default mode network should become increasingly negative if the role of the default mode network operates as originally proposed. Taken together, these analyses provide further insight into the underlying factors affecting typical measurements of functional connectivity using fMRI, and increase our understanding of the behaviors of the working memory and default mode networks and their role in the normal brain.
MATERIALS AND METHODS
Subjects
Ten healthy subjects were recruited for participation in this study. All were right handed by self report. The subject pool consisted of eight female and two male volunteers, with an age range of 20–36 years (median = 27 years). All subjects provided informed consent in accordance with procedures approved by the Institutional Review Board at Vanderbilt University, and were compensated for their participation. Two of the ten subjects were removed from the study for failure to complete all necessary tasks, yielding data from eight subjects which are presented here.
Imaging and Initial Processing
All subjects were imaged using a Philips Achieva 3T MR scanner, using an eight channel SENSE coil. Four functional data sets were acquired in each subject using a single shot, gradient‐echo echo planar imaging (EPI) pulse sequence (TR = 2 s, TE = 35 ms) with a SENSE acceleration factor of 1.8. The multislice images covered 12.2 cm of brain and were acquired axially (matrix size: 64 × 64), using a field of view of 240 mm. Voxels measured 3.75 mm × 3.75 mm × 3.5 mm. All functional data had slice timing and motion artifacts corrected, were coregistered within each subject, were normalized to the MNI 152 template using the SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/), and were spatially smoothed (Gaussian kernel, FWHM = 8 mm). High resolution T1 weighted anatomic images were acquired using conventional parameters, and were used to aid spatial normalization.
Cognitive Tasks
Each subject completed four fMRI runs: three N‐back runs, and one resting state run. The resting state run consisted of 100 images of each slice acquired over 200 s, and subjects were instructed to lie still with their eyes closed. Each N‐back run consisted of a 300‐s (150 image volumes) block‐design portion with N‐back and 0‐back conditions in alternating 30‐s blocks (15 letters, 3 targets per 30‐s block), followed by a 200‐s (100 image volumes) steady‐state portion of the N‐back task only containing 20 targets (Fig. 1A). These N‐back runs were performed with a verbal identity working memory N‐back task (1‐back, 2‐back, and 3‐back tasks in random order). Stimuli were a single letter presented visually at the beginning of the acquisition of each image volume, and subjects were asked to respond with a button press when the current letter was the same as that N‐letters back.
Figure 1.
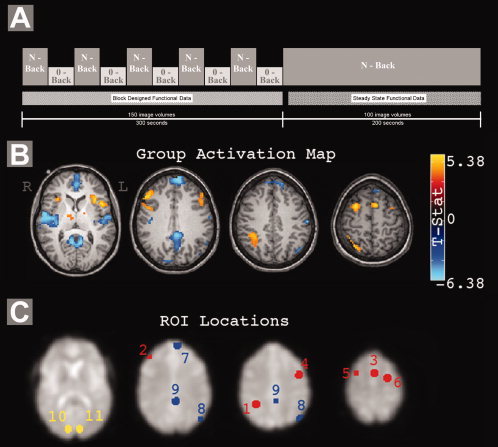
A: Summary of the N‐back tasks. Block designed task data were used for activation map calculation while steady state data were used for functional connectivity analyses. B: Group activity map, showing the main effect of task across loads. C: ROI locations. Chosen regions generally coincide with those identified by previous working memory and default mode studies. Red, task positive network; blue, task negative network; yellow, control regions. ROI number key is in Table I.
Regions of Interest
Regions of interest (ROIs) were defined based on statistical parametric mapping of the block‐design portions of the N‐back runs, and were classified into three separate groups: task positive regions, activated by the working memory task; task negative regions, deactivated by the working memory task; and control regions, where signal showed no significant response to the task manipulation. These definitions were made using the results of fitting a general linear model to the block‐design data of all N‐back tasks at the individual subject level, and entering the results into a second level random effects analysis across subjects. Regions of significant activation and deactivation during working memory performance were identified from the group‐level statistical contrast of average N‐back signal versus 0‐back. To identify positively activated clusters, an uncorrected one‐tailed voxel‐level threshold of P < 0.001 was applied, followed by a cluster size threshold to achieve correction for multiple comparisons at a false discovery rate (FDR) of 0.001. [Curran‐Everett,2000; Genovese et al.,2002] Positive activations generally coincided with previously reported working memory regions [D'Esposito et al.,1995; McCarthy et al.,1994; Owen et al.,2005]. For negatively activated clusters, FDR was held to 0.005; deactivations generally coincided with the default mode network [Greicius et al.,2003; Raichle et al.,2001]. The statistical threshold applied to task negative regions was raised in order to capture weaker deactivation in left parietal cortex, a commonly identified default mode region. [Fransson,2005] ROIs were defined within these clusters by retaining all significant voxels within 8 mm of the cluster peak. Control regions were defined in the occipital cortex using maps showing voxels with no significant positive or negative activation with respect to the task (voxel‐level P > 0.05 uncorrected), retaining all voxels within 8 mm of the specified coordinates. Table I lists all eleven ROIs, their locations (MNI coordinates), and abbreviations of our nomenclature. Figure 1C illustrates the location of each ROI.
Table I.
ROI locations
| # | Location | MNI coordinates | Description | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1 | Right parietal | 33 | −45 | 39 | Working memory (Task positive) |
| 2 | Right Frontal | 33 | 12 | 63 | Working memory (Task positive) |
| 3 | Pre‐SMA (pSMA) | 0 | 12 | 60 | Working memory (Task positive) |
| 4 | Left Frontal | −24 | 3 | 54 | Working memory (Task positive) |
| 5 | Right Prefrontal | 45 | 42 | 24 | Working memory (Task positive) |
| 6 | Left Prefrontal (LPFC) | −45 | 9 | 39 | Working memory(Task positive) |
| 7 | Prefrontal Dorsal | −6 | 63 | 30 | Default mode (Task negative) |
| 8 | Left Parietal | −48 | −72 | 36 | Default mode (Task negative) |
| 9 | Posterior Cingulate (PCC) | −3 | −39 | 33 | Default mode (Task negative) |
| 10 | Right occipital cortex (ROCC) | 10 | −90 | 2 | Visual (control) |
| 11 | Left occipital cortex | −10 | −90 | 2 | Visual (control) |
Activation Analysis
To compare load‐related changes in connectivity to underlying changes in mean activation, a simple analysis of activation in each of the block‐designed N‐back tasks was performed at the individual subject level. For each subject, the percent signal change [(S N‐back − S 0‐back)/S 0‐back × 100] was calculated for each of the N‐back conditions (N = 1, 2, and 3) in each of the defined ROIs (Table I). The signals used in this calculation were the average of the final 10 s of each condition across all blocks (to allow for settling of the hemodynamic response after transitions from one condition to another), and the mean percent signal change across subjects was plotted verses cognitive load for each ROI. An ANOVA was used to determine the effect of load on activation in each ROI.
Functional Connectivity Analysis
Functional connectivity measures were calculated based on the steady‐state data after discarding the first five volumes from each run. All time series had a discrete cosine basis high‐pass filter with period of 100 s applied to remove very low frequency trends and drifts, and the effects of residual correlates of motion (remaining in the data after image realignment and normalization) were minimized through linear regression of the first‐order estimated motion parameters. Cardiac, respiratory, and global trends were minimized through regression of the average signal across the entire brain. [Weissenbacher et al.,2009] A 0.1 Hz Chebyshev Type II low‐pass filter was also applied. The connectivity measure used was the Pearson correlation coefficient, converted to an approximately normal random variable via the Fisher Z transformation [Fisher,1928; Hampson et al.,2006b] according to Eq. ( 1), where r is the Pearson's correlation and N denotes the degrees of freedom (estimated as being equal to the number of acquired images here).
| (1) |
Three separate analyses of connectivity were performed. In the first, a mean time series was calculated for each ROI at each load, and these representative time courses were tested for significant effects of load on their pair‐wise correlation using a one way ANOVA using the R statistical package. (http://www.r-project.org/) Each combination of ROIs (i.e. path) was tested for this overall effect of load. Load effects for a single path were considered significant at P < 0.05 (# paths tested = 54), and significant paths had their individual measurements at each load compared using paired t‐tests.
In the second connectivity analysis, within network changes of functional connectivity were measured by calculating the average correlation between all combinations of regions within each network, at each load. This measurement was termed the path independent connectivity (PIC) as it is a measure of overall connectivity within each network. The distribution of values across subjects was tested for effects of load within the working memory, default mode, and control networks.
In the third connectivity analysis, we focused on maps of functional connectivity to each of the working memory ROIs as a method of identifying whether there was tissue outside of our ROIs that showed load‐related changes in functional connectivity to the working memory network as a whole. Typical maps of functional connectivity using each working memory ROI as a separate seed region were calculated for each subject at each load. This resulted in six maps of functional connectivity for each subject and load (192 maps total). These maps were then input into a full factorial ANOVA with factors for subject, load, and seed region. A map of the main effect of load was calculated, as were four maps of negative correlations at each load across subjects and seed regions. Conceptually, these maps identify voxels that are significantly negatively correlated to the working memory network as a whole, accounting for differences between seed regions and subjects. Load effects were considered to be significant at an FDR corrected P < 0.05 with no minimum cluster size.
RESULTS
In five subjects, performance was assessed during steady state N‐back acquisitions as the percent of targets correctly identified (1 back: 98 ± 2.7%; 2 back: 79 ± 11%; 3 back: 52 ± 15%), confirming the increase in task difficulty. A map of activation/deactivation to the blocked task at the group level can be seen in Figure 1B. Percent signal changes were measured during the block designed portion of each N‐back task for each ROI, and are plotted in Figure 2. Load‐related increases in activity were observed in all working memory regions at significant or nearly significant levels, with negligible changes occurring when increasing load from 2 Back to 3 Back conditions.
Figure 2.
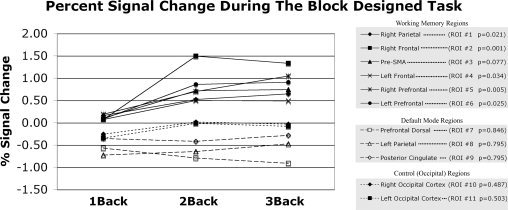
Percent signal changes were measured during the block designed portion of each N‐back task. ROI numbers (see Table I) and significance of the effect of task (measured via one way ANOVA) are listed alongside ROI names. Note that only working memory regions had significant or nearly significant effects of load on activation during the block designed task.
Six paths (pairs of ROIs) were found to show changes in functional connectivity related to cognitive load. These included paths within the working memory network (Left Frontal ‐ Left Prefrontal, P = 0.007; PreSMA ‐ Right Frontal, P = 0.022; PreSMA ‐ Left Prefrontal, P = 0.025), within the default mode network (Prefrontal Dorsal ‐ Posterior Cingulate, p = 0.006), and between the working memory and the default mode network (Right Parietal ‐ Posterior Cingulate, P = 0.030). One path showed load effects between the left frontal working memory (task positive) region and the left occipital region of interest (P = 0.047). None of these statistics remain below the 0.05 threshold when Bonferroni corrections for the number of region pairs tested are applied. However, the ensuing paired t‐tests confirmed individual differences between some loads for each path, with the most consistent difference being between the resting state condition and the 2back condition (5/6 paths, P < 0.05). This difference represented an increase for all paths except the one spanning between the working memory and default mode networks (Right Parietal ‐ Posterior Cingulate), in which case there as a connectivity decrease as load increased.
Analyzing the average within network changes in functional connectivity, Figure 3 shows functional connectivity within the working memory and within the default mode networks as a whole increased as a function of load (P = 0.063 and P = 0.037, respectively), with no such load effect within the control network (P = 0.12) or between the working memory network as a whole and the default mode network as a whole (P = 0.73).
Figure 3.
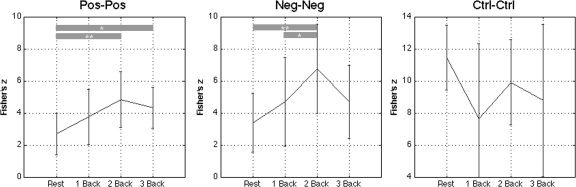
Mean ± standard deviation across subjects of the path independent connectivity (PIC) measure within networks. The PIC was calculated as the mean connectivity across all possible pairs of ROIs within each specified network (i.e. Pos ‐ Pos reflects the average connectivity of every working memory ROI to every other working memory ROI. Grey bars link loads that are significantly different (**P < 0.05) or nearly significantly different (*P < 0.1) as defined by paired t‐tests. Results show increases in functional connectivity within the working memory and within the default mode network as load increases, with no significant changes between the control regions. Pos, task positive; Neg, task negative; Ctrl, control network.
Collective analysis of the connectivity maps for each of the defined working memory regions of interest showed a cluster of voxels with load dependent functional connectivity in the posterior cingulate cortex (FDR, P < 0.05; Fig. 4). Inspection of the negative correlations to the working memory network shows that this dorsal and anterior region of the PCC presents increasingly negative correlations to the working memory network as working memory load increases (see Fig. 5), representing changes in connectivity between the working memory network and part of the default mode network. Furthermore, this region of the posterior cingulate is spatially distinct from any region showing load‐related changes in gross activity in response to the block designed task, shown by Figure 6.
Figure 4.
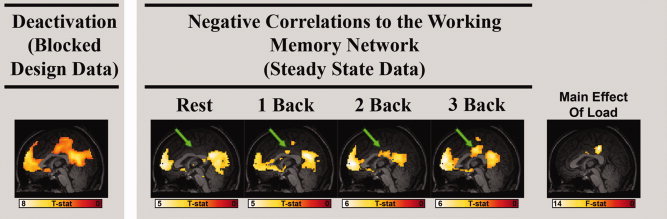
(Left) Regions of the default mode network visualized in a sagittal view of group deactivations to the block designed task (FDR P < 0.05, no minimum cluster size). (Right) The results of a full factorial ANOVA performed on maps of functional connectivity to all of the working memory ROIs (ROI 1–6, Table I). The voxels containing significant negative correlations to the working memory network are shown for each load, and the arrow calls attention to a part of the posterior cingulate cortex whose negative correlations become increasingly significant as a load increases, verified in the F‐contrast showing regions significantly affected by load.
Figure 5.
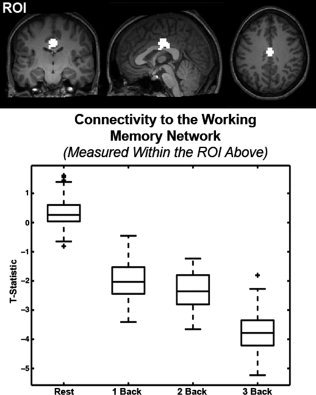
Quantification of the effect of load on functional connectivity between the region highlighted in Figure 4 and the working memory network. (top) An ROI was defined as the overlap between those cingulate voxels that are negatively correlated to the working memory network (across loads, P < 0.05, FDR corrected) and whose connectivity was significantly affected by load (P < 0.05 uncorrected). (bottom) A standard box plot of the ROI's connectivity to the working memory network at each steady state load. Box lines are at the lower quartile, median, and upper quartile of the data. Whiskers extend from each end of the box to the adjacent values in the data. Outliers are data with values beyond the ends of the whiskers. Note that functional connectivity to the working memory network becomes increasingly negative within the ROI as load increases.
Figure 6.
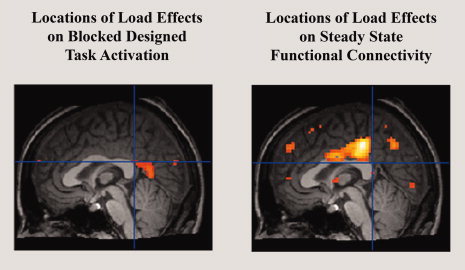
A comparison highlighting the differences between load effects on activation and load effects on connectivity. Shown are sagittal views of the results of an ANOVA of activation maps (left) and working memory functional connectivity maps (right) in all eight subjects. The position of the cross‐hairs highlights the spatial differences between the regions of the posterior cingulate cortex identified as being affected by load even when region boundaries are liberally defined with a relaxed statistical threshold (shown above, P < 0.01, uncorrected).
DISCUSSION
We have presented results from three analyses of steady state functional connectivity in the working memory and default mode networks. As prior work demonstrated graded load effects in the motor network [Newton et al.,2007], this study suggests that a similar behavior occurs in a cognitive domain (working memory) across a range of cognitive loads. Comparing connectivity measured in the resting state to that measured during steady‐state task performance, these data confirm previously reported increases in steady‐state connectivity within the working memory network across a wider range of conditions. [Hampson et al.,2006a]
Building on the confirmation of dynamic functional connectivity within the working memory network, the second goal of this work was to determine how connectivity changes within the default mode network and potentially between the default mode network and the working memory network in the context of a continuous working memory task. When we focus on average connectivity measured between all pairs of regions belonging to the same network (the PIC, Fig. 3), functional connectivity within both the default mode and the working memory networks increases as a function of load, supporting the view that functional connectivity dynamically changes within cognitive networks according to the cognitive demands present. Figure 3 demonstrates increased synchrony within the default mode network as working memory load increases which is contrary to some previous reports [Fransson2006] that suggest that correlations within the default mode network trend towards zero as cognitive load increases. However, our data replicate previously reported findings of negative correlations between working memory regions and the posterior cingulate cortex (see Fig. 4), a default mode region previously identified. [Fox et al.,2005; Greicius et al.,2003] Our results in the posterior cingulate cortex expand on these findings by suggesting that the relationship between the working memory and default mode networks is affected not just by the presence of a task but also by the task load. Functional connectivity measured under conditions other than rest may provide new information augmenting that obtained from passive subjects alone.
Whether or not these changes in functional connectivity occur linearly, or are subject to a ceiling effect remains unclear. Figure 2 suggests that working memory regions experience load‐related increases in the mean BOLD signal that taper off at very high loads (N = 2; N = 3), an effect that was relatively uniform across working memory regions. This may be the result of drops in the accuracy of task performance during the three back task, which could affect the measurements of steady state functional connectivity at these high loads as well. However, this cannot be confirmed given these data.
However, our data do suggest that load‐related changes in mean activity do not necessarily predict the presence of load‐related changes in connectivity. Load‐related changes in the mean BOLD signal were not observed among most default mode regions (see Fig. 2), while load‐related changes in functional connectivity were observed in the posterior cingulate cortex (see Fig. 4). The results presented in Figure 6 also speak to this point in that they show spatially distinct regions of the posterior cingulate cortex that present load‐related changes in activity and connectivity respectively. Because the current study simply focused on whether mean connectivity fluctuated as a function of load, this spatial mismatch between activity and connectivity load effects could be better supported by future studies more rigorously comparing block designed activation and steady state functional connectivity, potentially including measurements of connectivity variability over time as has been recently suggested. [Chang and Glover,2010]
While we have confirmed the presence of load‐related changes in functional connectivity in the working memory/default mode networks, the logical next step is to detail the origin of load‐related changes in functional connectivity. Presumably, some aspect of the cognitively demanding task is changing the low frequency variance in the measured BOLD signals, though what aspect of the task is responsible for this change remains unclear. Possible explanations include task performance whose variability may increase as a function of load. However, other factors like attention and oddball effects related to target identification ought to be considered as well.
One interpretation of the different locations of activity and connectivity load effects is that there are functional subdivisions within the large region of deactivation generally covering the posterior cingulate cortex. Evidence already exists that there may be at least two areas encompassed by this deactivation including the posterior cingulate cortex and restrosplenial cortex, with the latter lying more posterior and inferior to the other. [Buckner et al.,2008; Kobayashi and Amaral,2007] Evidence in other species suggests that regions of the posterior cingulate cortex modulate their activity based on visual input [Olson and Musil,1992], based on the processing of eye movements [Olson and Musil,1992; Olson et al.,1996; Sikes et al.,1988], and based on spatial orientation/memory and learning [Markowska et al.,1989; Murray et al.,1989]. Continued subdivision along the cingulate gyrus in humans may be possible as well, evidenced by studies of the anterior cingulate cortex. [Margulies et al.,2007] Functional subdivision along the posterior cingulate cortex may be important to understand in light of the large number of studies of the default mode network. [Buckner et al.,2008]
The dynamic nature of changes in our measured functional connectivity may have particular relevance for interpreting the behavior of the default mode network. The basic default mode hypothesis distinguishes between those brain regions that are primarily engaged in the resting state (default mode), and those that are not. Speculations on the underlying cognitive differences between the resting state and other ‘active’ conditions have focused on classifying thoughts into different types. For example, it has been posited that task‐related (default mode) deactivations may be related to switching from introspective, self‐oriented thought to attention demanding, goal‐oriented thought [Gusnard et al.,2001; Raichle et al.,2001]. Fransson suggests that there may be more than one type of cognition being accomplished by the default mode network, with example functions being self‐representation and self‐referential mental events [Fransson,2006], or self‐reflective and self‐referential thought [Fransson2005], though these hypotheses still operate under the assumption that the default mode network is either active or not depending on the type of thought. Figure 4 suggests that functional connectivity may not be modulated in a binary fashion (representing a “turning on” or a “turning off” of the synchronization within a given network), but instead may be modulated in a gradual fashion as cognitive load changes. This may also argue against a simple cessation of one type of thought in favor of another. It is also possible that this changing synchrony between brain regions represents inhibition modulated by a third region of the brain [Greicius et al.,2003], though the role of BOLD synchrony in measuring inhibition is beyond the scope of this study.
While our study highlights the variability of functional connectivity across different cognitive conditions, recent evidence suggests that connectivity is variable within long resting state periods as well [Chang and Glover,2010]. In that study, it was speculated that this variability over resting state acquisitions lasting as long as 15 min may result from unprompted changes in the cognitive state of the subjects. They go on to show that some regions of the brain are more variable than others, providing one explanation for the differences in the variability of connectivity measurements within the networks described in Figure 3 of this study. Our finding that connectivity modulates as a function of working memory load supports their claim that variable cognitive conditions could contribute to variable resting state connectivity. However, our finding also suggests that subtle, uncontrolled changes in cognitive conditions of subjects can be dominated by controlled changes in cognitive demand. Also, our findings of increased synchronization between some regions with increased loads may reflect a more controlled and, therefore less temporally variable brain state as they describe.
The practice of using global time course regression as a standard preprocessing step for correlation analyses (as we have done here) has recently been debated [Murphy et al.,2009]. The purpose of global time course regression is to remove sources of variance that are common to the entire brain that may decrease our sensitivity to underlying, neuronally driven signals. It was argued that regression of the global time course may artificially introduce negative correlations instead of revealing true negative correlations that underlie noise. For this reason, it has been suggested that extra caution be used when interpreting negative correlations between BOLD signals following global time course regression, particularly negative correlations involving signals from the default mode network [Murphy et al.,2009; Weissenbacher et al.,2009]. More recent studies report that the negative correlations between the working memory network and the default mode network have an underlying biological basis [Fox et al.,2009], and are present both before and after model based removal of cardiac and respiratory noise. [Chang and Glover,2009] It has also been asserted that the global time course is truly ‘global’, and thus not overly sensitive to signals from default mode regions [Fox et al.,2009], and that a large portion of its variance can be explained by cardiac and respiratory fluctuations. [Chang and Glover,2009] This suggests that methods employing global time course regression may not inappropriately manipulate the correlations within the brain, but are at least partially regressing out non‐neuronally driven signal variance. Still, the origin of the remaining variance (i.e. unexplained by respiration and cardiac pulsation) in the global time course remains to be determined, and may have implications for how these correlations are interpreted in the future. Measurement of load effects with and without global signal removal in future studies may provide insight into the efficacy of using global signal regression as a preprocessing tool.
Our finding that functional connectivity changes as a function of cognitive demand may also be important for studies developing methods for connectivity measurement in traditional fMRI data, as the temporal dynamics of these connectivity changes remain unclear. Measuring connectivity during traditional fMRI protocols would be useful because successful techniques could greatly increase the amount of candidate data available for connectivity analysis. One method [Fair et al.,2007] uses short segments of steady‐state data from within larger blocks of a condition acquired during a block designed experiment. In this case, our results suggest that functional connectivity would not remain constant as a subject transitions from one condition to another, and some time must be required to achieve the associated load‐related changes in functional connectivity. If the demand induced changes in functional connectivity occur slowly compared to the length of task blocks (i.e. blocks were short relative to the time required for connectivity changes to occur), then estimating connectivity over the entire length of those blocks may produce poor estimates of the true condition dependant functional connectivity. The temporal dynamics of these changes remain unclear and should be investigated further.
This study presents several other opportunities for extension beyond those already mentioned. First, the complicated functional connectivity of the posterior cingulate cortex, and the potential for functional subdivision along the cingulate gyrus warrants further investigation. Second, the relatively low number of subjects used in this study and the low accuracy of the three back performances necessitates further work to confirm whether modulation of functional connectivity tapers off at high cognitive loads, as our data suggest. Last, there is an opportunity to expand our investigation of the functional connectivity of the default mode network beyond just these “central” regions. This expansion may include other regions that are more peripherally associated with the default mode network or may include regions from other networks throughout the brain. This could lead to a better understanding of the underlying functional role played by the default mode network.
CONCLUSIONS
In conclusion, we have demonstrated that functional connectivity within the working memory network is modulated by working memory load (i.e. task condition), culminating in increased synchrony between signals from various working memory regions. Similar results were shown for functional connectivity within the default mode network. Furthermore, our results in the posterior cingulate cortex suggest that the functional connectivity between working memory regions and some default mode regions changes with load as well.
REFERENCES
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME ( 2000): Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging 18: 921–930. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM ( 2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS ( 1997): Simultaneous assessment of flow and BOLD signals in resting‐state functional connectivity maps. NMR Biomed 10( 4–5): 165–170. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH ( 2009): Effects of model‐based physiological noise correction on default mode network anti‐correlations and correlations. Neuroimage 47: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH ( 2010): Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. Neuroimage 50: 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME ( 2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22: 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Curran‐Everett D ( 2000): Multiple comparisons: Philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol 279: R1–R8. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M ( 1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, Tedeschi G, Cirillo S, Goebel R, Di Salle F ( 2006): Independent component model of the default‐mode brain function: Assessing the impact of active thinking. Brain Res Bull 70( 4–6): 263–269. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE ( 2007): A method for using blocked and event‐related fMRI data to study “resting state” functional connectivity. Neuroimage 35: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA ( 1928): The General Sampling Distribution of the Multiple Correlation Coefficient. Proceedings of the Royal Society of London. Series A, Containing Papers Mathematical Physical Character 121:20.
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME ( 2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101: 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P ( 2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44: 2836–2845. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V ( 2008): Persistent default‐mode network connectivity during light sedation. Hum Brain Mapp 29: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT ( 2006a): Brain connectivity related to working memory performance. J Neurosci 26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC ( 2004): Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport 15: 1315–1319. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson B, Gore J ( 2001): Detection of low frequency temporal correlations in steady state and block design data. Neuroimage 13: S144. [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT ( 2006b) : Connectivity‐behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. Neuroimage 31: 513–519. [DOI] [PubMed] [Google Scholar]
- Honey GD, Fu CH, Kim J, Brammer MJ, Croudace TJ, Suckling J, Pich EM, Williams SC, Bullmore ET ( 2002): Effects of verbal working memory load on corticocortical connectivity modeled by path analysis of functional magnetic resonance imaging data. Neuroimage 17: 573–582. [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH ( 2008): Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG‐fMRI study. Hum Brain Mapp 29: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG ( 2008): Macaque monkey retrosplenial cortex. III. Cortical efferents. J Comp Neurol 502: 810–833. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Dzemidzic M, Lurito JT, Mathews VP, Phillips MD ( 2000): Correlations in low‐frequency BOLD fluctuations reflect cortico‐cortical connections. Neuroimage 12: 582–587. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA ( 1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP ( 2007): Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37: 579–588. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Murray EA, Gaffan D ( 1989): A comparative analysis of the role of fornix and cingulate cortex in memory: Rats. Exp Brain Res 74: 187–201. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman‐Rakic P, Shulman RG ( 1994): Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA 91: 8690–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Baese A, Wismueller A, Lange O ( 2004): Comparison of two exploratory data analysis methods for fMRI: Unsupervised clustering versus independent component analysis. IEEE Trans Inf Technol Biomed 8: 387–398. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA ( 2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Davidson M, Gaffan D, Olton DS, Suomi S ( 1989): Effects of fornix transection and cingulate cortical ablation on spatial memory in rhesus monkeys. Exp Brain Res 74: 173–186. [DOI] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Gore JC ( 2007): Task demand modulation of steady‐state functional connectivity to primary motor cortex. Hum Brain Mapp 28: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, Musil SY ( 1992): Posterior cingulate cortex: Sensory and oculomotor properties of single neurons in behaving cat. Cereb Cortex 2: 485–502. [DOI] [PubMed] [Google Scholar]
- Olson CR, Musil SY, Goldberg ME ( 1996): Single neurons in posterior cingulate cortex of behaving macaque: Eye movement signals. J Neurophysiol 76: 3285–3300. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E ( 2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging. Hum Brain Mapp 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohovnik I, Hakansson K, Risberg J ( 1980): Observations on the functional significance of regional cerebral blood flow in “resting” normal subjects. Neuropsychologia 18: 203–217. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2004): Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23: 752–763. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2008): Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex 18: 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC ( 2007): Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes RW, Vogt BA, Swadlow HA ( 1988): Neuronal responses in rabbit cingulate cortex linked to quick‐phase eye movements during nystagmus. J Neurophysiol 59: 922–936. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE ( 2004): Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 22: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C ( 2009): Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 47: 1408–1416. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET ( 2006): Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience 139: 317–325. [DOI] [PubMed] [Google Scholar]


