Abstract
We randomly assigned 23 older adults with Mild Cognitive Impairment to either a high carbohydrate or very low carbohydrate diet. Following the six-week intervention period, we observed improved verbal memory performance for the low carbohydrate subjects (p = 0.01) as well as reductions in weight (p < 0.0001), waist circumference (p < 0.0001), fasting glucose (p = 0.009), and fasting insulin (p = 0.005). Level of depressive symptoms was not affected. Change in calorie intake, insulin level, and weight were not correlated with memory performance for the entire sample, although a trend toward a moderate relationship between insulin and memory was observed within the low carbohydrate group. Ketone levels were positively correlated with memory performance (p = 0.04). These findings indicate that very low carbohydrate consumption, even in the short-term, can improve memory function in older adults with increased risk for Alzheimer’s disease. While this effect may be attributable in part to correction of hyperinsulinemia, other mechanisms associated with ketosis such as reduced inflammation and enhanced energy metabolism also may have contributed to improved neurocognitive function. Further investigation of this intervention is warranted to evaluate its preventive potential and mechanisms of action in the context of early neurodegeneration.
Keywords: ketosis, low carbohydrate diet, memory, MCI, Alzheimer’s disease
1. Introduction
Currently, there are 5.3 million cases of Alzheimer’s disease (AD) with projections of as many as 16 million cases by the year 2050 (Alzheimer’s Association, 2009). There is no remedy for dementia, and it is not clear when or if effective therapy will be developed. Accordingly, prevention and mitigation of risk will be essential to reduce the impact of this ominous public health problem. Mild Cognitive Impairment (MCI) is a clinical construct that identifies individuals with increased risk for dementia and represents the first manifestation of neurodegeneration for a substantial subset of individuals who will progress to AD (Mitchell and Shiri-Feshki, 2009; Petersen, 2004). It has been proposed that interventions initiated in individuals with pre-dementia conditions such as MCI might forestall progression of cognitive decline, and that MCI may represent the final point at which intervention might be effective (Cotman, 2000).
Contemporaneous with the developing dementia epidemic is an epidemic of obesity and associated metabolic disturbance. Currently, 64% of the US adult population is overweight and 34% obese (Flegal et al., 2010). It is projected that by the year 2030, 86% will be overweight and 51% of adults in the US will be obese (Wang, 2008). Likewise, diabetes prevalence is accelerating, particularly in the aging population (National Institute of Diabetes and Digestive and Kidney Diseases, 2008). Hyperinsulinemia, which is a precursor to type 2 diabetes, occurs in more than 40% of individuals aged 60 and older (Craft, 2005; Ford et al., 2002).
The co-occurrence of dementia and metabolic disease reflects the fact that metabolic disturbance is a fundamental factor contributing to neurodegeneration (Craft, 2005). Type 2 diabetes increases the risk for dementia (Biessels and Kappelle, 2005; Xu et al., 2007), and the risk for AD attributable solely to hyperinsulinemia was determined to be as high as 39% in one longitudinal study (Luchsinger et al., 2004). Hyperinsulinemia develops as a compensatory adaptation in the context of insulin resistance to overcome receptor insensitivity and maintain glucose homeostasis. Insulin receptors are densely expressed in brain regions vulnerable to neurodegeneration including the medial temporal lobe and prefrontal cortex, regions mediating long-term memory and working memory. However, insulin must be transported from the periphery because very little is synthesized in the brain. Paradoxically, peripheral compensatory hyperinsulinemia resulting from insulin resistance is associated with central (brain) hypoinsulinemia because of saturation of the blood-brain barrier transport mechanism (Baura et al., 1996; Wallum et al., 1987; Woods et al., 2003), and individuals with Alzheimer’s disease have lower cerebrospinal fluid (CSF) to plasma insulin ratios relative to healthy older adults (Craft et al., 1998). Insulin plays a direct role in regulating pro-inflammatory cytokines and neurotrophic and neuroplastic factors essential to memory function and in the generation and clearance of beta-amyloid from the brain. Accordingly, central hypoinsulinemia can accelerate neurodegenerative processes associated with dysregulation of these factors (Craft et al., 2003; Craft, 2005; Reagan, 2010).
Dietary approaches to dementia prevention represent interesting and under-utilized interventions that offer the possibility of effective, low risk interventions. For example, epidemiological evidence supports the notion that relatively greater consumption of polyphenol-containing fruits and vegetables mitigates risk for neurocognitive decline and dementia in western cultures (Letenneur et al., 2007; Solfrizzi et al., 2003). A substantial body of pre-clinical evidence suggests that such protection may be related to neuronal signaling effects and anti-inflammatory and antioxidant actions (Williams et al., 2008). In addition, certain of these compounds enhance metabolic function (Martineau et al., 2006; Tsuda, 2008), and recent preliminary human data demonstrated memory enhancement with moderate-term berry fruit supplementation in association with improvement in metabolic parameters (Krikorian et al. 2010). Given that hyperinsulinemia has been implicated as a promoter of central inflammation and other aspects of neurodegeneration, dietary interventions aimed specifically at improving metabolic function may influence fundamental neurodegenerative processes.
In the past, the ketogenic diet has been prescribed primarily as a means of suppressing seizures (Vining et al., 1998). However, there are indications that ketone metabolism may be beneficial in other clinical conditions (Gasior et al., 2006; Veech et al., 2004) by protecting against neuronal insults (Noh et al., 2006), increasing metabolic efficiency relative to glucose metabolism (Veech, 2001), and mitigating neurodegenerative mechanisms (Sullivan et al., 2004; Yudkoff et al., 2001). Also, very low carbohydrate diets have been shown to reduce inflammatory factors, including pro-inflammatory mediators associated with neurodegeneration (Forsythe et al., 2008). Indirect support for a neurotrophic effect of ketone metabolism has been observed in ketone feeding studies demonstrating acute cognitive benefit in patients with Alzheimer’s disease (Henderson et al., 2009; Reger et al., 2004).
We sought to undertake an initial assessment of the potential cognitive benefit of ketone metabolism in older adults with mild memory decline and increased risk for neurodegeneration. We administered a short-term dietary intervention involving strict carbohydrate restriction to induce adaptation to ketosis in order to assess effects on neurocognitive function and to determine the feasibility of maintaining this intervention in older adults.
2. Methods
2.1 Participants
The study protocol was approved by the University of Cincinnati Medical Institutional Review Board, and each enrolled participant signed the informed consent document. Older adult men and women were recruited from the greater Cincinnati community with print advertising in the form of flyers posted at senior centers and advertisements placed in the Cincinnati Enquirer, the major daily newspaper. The recruitment ads solicited participation of older adults with mild, acquired memory decline for a dietary intervention study. There was no stipulation in the recruitment material as to weight or metabolic status. We enrolled 23 participants (10 men, 13 women) who had experienced age-related memory decline such as forgetfulness and prospective memory lapses with inefficiencies in everyday activities but not substantial functional decline. The mean (± SD) age of the sample was 70.1 (± 6.2) years, and the mean (± SD) educational level was 15.3 (± 2.8) years.
2.2 Procedure
Prospective participants were assessed with structured interview instruments to determine eligibility for study inclusion. The Academic and Medical History Questionnaire (Krikorian et al., 2004; Krikorian et al., 2010) was used to obtain demographic information and information regarding academic attainment, current and past medical conditions, and medication and substance use. Those with diabetes, substance abuse disorder, or diagnosed psychiatric or neurological condition were excluded as well as those using medications that might affect outcome measures such as benzodiazepine and stimulant drugs. Level of memory impairment as manifested in everyday activities was determined with the Clinical Dementia Rating (CDR), which elicits information from the participant and an informant (typically, spouse or adult child) concerning the nature and extent of cognitive decline at home and in the community (Hughes et al., 1982). The domains memory, orientation, problem solving, community affairs, home activities, and personal care were evaluated, and the ratings for each domain contributed to a global CDR classification with the memory domain weighted most heavily. CDR classifications include no impairment, mild decline, and mild, moderate, and severe dementia. We enrolled individuals with mild decline corresponding to Mild Cognitive Impairment and excluded those with CDR classifications indicating no impairment and dementia. In addition to the global CDR classification, we derived the sum of boxes score from the arithmetic sum of the category ratings across the six domains of functioning, which served as a means of quantifying overall level of functional decline (Lynch et al., 2006).
The primary outcomes included measures of executive ability, long-term memory, and mood and were obtained at pre-treatment baseline and after the sixth week of the intervention. The Trail Making Test part B (Reitan, 1992) was used to assess working memory and set switching aspects of executive ability (Sanchez-Cubillo et al., 2009). In this paper and pencil task subjects were presented with a two dimensional array of randomly arranged digits and letters of the alphabet and asked to alternately sequence the digits and letters by drawing connecting lines with a pencil. Time on task served as the outcome score. We assessed secondary or long-term memory with the Verbal Paired Associate Learning Test (V-PAL; Krikorian, 1996). Paired associate tasks have demonstrated diagnostic utility in early and more advanced Alzheimer’s disease (Spaan et al., 2005). The V-PAL has been shown to be sensitive to developmental performance changes among young, middle-aged, and elderly individuals (Krikorian, 2006) and has been utilized in the context of other intervention studies in this population (Krikorian, 2010). This task calls for the subject to learn novel associations between common one- and two-syllable, semantically unrelated words (e.g., help-years). The V-PAL performance score represents the cumulative number of correct responses summed across four learning trials. Alternate forms of the V-PAL were administered at the baseline and final visits so that the specific test item content was not repeated. Mood was assessed with the Geriatric Depression Scale (GDS; Yesavage, 1983), a 30-item inventory designed to evaluate symptoms of depression in older adults. The GDS has the advantage of being a largely unitary measure of mood and has been used to identify depression among elderly patients with and without cognitive decline (Burke et al., 1989).
We also gathered data on potential mediators of neurocognitive function and of the dietary intervention. We measured waist circumference at the narrowest waist between the lowest rib and iliac crest as well a body weight. Blood samples were obtained after overnight fast for determinations of serum glucose and insulin values by radioimmunoassay and enzymatic assay at the biochemistry laboratory of the General Clinical Research Center. Fasting insulin level tends to be positively correlated with waist circumference, particularly in the elderly (Tabata et al., 2009; Wahrenberg et al., 2005). All subjects also provided urine samples at the baseline and final visits for urinary ketone assessment. These determinations were made with test strips containing sodium nitroprusside for semi-quantitative determination of acetoacetic acid levels. This method was used because of its validity, convenience, and modest subject burden and because it has been utilized as an indicator of adherence to low carbohydrate regimens in several human trials (Cassady et al., 2007; Sharman et al., 2002; Volek et al., 2002; Volek et al., 2004).. Urine reagent strip measures are correlated with serum and capillary ketone determinations and provide detection for concentrations as low as 5 mg/dl (Henday et al., 1997; Rukkwamsuk et al., 2008), levels expected for subjects in the low carbohydrate group
2.3 Dietary Intervention
Consumption was monitored with daily diet diaries throughout the intervention. In addition, subjects completed a seven-day diet diary during the week before the pre-intervention baseline visit in order to establish a record of consumption habits. Subjects received oral and written instruction for estimation of food and beverage portions using a portion poster (Nutrition Consulting Enterprises, Framingham, MA) as well as oral and written instruction for recording quantities of all foods and beverages in the diet record. Diet diary information from the seven-day periods before the baseline and final visits were used to derive pre- and post-intervention data concerning change in calorie and macronutrient intake. A registered dietician reviewed the completed diet records with each participant to clarify ambiguities related to foods listed and portion size. Food records were analyzed for calorie and macronutrient content using the Nutrition Data Systems software (University of Minnesota, Minneapolis, MN).
The prescribed dietary interventions consisted of high carbohydrate (50% of calories) and very low carbohydrate (5% to 10% of calories) diets, the latter intended to induce ketosis. Dietary regimens with 20 g to 50 g carbohydrate per day have been shown to produce a shift to ketone metabolism and are associated with the detectable presence of ketones in urine (Westman et al., 2007). Adaptation to ketone metabolism begins after several hours of carbohydrate restriction. Within three to four days, the brain begins to utilize ketones for energy with full adaptation occurring after two to three weeks (Cahill, 1970; McDonald, 1998). Carbohydrate consumption was the only macronutrient constrained in either diet. Intake of protein and fat was allowed to vary, and total calorie intake was not restricted. Very high levels of fat (90% of total calorie intake) have been prescribed traditionally to induce ketosis for seizure management (Vining, 1998). However, recent trials have indicated that protein restriction is not necessary to achieve ketosis (Boden et al., 2005; Cassady et al., 2007) or effective seizure control (Kossoff, et al., 2003; Kossoff and Dorward, 2008), allowing for a less severe regimen.
We provided dietary education and counseling at the baseline enrollment visit to assist the subjects with practical aspects of the diet. We also maintained weekly contact with subjects throughout the intervention to answer questions and promote adherence to the protocol. Subjects were given information as to the macronutrient constituents of common foods and counseled as to sources of carbohydrate, fat, and protein. We endeavored to represent each dietary approach as potentially providing health benefits. The high carbohydrate diet approximated the macronutrient profile consumed at the time of enrollment for most subjects, which included at least 50% of calories from carbohydrates. Those assigned to the low carbohydrate diet were advised to consume not more than 20 g carbohydrate per day as a target level in order to help insure adaptation to ketone metabolism. Specific foods that might be included or eliminated were discussed in light of information as to food items typically consumed based on the pre-intervention diet diaries and consultation with each subject. We advised the high carbohydrate subjects to consume fruits and vegetables as carbohydrate sources as much as possible. Those in the low carbohydrate diet were restricted from fruit and instructed to limit carbohydrate consumption to small portions of vegetables. All subjects were advised to choose monounsaturated fats when possible, although this was not controlled.
2.4 Statistical Analyses
We performed analysis of covariance (ANCOVA) for the primary outcomes. These analyses isolated the effect of the intervention using the outcome score from the final visit as the dependent measure and the corresponding score from the baseline visit as covariate (Cohen, 1988). Cohen’s f represents the effect size statistic for these analyses, which was computed from the eta squared value and is characterized as small (0.10), medium (0.25), and large (0.40) (Cohen, 1988). We also performed multiple regression analysis and bivariate correlations to investigate contributions of dietary, metabolic, and anthropometric factors to memory performance. Univariate t-tests were used to assess group differences with respect to pre-intervention subject sample characteristics and post-intervention macronutrient parameters.
3. Results
Table 1 contains information concerning the subject sample characteristics. There was no difference between the high and low carbohydrate groups with respect to age, education, level of memory-related functional impairment, depressive symptoms, or anthropometric and metabolic parameters prior to the evaluation. The level of depressive symptoms was well below the threshold for mild depression (Yesavage et al., 1983). Average waist circumference values for each group were high and consistent with base rates for overweight and hyperinsulinemia (Ford et al., 2002; Hans et al., 1995). Mean fasting glucose levels for subjects in both groups were within the normal range (Gabir et al., 2000). However, mean fasting insulin levels were at the upper threshold of the normal laboratory range (15 μU/mL), indicating hyperinsulinemia. As expected, fasting insulin was correlated with waist circumference, r = 0.65, p = 0.01.
Table 1.
Pre-intervention subject sample characteristics
| High carb (n = 11) | Low carb (n = 12) | t(21) | p | |
|---|---|---|---|---|
| Age, years | 71 (8) | 68 (3) | 1.07 | 0.29 |
| Education, years | 15.5 (3) | 15.2 (2) | 0.27 | 0.78 |
| CDR sum boxes | 0.63 (0.3) | 0.75 (0.5) | 0.64 | 0.52 |
| GDS | 5.4 (2) | 6.4 (4) | 0.68 | 0.49 |
| Weight, kg | 79 (18) | 84 (17) | 0.75 | 0.45 |
| Waist, cm | 93 (15) | 99 (16) | 0.81 | 0.42 |
| Glucose, mg/dl | 97 (9) | 95 (9) | 0.51 | 0.61 |
| Insulin, μU/mL | 14.4 (6) | 16.9 (6) | 0.85 | 0.40 |
| Total energy, kcal | 1697 (417) | 1762 (481) | 0.34 | 0.73 |
| Carb, g | 207 (63) | 190 (56) | 0.65 | 0.51 |
| Protein, g | 60 (21) | 64 (17) | 0.42 | 0.67 |
| Fat, g | 61 (24) | 78 (27) | 1.60 | 0.12 |
| Urinary ketone, mg/dl | 0 | 0 | - | - |
Note. CDR = Clinical Dementia Rating. GDS = Geriatric Depression Scale.
High carb = high carbohydrate group. Low carb = low carbohydrate group. Urinary ketone = acetoacetic acid. Data represent mean (SD) values.
There was no baseline group difference with respect to total daily calorie intake or carbohydrate, protein, or fat intake. Ketone bodies were not detected for any subject at the baseline visit.
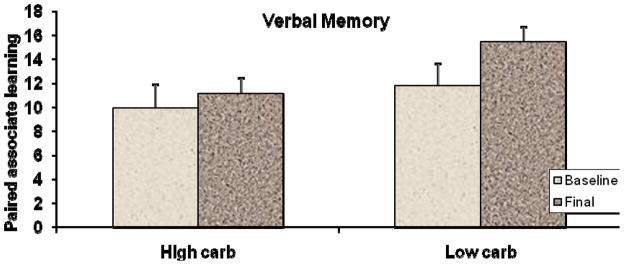
ANCOVA analyses were performed to isolate the effects of the intervention on the main outcome measures. The primary finding indicated improved secondary memory performances for the low carbohydrate subjects. As shown in Figure 1, at six weeks, performance on the paired associate learning task did not change appreciably for the high carbohydrate subjects but improved significantly for the low carbohydrate subjects (11.8 v 14.6, adjusted mean scores), F(1,20) = 6.45, p = 0.01, with a medium effect size, Cohen’s f = 0.26.
Figure 1.
Pre- and post-intervention memory performances for the low and high carbohydrate groups as measured by the Verbal Paired Associate Learning Test (Krikorian, 1996). Values are unadjusted means of the cumulative number of correct immediate recall responses summed across four learning trials. Vertical bars represent standard error. The ANCOVA analysis indicated improved learning for the low carbohydrate subjects, F(1,20) = 6.45, p = 0.01, Cohen’s f = 0.26.
There was no effect of the intervention on the Trail Making Test part B (79.2 s v 82.9 s, adjusted means), F(1,20) = 0.46, p = 0.50). In addition, depressive symptoms as measured by the Geriatric Depression Scale were not affected by the intervention (5.3 v 5.8, adjusted mean scores), F(1,20) = 0.34, p = 0.56.
There were significant changes in anthropometric and metabolic values and in dietary parameters. After the intervention, weight (81 kg v 77 kg, adjusted means), F(1,20) = 30.45, p < 0.0001, Cohen’s f = 0.12, and waist circumference (95 cm v 90 cm, adjusted means), F(1,20) = 15.00, p < 0.001, Cohen’s f = 0.14, were reduced for the low carbohydrate group. Likewise, fasting glucose (96 mg/dl v 86 mg/dl, adjusted means), F(1,20) = 8.40, p = .009, Cohen’s f = 0.49, and fasting insulin values (14.5 μ/mL v 11.9 μ/mL, adjusted means), F(1,20) = 9.88. p = 0.005, Cohen’s f = 0.26, were lower for the low carbohydrate but not high carbohydrate group. Urinary ketone bodies were not detected for the high carbohydrate subjects but were present for the low carbohydrate subjects (Table 2), and ketone body levels were related to memory performance, r = 0.45, p = 0.04.
Table 2.
Post-intervention dietary parameters by group
| High carb | Low carb | t(21) | p | |
|---|---|---|---|---|
| Total energy, kcal | 1592 (395) | 1042 (347) | 3.55 | 0.001 |
| Carb, g | 197 (53) | 34 (18) | 9.94 | < 0.0001 |
| Protein, g | 58 (12) | 67 (19) | 1.32 | 0.20 |
| Fat, g | 61 (24) | 69 (27) | 0.74 | 0.49 |
| Urinary ketone, mg/dl | 0 | 5.4 (3.3) | 4.54 | < 0.001 |
Note: High carb = high carbohydrate group. Low carb = low carbohydrate group.
Urinary ketone = acetoacetic acid. Data represent unadjusted, mean (SD) daily values.
Over the course of the intervention, daily calorie intake declined substantially for the low carbohydrate subjects but only modestly and nonsignificantly for the high carbohydrate group (1599 v 1036, adjusted means), F(1,20) = 13.30, p < 0.001, Cohen’s f = 0.79. Table 2 shows between group comparisons of daily energy and macronutrient intake at study termination. Total calorie intake was significantly lower for the low carbohydrate group. This difference was attributable to the large reduction in daily carbohydrate consumption (197 g v 34 g), while fat and protein intake were slightly but not significantly higher for the low carbohydrate subjects.
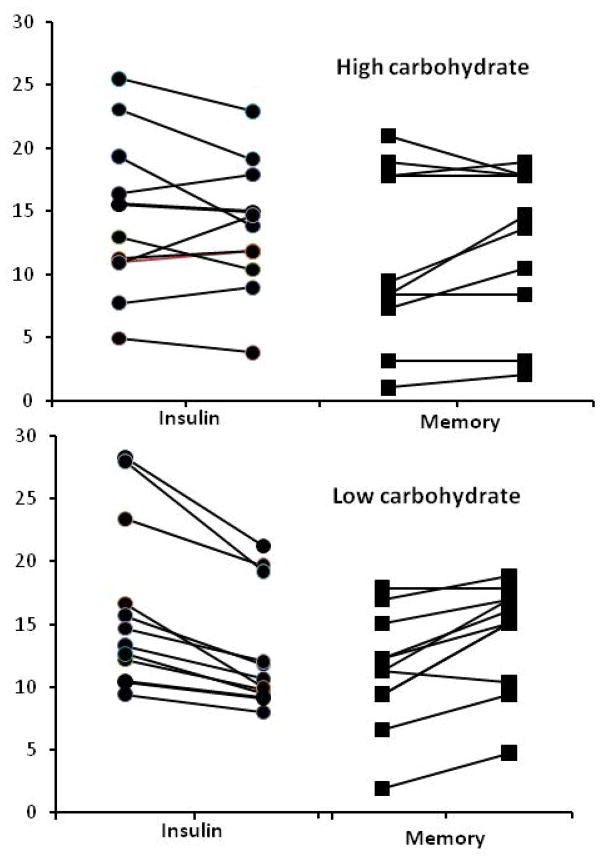
Given the reductions in calorie intake, metabolic parameters, and in anthropometric values, we investigated the possibility that improvement in memory performance might have been associated with changes in these factors with a multiple regression analysis. The overall effect was not significant, Multiple R = .29, p = 0.61. Similarly, the semi-partial correlations indicated weak and nonsignificant relationships to memory performance for each of these factors: change in calories (rsp = −0.16, p = 0.46); change in insulin (rsp = −0.26, p = 0.24); and change in weight (rsp = 0.28, p = 0.20). However, within the low carbohydrate group, the relationship between change in insulin and change in memory performance was stronger although not statistically significant, r = 0.47, p = 0.11. Figure 2 shows the pre- and post-intervention insulin values and memory scores for each subject in the high and low carbohydrate groups. While there was variability across subjects for these factors in each group, the pre- and post-intervention trends toward lower insulin and improved memory performance are more consistently represented for the subjects in the low carbohydrate group.
Figure 2.
Individual values for fasting insulin (μU/mL) and long-term memory performance (paired associate learning score) plotted from left to right to show change from pre-intervention baseline visit to final visit, respectively. Upper panel shows values for the high carbohydrate subjects and the lower panel for low carbohydrate subjects. Variability across the intervention is apparent for insulin and memory for subjects in both groups, although the trends toward lower insulin and higher memory performance are more consistently demonstrated among the low carbohydrate subjects.
4. Discussion
We performed this very low carbohydrate diet trial to investigate effects on neurocognitive function and to assess its feasibility in the older adult population. Our findings indicated improved memory function with a medium effect size in individuals with Mild Cognitive Impairment in response to a relatively brief period of carbohydrate restriction designed to reduce insulin levels and induce ketone metabolism. To our knowledge, these data demonstrate for the first time that carbohydrate restriction can produce memory enhancement in this at-risk population. Medium chain triglyceride feeding has been shown to induce ketone metabolism rapidly with acute memory and functional improvement in MCI and AD (Henderson et al., 2009; Reger et al., 2004); findings which are corroborated by our data and support the notion that ketosis can produce enhancement of neurocognitive function in a brief timeframe. The absence of effects for the Trail Making Test and the Geriatric Depression scale indicated that working memory and executive function and mood were not affected by the intervention and supports the notion of a discrete secondary memory effect. This would be associated with enhanced hippocampal and paraphippocampal function mediating paired associate learning and binding of unrelated semantic items (Badgaiyan et al., 2003). This would be consistent with pre-clinical studies indicating greater neuroprotection and increased energy output in hippocampal tissue in the context of ketone metabolism (Kashiwaya et al., 2000; Noh et al., 2006; Puchowizt et al., 2005; Veech et al., 2001).
A number of mechanisms might be considered with respect to our memory finding. There are indications that central ketone metabolism may confer neurocognitive benefit and mitigate neurodegenerative processes in conjunction with, but also independent of, effects on insulin. Mean fasting insulin levels prior to the intervention indicated that, on average, subjects were hyperinsulinemic. We observed a significant reduction of insulin among the low carbohydrate subjects, suggesting that the memory improvement was related, in part, to increased insulin transport into the CNS as a consequence of correction of peripheral hyperinsulinemia. There was a trend toward a moderate relationship between fasting insulin and memory performance within the low carbohydrate group would be expected to reach statistical significance in a larger sample. It is noteworthy that a recent trial involving 12 weeks’ calorie restriction in a sample of 50 middle-aged and older adults demonstrated improvement in memory function related to change in fasting insulin (Witte et al., 2009). Also, van der Auwere et al. (2005) have shown in a mouse model of Alzheimer’s disease that dietary ketosis by means of carbohydrate restriction maintained for 43 days was associated with reduction of soluable beta-amyloid (Abeta), presumably resulting from increased Abeta clearance mediated by increases in insulin degrading enzyme associated with brain insulin level (Qui et al., 1998). Conversely, in a study involving healthy older adults, induced peripheral hyperinsulinemia acutely decreased CSF levels of Abeta 42, reflecting reduced cerebral clearance (Fishel et al., 2005).
The absence of a strong relationship between insulin reduction and memory improvement suggests that neurocognitive benefit also might be associated with other aspects of the ketotic condition. Ketone metabolism has been shown to protect hippocampal neurons from Abeta toxicity (Kashiwaya et al., 2000), glutamate toxicity and apopotosis (Noh et al., 2006), as well as other insults such as kainic acid (Noh et al., 2003) and hypoxia (Puchowizt et al., 2005). As compared with glucose metabolism, central ketone metabolism generates lower levels of oxidative stress (Prins, 2008) and has been shown to produce greater cellular energy output and antioxidant capacity, the latter by increasing glutathione peroxidase in hippocampal cells (Veech et al., 2001; Ziegler et al,. 2003). In addition, the presence of cerebral ketones is associated with decreased apoptosis and inflammation (Gasior et al., 2006; Malouf et al., 2009), which along with oxidative stress, have been identified as fundamental factors contributing to neurodegeneration (Cotman, 2000). These several benefits for neural function imply that ketosis can mitigate neurodegeneration, and it will be important to demonstrate enhancement of memory function in conjunction with change in these neuroprotective factors in future studies.
We also observed reductions in weight, waist circumference and fasting insulin and glucose. Given the relatively brief duration of the intervention and the small sample size, these effects are notable, especially given the medium effect sizes for the memory and metabolic findings. However, in the context of the relatively strong group effects, it should be noted that the intervention-induced changes both with respect to insulin and memory were variable across subjects (Figure 2), suggesting that individual responses may have been moderated by other, unmeasured factors.
Reduction in calorie intake is a common observation in studies utilizing the low carbohydrate approach and typically occurs as a consequence of the reduction of carbohydrate intake exclusively (Boden et al., 2005), as was the case in our trial. Several factors inherent in the low carbohydrate intervention likely contributed to the large calorie decrement exhibited in the low carbohydrate group. The relative greater percentage of protein consumption associated with exceedingly low carbohydrate intake has been related to increased thermogenesis and metabolic advantage (Feinman and Fine, 2004; Lejeune et al., 2006) as well as to greater satiety and reduced overall consumption (Weigle et al., 2005; Westerterp-Plantenga et al., 2006). Satiety also has been associated with improved central insulin sensitivity (Bruning et al., 2000). In human trials, higher insulin levels have been associated with increased consumption and lower levels with reduced consumption (Holt and Miller, 1995; Rodin et al., 1985).
It is noteworthy that the behavioral demands of the low carbohydrate intervention were maintained by our older adult subjects. The dietary intervention involved carbohydrate restriction only, as opposed to the high fat, low protein, and low carbohydrate approach traditionally prescribed for seizure control. While carbohydrate restriction itself can be difficult to maintain in western cultures, the fact that benefit was obtained without the additional burden of protein restriction and very high fat intake increases the probability that this intervention can be applied effectively. However, there may be concern regarding long-term application of severe carbohydrate restriction. Aside from the burden of dietary constraint, very low carbohydrate intake will reduce consumption of fiber and phytonutrients de facto, resulting in loss of attendant health benefits. In general, weight loss in low carbohydrate interventions tends to be restricted to loss of body fat as opposed to lean mass (Volek et al., 2002) but not in all studies (Meckling et al., 2004). This may be a prominent issue for older adults for whom there is substantial risk of sarcopenia. There also may be concern regarding kidney stress related to increase protein intake. Protein intake among our low carbohydrate subjects was, on average, 0.83 g/kg, at the standard recommended level and well below the recommended limit of 2 to 2.5 g/kg based on the hepatic urea synthesis limit of 2.6 to 3.6 g/kg (Bilsborough and Mann, 2006). Several controlled trials and epidemiological investigations have indicated that relatively high protein intake is not associated with kidney dysfunction in subjects without existing kidney disease (Knight et al., 2003; Poortmans and Dellalieux, 2000; Skov et al., 1999; Wrone et al., 2003). However, again, these studies generally were performed with younger subjects and more information is needed with respect to aging subjects for whom metabolic disturbance and related kidney dysfunction will be more prevalent.
In general, the subjects participating in the low carbohydrate intervention tolerated the intervention well and, as noted, there was no increase in mood symptoms. Nonetheless, there were disparate responses and attitudes toward the intervention. Most were pleased with the weight loss aspect and found this to be motivating. On the other hand, maintaining the daily diet diaries was perceived as burdensome. A few were relieved when the trial was over and mentioned cravings for bread and fruit that had persisted throughout. However, several mentioned improved sense of well being characterized as having more mental energy and feeling more clear-headed. Just one subject expressed the wish to continue with a low carbohydrate approach beyond the end of the intervention, albeit with less severe restriction.
The use of urinary reagent strips was necessary in this study given the study design and resource limitations. The robust anthropometric findings and the diet records support the validity ketone measurements obtained in this study. In addition, this method has been utilized successfully in a number of other trails involving carbohydrate restriction (Cassady et al., 2007; Sharman et al., 2002; Volek et al., 2002; Volek et al., 2004). However, there are indications that reagent strips may be less sensitive than blood-based methods specifically for detection of pathologically high ketone body levels observed in the context of metabolic disorders (Taboulet et al., 2007). This would not be an issue for nondiabetic individuals who will experience controlled increase in ketone bodies in response to carbohydrate restriction. However, because urine ketone strips are susceptible to reduced sensitivity under certain conditions, their use represents a potential methodological limitation. It will be important in future research to corroborate findings obtained with reagent strips measuring acetoacetic acid with plasma or capillary assays of ß-hydroxybutyrate (Guerci et al., 2003) to evaluate reliability and validity of these measures in the older adult population, particularly given our observation of individual response variation and the generally greater risk for metabolic disturbance with aging.
On balance, these preliminary data provide evidence that dietary ketosis by means of carbohydrate restriction can provide neurocognitive benefit for older adults with early memory decline and increased risk for neurodegeneration. Replication of our findings in larger samples will be essential to evaluate the preventive potential of this intervention more comprehensively. Also, demonstrating and distinguishing mechanisms of neural action, including metabolic and neuroprotective factors in conjunction with neurocognitive effects, will be of particular interest and should have implications for understanding the etiology of neurodegeneration. A prominent issue will be investigation of whether there is persistence of benefit beyond the period of active intervention. Should this approach prove to be effective and to have benefit beyond the period of intervention, it might be applied briefly and intermittently as a prophylactic strategy, an approach that would mitigate many concerns about chronic, severe carbohydrate restriction.
Acknowledgments
Funding for this research was provided by grants from the Robert C and Veronica Atkins Foundation and the NIH National Institute on Aging Indiana Alzheimer’s Disease Center.
Footnotes
Disclosure Statement
None of the authors has a conflict of interest influencing this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marcelle D Shidler, Email: marcelle.shidler@uc.edu.
Krista Dangelo, Email: krista_dangelo@yahoo.com.
Sarah C Couch, Email: sarah.couch@uc.edu.
Stephen C Benoit, Email: stephen.benoit@uc.edu.
Deborah J Clegg, Email: deborah.clegg@utsouthwestern.edu.
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2009;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Schacter DL, Alpert NM. Priming of new associations: a PET study. Neuroreport. 2003;14:2475–2479. doi: 10.1097/00001756-200312190-00036. [DOI] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Kaiyala K, Porte D, Kahn SE, Schwartz MW. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45:86–90. doi: 10.2337/diab.45.1.86. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kappelle LJ. Increased risk of Alzheimer’s disease in type II diabetes. Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- Bilsborough S, Mann N. A review of issues of dietary protein intake in humans. Int J Sport Nutr Exerc Metab. 2006;16:129–152. doi: 10.1123/ijsnem.16.2.129. [DOI] [PubMed] [Google Scholar]
- Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucoselevels, and insulin resistance in obese patients with Type 2 Diabetes. Ann Intern Med. 2005;142:403–411. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2121. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Houston MJ, Boust SJ, Rocaforte WH. Use of the Geriatric Depression Scale in dementia of the Alzheimer type. J Am Geri Soc. 1989;37:856–860. doi: 10.1111/j.1532-5415.1989.tb02266.x. [DOI] [PubMed] [Google Scholar]
- Cahill G. Starvation in man. New Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cassady BA, Charboneau NL, Brys EE, Crouse KA, Beitz DC, Wilson T. Effects of low carbohydrate diets high in red meats or poultry, fish, and shellfish on plasma lipids and weight loss. Nutr Met. 2007;4:23. doi: 10.1186/1743-7075-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; New York: 1988. [Google Scholar]
- Cotman CW. Homeostatic processes in brain aging: The role of apoptosis, inflammation, and oxidative stress in regulating healthy neural circuitry in the aging brain. In: Stern P, Carstensen L, editors. The aging mind: Opportunities in cognitive research. National Academy Press; Washington DC: 2000. pp. 114–143. [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer’s disease: Age- and obesity related effect on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26S:S65– S69. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: Interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- de Mello VD, Zelmanovitz T, Perassolo MS, Azevedo MJ, Gross JL. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr. 2006;83:1032–1038. doi: 10.1093/ajcn/83.5.1032. [DOI] [PubMed] [Google Scholar]
- Feinman RD, Fine EJ. “A calorie is a calorie” violate the second law of thermodynamics. Nutr J. 2004;3:9. doi: 10.1186/1475-2891-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and β-amyloid in normal adults. Arch Neurol. 2005;62:1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults. J Amer Med Assoc. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. J Amer Med Assoc. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carhohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–1112. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JL, Zelmanovitz T, Moulin CC, de Mello V, Perassolo M, Leitao C, Hoefel A, Paggi A, Azevedo MJ. Effect of a chicken-based diet on renal function and lipid profile in patients with type 2 diabetes: a randomized crossover trial. Diabetes Care. 2002;25:645–651. doi: 10.2337/diacare.25.4.645. [DOI] [PubMed] [Google Scholar]
- Guerci B, Benichou M, Floriot M, Bohme P, Fougnot S, Franck P, Drouin P. Accuracy of an electrochemical sensor for measuring capillary blood ketones by fingerstick samples during metabolic deterioration after continuous subcutaneous insulin infusion interruption in type 1 diabetic patients. Diebetes Care. 2003;26:1137–1141. doi: 10.2337/diacare.26.4.1137. [DOI] [PubMed] [Google Scholar]
- Hans TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:140–145. doi: 10.1136/bmj.311.7017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind placebo-controlled, multicenter trial. Nutr Metab. 2009;3:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendey GW, Schwab T, Soliz T. Urine ketone dip test as a screen for ketonemia in diabetic ketoacidosis and ketosis in the emergency department. Ann Emerg Med. 1997;29:735–738. doi: 10.1016/s0196-0644(97)70193-8. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Brit J Psychia. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-β-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97:5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–1791. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Dorward JL. The modified Atkins diet. Epilepsia. 2008;49S:37–41. doi: 10.1111/j.1528-1167.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- Krikorian R, Shidler MD, Nash TA, Kalt W, Vinqvist-Tymchuk MR, Shukitt-Hale B, Joseph JA. Blueberry supplementation improves memory in older adults. J Agr Food Chem. 2010;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R, Zimmerman ME, Fleck DE. Inhibitory control in Obsessive- Compulsive Disorder. Brain Cognition. 2004;54:257–259. doi: 10.1016/j.bandc.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Krikorian R. Independence of verbal and spatial paired associate learning. Brain Cognition. 1996;32:219–223. [Google Scholar]
- Krikorian R. Cognitive changes in perimenopause. In: Liu J, Gass M, editors. Management of the perimenopause. McGraw-Hill; New York: 2007. pp. 57–74. 2006. [Google Scholar]
- Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94. doi: 10.1093/ajcn/83.1.89. [DOI] [PubMed] [Google Scholar]
- Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JG, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Ming-Xiu T, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- Lynch CA, Walsh C, Blanco A, Moran M, Coen RF, Walsh JB, Lawlor BA. The clinical dementia rating sum of box score in mild dementia. Dement Geriatr Cogn. 2006;21:40–43. doi: 10.1159/000089218. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, Le PM, Prentki M, Bennett SA, Arnason JT, Haddad PS. Anti-diagetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13:612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- McDonald L. The ketogenic diet. McDonald; Austin, TX: 1998. [Google Scholar]
- Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiat Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Mechling KA, O’Sullivan C, Saari D. Comparison of low-fat diet to a low-carbohdyrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endo Met. 2004;89:2717–2723. doi: 10.1210/jc.2003-031606. [DOI] [PubMed] [Google Scholar]
- Möllstein AV, Dahlquist GG, Stattin EL, Rudberg S. Higher intakes of fish protein are related to a lower risk of microalbuminuria in young Swedish type 1 diabetic patients. Diabetes Care. 2001;24:805–810. doi: 10.2337/diacare.24.5.805. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health; 2008. [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Noh HS, Hah Y, Nilufar R, Han J, Bong J, Kang S, Cho G, Choi W. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J Neurosci Res. 2006;83:702–709. doi: 10.1002/jnr.20736. [DOI] [PubMed] [Google Scholar]
- Pecis M, de Azevedo MJ, Gross JL. Chicken and fish diet reduces glomerular hyperfiltration in IDDM patients. Diabetes Care. 1994;17:665–672. doi: 10.2337/diacare.17.7.665. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cerebr Blood F Met. 2008;28:1–16. doi: 10.1038/sj.jcbfm.9600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poortmans JR, Dellalieux O. Do regular high protein diets have potential health risks on kidney function in athletes? Int J Sport Nutr Exerc Metab. 2000;10:28–38. doi: 10.1123/ijsnem.10.1.28. [DOI] [PubMed] [Google Scholar]
- Puchowicz MA, Emancipator DS, Xu K, Mangess Dl, Ndubuizu OI, Lust WD, LaManna JC. Adaptation to chronic hypoia during diet-induced ketosis. Adv Exp Med Biol. 2005;566:51–57. doi: 10.1007/0-387-26206-7_8. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Insulin-mediated neuroplasticity in the central nervous system. In: Craft S, Christen Y, editors. Diabetes, insulin and Alzheimer’s disease. Springer-Verlag; Berlin: 2010. [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of β-hydroxbutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Train Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. [Google Scholar]
- Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34:826–831. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- Rukkwamsuk T, Suksiri J, Chutiyanawat N, Kaewsakhorn N, Rungruang S. Relationship between the sodium nitroprusside test for ketone bodies in urine and serum β-hydroxybutyrate concentrations in dairy cows. Kasetsart J (Nat Sci) 2008;42:457–462. [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsycholo Soc. 2009;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Sharman JJ, Kraemer WJ, Love DM, Avery NG, Gomez AL, Scheett TP, Volek JS. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002;132:1879–1885. doi: 10.1093/jn/132.7.1879. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003;110:95–110. doi: 10.1007/s00702-002-0766-8. [DOI] [PubMed] [Google Scholar]
- Spaan PJ, Raaijmakers JG, Jonkers C. Early assessment of dementia: The contribution of different memory components. Neuropsychology. 2005;19:629–640. doi: 10.1037/0894-4105.19.5.629. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepsion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Tabata S, Yoshimitsu S, Hamachi T, Hiroshi A, Ohnaka K, Kono S. Waist circumference and insulin resistance: a cross-sectional study of Japanese men. BMC Endocrine Dis. 2009;9:1. doi: 10.1186/1472-6823-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboulet P, Deconinck N, Thurel A, Haas L, Manamani J, Porcher R, Schmit C, Fontaine J-P, Gautier J-F. Correlation between urine ketones (acetoacetate) and capillary blood ketones (3-beta-hydroxybutyrate) in hyerglycaemic patients. Diab Met. 2007;33:135–139. doi: 10.1016/j.diabet.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tsuda T. Regulation of adipocyte function by anthocyanins; Possibility of preventing the metabolic syndrome. J Agr Food Chem. 2008;56:642–646. doi: 10.1021/jf073113b. [DOI] [PubMed] [Google Scholar]
- Skov AR, Toubro S, Bulow J, Krabbe K, Parving HH, Astrup A. Changes in renal function during weight loss induced by high vs. low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord. 1999;23:170–177. doi: 10.1038/sj.ijo.0801048. [DOI] [PubMed] [Google Scholar]
- Van der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab. 2005;2:28. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF. Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–247. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies. Prostag Leukotr ESS. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55:1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- Volek JS, Sharman MlJ, Love DM, Avery NG, Gomez AL, Scheett TP, Kraemer WJ. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism. 2002;51:864–870. doi: 10.1053/meta.2002.32037. [DOI] [PubMed] [Google Scholar]
- Volek JS, Sharman MJ, Gomez AL, Judelson DA, Rubin MR, Watson G, Sokmen B, Silvestre R, French DN, Draemer WJ. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Met. 2004;1:13. doi: 10.1186/1743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volek JS, Sharman MJ, Forsythe CE. Modification of lipoproteins by very low-carbohydrate diets. J Nutr. 2005;135:1339–1342. doi: 10.1093/jn/135.6.1339. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Obesity. 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- Wahrenberg H, Hertel K, Leijonhufvud B, Perrson L, Toft E, Arner P. Use of waist circumference to predict insulin resistance: retrospective study. BMJ. 2005;330:1363–1364. doi: 10.1136/bmj.38429.473310.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz P, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocr Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- Weigle DS, Breen PA, Matthys CC, Callahan JS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Luscombe-Marsh N, Legeune MP, Diepvens K, Neiuwenhuizen A, Engelen MP, Duetz NE, Assout-Marniche D, Tome D, Westerterp KR. Dietary protein, metabolism, and body-weight regulation: dose-response effects. Int J Obesity. 2006;30:S16–S23. [Google Scholar]
- Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, Yancy WS, Phinney SD. Low-carbohydrate nutrition and metabolism. Am J Clin Nutri. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer J. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Bio Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Baskin DG, Schwartz M. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. PNAS. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrone EM, Carnethon MR, Palaniappan L, Fortmann SP. Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580–587. doi: 10.1053/ajkd.2003.50119. [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56:211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL. Development and validation of a geriatric depression rating scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Grunstein R, Nissim I. Ketogenic diet, amino acid metabolism and seizure control. J Neurosci Res. 2001;66:931–940. doi: 10.1002/jnr.10083. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Tibeiro LC, Hagenn M, Siqueira IR, Araujo E, Torres IL. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem Res. 2003;28:1793–1797. doi: 10.1023/a:1026107405399. [DOI] [PubMed] [Google Scholar]