Abstract
Markers of GABA neurotransmission between chandelier neurons and their synaptic targets, the axon initial segment (AIS) of pyramidal neurons, are altered in the dorsolateral prefrontal cortex (DLPFC) of subjects with schizophrenia. For example, immunoreactivity for the GABA membrane transporter (GAT1) is decreased in presynaptic chandelier neuron axon terminals, whereas immunoreactivity for the GABAA receptor α2 subunit is increased in postsynaptic AIS. These alterations are most marked in cortical layers 2–3. In addition, other determinants of the function of chandelier cell-pyramidal neuron synapses, such as ankyrin-G (which regulates the recruitment of sodium channels to the AIS), are also selectively altered in superficial layer pyramidal neurons in subjects with schizophrenia. Each of these components of chandelier cell-pyramidal neuron connectivity exhibits distinctive developmental trajectories in the primate DLPFC, suggesting that disturbances in these trajectories could contribute to the pathogenesis of schizophrenia. Recent findings that inputs from neocortical chandelier neurons are excitatory provide new ideas about the role of this circuitry in the pathophysiology of cortical dysfunction in schizophrenia.
Keywords: ankyrin-G, axon initial segment, GABA, prefrontal cortex
Schizophrenia is a devastating illness that afflicts 0.5–1% of the world's population (Lewis and Lieberman, 2000). Affected individuals frequently come to clinical attention during late adolescence or early adulthood, 5–10% eventually die by suicide, and most experience a lifetime of disability (Lewis and Sweet, 2009). As a result, schizophrenia ranks as one of the leading causes of years of life lost to disability and premature mortality (Insel and Scolnick, 2006).
Among the clinical manifestations of schizophrenia, deficits in cognitive abilities appear to be the core features of the illness (Elvevag and Goldberg, 2000). For example, a characteristic pattern of cognitive deficits occurs with high frequency in individuals with schizophrenia, is relatively stable over the course of the illness, is independent of the psychotic symptoms of the disorder, and is present in a milder form in individuals at genetic risk who do not become clinically ill (Gold, 2004). Furthermore, the degree of cognitive impairment is the strongest determinant of functional outcome for individuals with schizophrenia (Green, 1996).
Of the different domains of cognitive dysfunction in schizophrenia, working memory, the ability to transiently maintain and manipulate a limited amount of information in order to guide thought or behavior, has been studied extensively. Working memory function, especially the ability to manipulate transiently stored information, has been consistently shown to be disturbed in subjects with schizophrenia (Cannon et al., 2005; Tan et al., 2005), and this dysfunction is accompanied by altered activation of the dorsolateral prefrontal cortex (DLPFC) (Van Snellenberg et al., 2006). Altered DLPFC activity during such cognitive tasks might be specific to the disease process of schizophrenia because these disturbances are present in medication-naïve individuals with schizophrenia, but are absent (or seen only to a milder degree) in subjects with other psychotic disorders or major depression (Barch et al., 2003; MacDonald, III et al., 2005). Furthermore, a lag in the development of working memory was present in children who were diagnosed with schizophrenia as adults, whereas children who later developed recurrent depression did not exhibit these premorbid cognitive disturbances (Reichenberg et al., 2010).
Markers of GABA neurotransmission are altered in DLPFC in schizophrenia
Substantial evidence indicates that cortical GABA neurotransmission is altered in schizophrenia, and that these disturbances are prominent in the chandelier cell subpopulation of GABA neurons. Early postmortem studies of schizophrenia revealed evidence of decreased GABA synthesis (Bird et al., Lancet, 1978) and uptake (Simpson et al., 1989), and increased binding to GABAA receptors in the neocortex (Benes et al., 1996; Hanada et al., 1987). Studies conducted over the past decade, using DNA microarray, real-time quantitative PCR or in situ hybridization, have consistently found reduced levels of the transcript for the 67 kilodalton isoform of glutamic acid decarboxylase (GAD67), the enzyme responsible for most GABA synthesis (Asada et al., 1996; Asada et al., 1997), in the DLPFC of subjects with schizophrenia (Akbarian et al., 1995; Duncan et al., 2010; Guidotti et al., 2000; Hashimoto et al., 2005; Hashimoto et al., 2008a; Hashimoto et al., 2008b; Mellios et al., 2009; Mirnics et al., 2000; Straub et al., 2007; Vawter et al., 2002; Volk et al., 2000). Although these studies show a remarkable uniformity in demonstrating lower mean GAD67 levels in subjects with schizophrenia relative to comparison subjects, the variance of GAD67 levels across schizophrenia subjects suggests the possibility of different subtypes of schizophrenia, with a large proportion of subjects having low GAD67 expression, but with smaller subgroups characterized by normal, or perhaps even elevated, levels of GAD67.
Importantly, the deficit in GAD67 mRNA expression in schizophrenia is not restricted to the DLPFC. For example, we recently reported similar reductions in GAD67 mRNA in the DLPFC, anterior cingulate cortex, primary motor cortex and primary visual cortex in the same subjects with schizophrenia (Hashimoto et al., 2008b), suggesting that GABA synthesis is altered in a similar manner across neocortical regions that markedly differ in cytoarchitecture, connectivity and function. Furthermore, reductions in GAD67 mRNA have been found in orbital frontal, superior temporal and anterior cingulate cortices in other subject cohorts (Impagnatiello et al., 1998; Thompson et al., 2009; Woo et al., 2004). Thus, disturbances in GABA neurotransmission could represent a common pathophysiology for different domains of cortical dysfunction in schizophrenia, raising the possibility that pharmacological agents with the appropriate specificity for certain GABA-related targets might be effective for a range of clinical features in the illness.
At the cellular level, the expression of GAD67 mRNA was not detectable in ~25–35% of GABA neurons in layers 2–5 of the DLPFC of subjects with schizophrenia, but the remaining GABA neurons exhibited normal levels of GAD67 mRNA (Akbarian et al., 1995; Volk et al., 2000). Furthermore, levels of the mRNA for the GABA membrane transporter (GAT1), a protein responsible for reuptake of released GABA into nerve terminals, was also decreased (Ohnuma et al., 1999) in a similar minority of GABA neurons (Volk et al., 2001). These findings suggest that both the synthesis and re-uptake of GABA are lower in a subset of DLPFC neurons in schizophrenia.
GABA neurons of the chandelier subclass are affected in schizophrenia
As in other cortical regions, subclasses of inhibitory GABA neurons in the primate DLPFC can be distinguished on the basis of a number of molecular, electrophysiological and anatomical properties. For example, the calcium-binding proteins, parvalbumin (PV) and calretinin, and the neuropeptide somatostatin are, with a few exceptions, expressed in separate populations of GABA neurons in the primate neocortex (Condé et al., 1994; DeFelipe, 1997; Gabbott and Bacon, 1996). In the monkey DLPFC, these subtypes exhibit different membrane firing properties and their axons have different arborization patterns and synaptic targets (Krimer et al., 2005; Zaitsev et al., 2005; Zaitsev et al., 2009). For example, the axon terminals of PV-containing chandelier and basket neurons principally target the axon initial segments (AIS) and cell body/proximal dendrites, respectively, of pyramidal neurons (Melchitzky et al., 1999; Williams et al., 1992), the somatostatin-containing Martinotti cells innervate the distal dendrites of pyramidal neurons (DeLima and Morrison, 1989; Kawaguchi and Kubota, 1996; Ma et al., 2006), and the calretinin-containing double-bouquet cells tend to synapse on the dendrites of other GABA cells (Melchitzky et al., 2005).
The affected GABA neurons in schizophrenia include the PV-positive neurons which comprise ~25% of GABA neurons in the primate DLPFC. For example, in individuals with schizophrenia the expression level of PV mRNA is reduced, although the number of PV neurons appears to be unchanged (Woo et al., 1997a); in addition, ~50% of PV mRNA-containing neurons lack detectable levels of GAD67 mRNA (Hashimoto et al., 2003). In contrast, the ~50% of GABA neurons in the primate DLPFC that express calretinin appear to be unaffected in schizophrenia (Hashimoto et al., 2003).
In the DLPFC of subjects with schizophrenia, GAT1 immunoreactivity appears to be preferentially reduced in the characteristic axon terminals (cartridges) of the chandelier class of PV-containing neurons (Woo et al., 1998). In the postsynaptic targets of these axon cartridges, the AIS of pyramidal neurons, immunoreactivity for the GABA-A receptor α2 subunit (which is present in most GABA-A receptors in AIS (Nusser et al., 1996)) is markedly increased in schizophrenia (Volk et al., 2002). These changes appear to be specific to the disease process of schizophrenia because they are not found in subjects with other psychiatric disorders or in monkeys exposed chronically to antipsychotic medications (Hashimoto et al., 2003; Volk et al., 2000; Volk et al., 2001; Volk et al., 2002).
The pre- and postsynaptic changes in GAT1 and GABA-A receptor α2 subunit immunoreactivity are inversely correlated (Volk et al., 2002), suggesting that they index the degree of alteration in GABA neurotransmission at the chandelier cell inputs to pyramidal neurons. Consistent with this interpretation, several lines of evidence suggest that the reductions in pre-synaptic GABA markers (GAT1 and PV) and increased post-synaptic GABA-A receptors could be compensatory responses to a deficit in GABA release from chandelier neurons. For example, PV is a slow calcium buffer that does not affect the amplitude, but accelerates the decay, of Ca2+ transients in GABA nerve terminals (Collin et al., 2005; Muller et al., 2007). Thus, PV decreases the residual Ca2+ levels that normally accumulate in nerve terminals and facilitate GABA release during repetitive firing (Collin et al., 2005). Studies in PV-deficient mice have demonstrated that a decrease in PV increases residual Ca2+ and favors synaptic facilitation (Collin et al., 2005; Vreugdenhil et al., 2003). Similarly, the blockade of GABA reuptake via GAT1 prolongs the duration of IPSCs when synapses located close to each other are activated synchronously (Overstreet and Westbrook, 2003); the resulting prolongation of IPSCs increases the probability of IPSC summation and enhances the total efficacy of IPSC trains. The up-regulation of the post-synaptic GABA-A receptors that contain α2 subunits would be expected to increase the efficacy of the GABA that is released from chandelier neurons. Thus, the combined reduction of PV and GAT1 proteins in chandelier cell axon cartridges, and the up-regulation of post-synaptic GABA-A receptors, would act synergistically to increase the efficacy of GABA neurotransmission at pyramidal neuron AIS during the types of repetitive neuronal activity associated with working memory. Although the persistence of cognitive impairments in individuals with schizophrenia suggests that these compensatory changes in GABA neurotransmission from chandelier neurons are insufficient to restore normal function, they do suggest that augmenting these responses might represent a novel therapeutic strategy for the treatment of cortical dysfunction in schizophrenia. Consistent with this hypothesis, a novel compound that enhances GABA neurotransmission at GABA-A receptors containing α2 subunits was associated with improved cognitive performance in subjects with schizophrenia (Lewis et al., 2008) and in an animal model of the cognitive deficits of schizophrenia (Castner et al., 2010) in proof-of-concept studies. However, it is important to note that existing methods have not yet permitted a direct demonstration that GAD67 mRNA expression is lower in chandelier neurons in schizophrenia; that is, it is possible that reduced GAD67 is restricted to the basket cell population of PV neurons.
Plasticity of the axon initial segment is altered in schizophrenia
Understanding the nature and functional significance of the pre- and postsynaptic alterations at chandelier cell-pyramidal cell connectivity in schizophrenia depends, at least in part, on other processes that regulate GABA neurotransmission at pyramidal neuron AIS. For example, the 480- and 270-kDa isoforms of ankyrin-G, members of a class of adaptor molecules that link various membrane proteins to the cytoskeleton, are localized to AIS (and nodes of Ranvier) of certain neurons, including cortical pyramidal neurons (Kordeli et al., 1995; Susuki and Rasband, 2008). In the cerebellum, ankyrin-G interacts with the neuronal cell adhesion molecule neurofascin to recruit and stabilize GABA synapses at the AIS of Purkinje cells (Ango et al., 2004). Binding to ankyrin G is also essential for the localization of many other membrane proteins to the AIS (Susuki and Rasband, 2008), including the voltage-gated Na+ channels that are required for action potential generation (Zhou et al., 1998). The cytoskeletal protein ßIV spectrin is also localized to the AIS of pyramidal neurons through its direct interaction with ankryin-G (Yang et al., 2007) and is a critical component in the maintenance of membrane structure and molecular organization (Lacas-Gervais et al., 2004), and thus the stability (Yang et al., 2007), of AIS.
In human DLPFC, immunoreactivity for both ankyrin-G and ßIV spectrin-SD is prominently localized to the AIS of pyramidal neurons in layers 2–6 (Cruz et al., 2009b). The density of ankyrin-G-immunoreactive (IR) AIS was significantly decreased in DLPFC layers 2–3 of subjects with schizophrenia relative to both matched normal comparison subjects and subjects with major depressive disorder (Cruz et al., 2009b). These differences were not attributable to other factors such as sex, the use of antipsychotic, benzodiazepine or antidepressant medications at the time of death, a history of a substance use disorder or other clinical features. In contrast, the density of ankyrin-G-IR AIS in layers 5–6 did not differ across subject groups, and the density of βIV spectrin-SD-IR AIS did not differ across subject groups in either the superficial or deep cortical layers. Because ankyrin-G is essential for the recruitment of sodium channels to the AIS (Yang et al., 2007), schizophrenia may be associated with a lower number of sodium channels at the AIS and a reduced capacity of layer 2–3 pyramidal neurons to generate action potentials.
The laminar specificity of the ankyrin-G-IR AIS alterations in schizophrenia matches that of the changes in GAT1-IR chandelier cell axon cartridges (Pierri et al., 1999) and AIS immunoreactive for the GABA-A receptor α2 subunit (Volk et al., 2002) described above which were also more prominent in the superficial than the deep layers of the DLPFC. Consistent with these findings, pyramidal neurons in layer 3, but not those in layers 5 or 6, have smaller somal volumes and lower spine density in both the DLPFC (Garey et al., 1998; Glantz and Lewis, 2000; Kolluri et al., 2005; Pierri et al., 2001; Rajkowska et al., 1998) and auditory cortex (Sweet et al., 2003; Sweet et al., 2004; Sweet et al., 2009). Interestingly, the densities of AIS immunoreactive for ankyrin-G (Cruz et al., 2009b) and for the GABAA receptor α2 subunit (Volk et al., 2002) in the superficial layers had a significant inverse correlation in schizophrenia subjects (r= −.690, p= .006), but not in control subjects (r= −.229, p= .430), suggesting that a common factor may contribute to the changes in both ankyrin-G and the GABA-A receptor α2 subunits at AIS in DLPFC layers 2–3 in subjects with schizophrenia. For example, if GABAergic innervation is required for the localization of ankyrin-G and the formation of the AIS (Ango et al., 2004; Hedstrom et al., 2008), then lower GABA neurotransmission from chandelier cells (due to a deficit in expression of GAD67) to pyramidal cell AIS would be reflected in both decreased ankyrin-G and a compensatory increase in GABA-A receptors containing the α2 subunit.
In concert, the findings of pre- and postsynaptic alterations at chandelier cell inputs to pyramidal neurons in layers 2–3, and the smaller somal size and reduced spine density of pyramidal neurons in these layers, raises the possibility that all of these changes reflect abnormalities intrinsic to superficial layer pyramidal neurons in schizophrenia. Consistent with this hypothesis, the expression of certain gene products involved in intracellular signaling mechanisms that regulate spine formation and maintenance (e.g., Duo and Cdc42) is altered in schizophrenia (Hill et al., 2006), and some of these molecules are preferentially expressed in DLPFC layer 3 (e.g., Cdc42 effector protein 3) (Arion et al., 2007; Ide and Lewis, 2010).
Chandelier neuron alterations in schizophrenia might reflect disturbances in their postnatal maturation
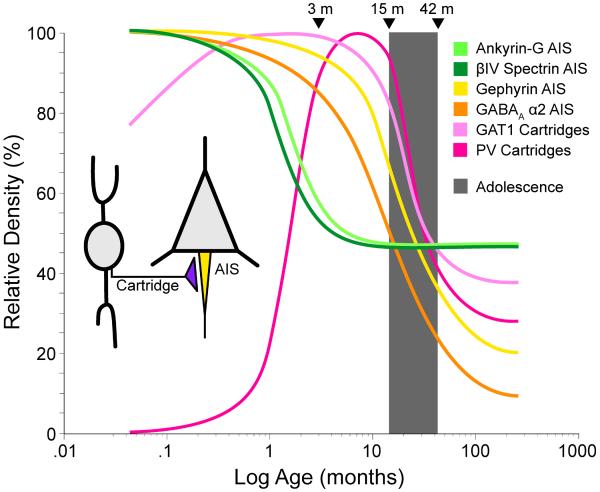
Environmental factors associated with an increased risk for schizophrenia have been found from the first trimester of gestation through early adolescence. Interestingly, chandelier neurons exhibit substantial developmental changes in the expression of biochemical markers both pre- and post-synaptically (Figure 1). During postnatal development, the density of chandelier neuron axon cartridges immunoreactive for either PV or GAT1 changes markedly in monkey DLPFC (Cruz et al., 2003). Although the precise time course differs for the two markers, the density of labeled cartridges is low in the newborn, increases to reach a peak prior to the onset of puberty, and then declines markedly to adult levels. Because cartridges are readily visualized with the Golgi technique over this same time period (Lund and Lewis, 1993), the changes in PV- and GAT-immunoreactive cartridges likely reflect developmental shifts in the concentration of these proteins. Interestingly, the peak and subsequent decline in the density of labeled cartridges appears concomitant with the pruning of intracortical excitatory connections (Woo et al., 1997b).
Figure 1.
Schematic drawing summarizing the developmental trajectories of pre- and post-synaptic molecular marker at pyramidal neuron axon initial segment (AIS) in monkey DLPFC. Cartridge indicates the axon terminals of chandelier neurons. Values for each marker are plotted as a percentage of the maximal value achieved between birth and adulthood. Note the marked differences in the developmental trajectories of these markers. Adapted from (Cruz et al., 2009a).
Postsynaptically, the expression of GABA-A receptor subunits changes substantially during cortical development. For example, the α2 subunit is most prevalent early in development and then declines with age, whereas expression of the α1 subunit progressively increases with age (Fritschy et al., 1994); in monkey DLPFC, these reciprocal changes extend through adolescence (Hashimoto et al., 2009). Consistent with these findings, the detectability of GABAA α2 subunits in pyramidal neuron AIS undergoes substantial changes during postnatal development (Cruz et al., 2003); the density of pyramidal cell AIS immunoreactive for the α2 subunit is very high in the postnatal period, and then steadily declines through adolescence into adulthood. Because GABA-A receptors including the α2 subunit have a higher affinity for GABA, faster activation times, and slower deactivation times than receptors containing the α1 subunit (Lavoie et al., 1997), this decrease in the density of α2-labeled AIS may be interpreted as a change in the kinetics (if α2 are replaced by α1 subunits) or the efficacy (if the density of GABA-A receptors is reduced) of GABA transmission at layer 2–3 pyramidal neuron AIS during postnatal maturation.
Immunoreactivity for ankyrin-G, βIV spectrin and gephyrin (a scaffolding protein that regulates the clustering of GABA-A receptors containing α2 subunits at AIS (Fritschy et al., 2008; Jacob et al., 2005; Levi et al., 2004)) also exhibit substantial changes during postnatal development (Cruz et al., 2009a). The densities of ankyrin-G and βIV spectrin immunoreactive AIS were greatest at birth and then sharply declined to reach relatively stable values by one year of age. In contrast, the relative density of gephyrin-IR AIS did not appear to change through the two postnatal years, but then sharply declined through adolescence and into adulthood.
The high density of AIS with detectable levels of ankyrin-G immunoreactivity in the first three postnatal months may reflect the recruitment to this location of a portion of the large number of GABA synapses that are formed in the monkey DLPFC during this developmental epoch (Bourgeois et al., 1994). Binding to ankyrin G is also essential for the localization of many other membrane proteins to the AIS (Susuki and Rasband, 2008), including the voltage-gated Na+ channels that are required for action potential generation (Zhou et al., 1998). Thus, the high levels of ankyrin G immunoreactivity may also indicate an increased capacity of pyramidal neurons for repetitive firing that parallels their increase in excitatory inputs during early postnatal development (Anderson et al., 1995; Bourgeois et al., 1994). The parallel relative densities of ankyrin-G-IR and βIV spectrin-SD-IR AIS likely reflect that ankyrin-G is required for the recruitment of βIV spectrin to AIS (Yang et al., 2007). Although βIV spectrin is not essential for the formation of the AIS, it does appear necessary for maintenance of membrane structure and molecular organization (Lacas-Gervais et al., 2004), and thus the stability (Yang et al., 2007), of AIS. Given the general role of spectrins in maintaining membrane integrity and elasticity (Susuki and Rasband, 2008), high levels of βIV spectrin during early postnatal development might insure the stability of AIS structure while prefrontal cortical thickness is increasing (Giedd et al., 1999; Rabinowicz, 1974).
The high density of gephyrin-IR AIS during early postnatal development is accompanied by a high density of AIS immunoreactive for GABA-A receptors containing the α2 subunit (Cruz et al., 2003), consistent with the role of gephyrin in clustering this type of GABA-A receptor (Tretter et al., 2008). In contrast, during this same developmental epoch, the densities of PV- and GAT1-IR chandelier cell axon cartridges (Cruz et al., 2003) are very low. At presynaptic terminals, PV is thought to reduce Ca2+-dependent GABA release (Vreugdenhil et al., 2003) and the amount of GAT1 is inversely correlated with the availability of GABA at the synapse (Isaacson et al., 1993; Mager et al., 1993). Together, these findings suggest that both the release of GABA from chandelier axon cartridges, and its persistence in the extracellular space at AIS, is very high during early postnatal development. In concert with the high density of both gephyrin- and GABA-A α2-immunoreactive AIS, these findings suggest that both pre- and post-synaptic factors are shifted to maximize GABA neurotransmission at pyramidal cell AIS during the first months of postnatal development.
This complex and protracted postnatal maturation of the connectivity between chandelier neurons and pyramidal neurons in the superficial layers of the DLPFC provides a number of opportunities for any disturbances even subtle ones, to have their effects amplified as they alter the trajectories of the developmental events that follow. In particular, the marked developmental changes in the axon terminals of PV-containing chandelier neurons, and their postsynaptic receptors, during the perinatal period and adolescence raises the possibility that the alterations in schizophrenia of these markers reflect a disturbance in these patterns of development. These temporal correlations may explain how a range of environmental factors (e.g., labor-delivery complications, urban place of rearing, and marijuana use during adolescence) are all associated with increased risk for the appearance of schizophrenia later in life. Although it seems unlikely that the GABA-related disturbances in schizophrenia represent an arrest of development, they may reflect an alteration of DLPFC circuitry that makes it unable to support higher levels of working memory load, rendering the impaired performance in schizophrenia analogous to the immature levels of working memory function seen in children (Crone et al., 2006; Diamond, 2002; Luna et al., 2004).
The output of chandelier neurons is excitatory in the neocortex
Chandelier neurons have been considered to be powerful inhibitors of pyramidal cell output, exercising “veto power” by virtue of the close proximity of their synaptic inputs to the site of action potential generation in pyramidal neurons. Like other cortical GABA neurons, the effect of GABA released from chandelier neuron axon terminals is mediated by binding to post-synaptic GABA-A receptors which results in the opening of chloride ion channels. In the adult brain, high expression of the potassium-chloride co-transporter (KCC2) results in the extrusion of chloride from the cell (Stein and Nicol, 2003). Thus, when GABA-A receptors are activated, chloride ions flow into the cell along a concentration gradient, resulting in hyperpolarization of the membrane, and a reduced probability of cell firing. However, a recent study (Szabadics et al., 2006) found that KCC2, while readily detectable in the cell body of adult pyramidal neurons, was apparently absent in the AIS of neocortical pyramidal neurons. Consistent with this observation, Szabadics and colleagues found that the release of GABA from chandelier neuron axon terminals resulted in depolarization of pyramidal cells in an in vitro slice preparation. In fact, the chandelier cell-mediated depolarization was so powerful that in ~50% of the cases in which a single chandelier cell was stimulated, the postsynaptic pyramidal cell was depolarized to the point of firing an action potential. Consistent with these findings, micro-application of GABA near the AIS of neocortical pyramidal cells was found to be excitatory (Khirug et al., 2008). Furthermore, the excitation of pyramidal cells by chandelier cells was found in both rodent and human neocortex (Molnar et al., 2008). Studies using recording techniques that were able to exclude potential methodological confounds showed that both basket and chandelier neurons are inhibitory in the hippocampus (Glickfeld et al., 2009), but that neocortical chandelier cells are able to produce GABA-mediated excitation (Woodruff et al., 2009).
Chandelier neurons and the pathophysiology of schizophrenia
As noted above (see “GABA neurons of the chandelier subclass are affected in schizophrenia”), current views of the role of chandelier neurons in the pathophysiology of schizophrenia are based on the idea that chandelier neurons potently inhibit pyramidal cells, and the presumed deficit of GABA in these neurons contributes to impaired synchronization of network activity in postsynaptic pyramidal cells. However, the idea that chandelier cell inputs can be excitatory suggests alternative considerations. For example, if chandelier cell inputs to pyramidal neurons are usually depolarizing, then reduced synthesis of GABA in these neurons could result in a marked decrease in the excitatory output of pyramidal neurons. Alternatively, it is possible that instead of providing a substantial fraction of background excitatory synaptic input over time, chandelier neurons excite pyramidal cells only during specific patterns of network activity. For instance, by virtue of the divergent connections of a given chandelier cell to several hundred pyramidal cells, then excitatory input from chandelier cells could provide a strong synchronization signal during network oscillations.
The alterations in pre- and post-synaptic markers of chandelier cell inputs to layer 3 pyramidal neurons in schizophrenia might also reflect a compensatory response to increase excitatory drive to pyramidal neurons that lack normal levels of excitation due to an intrinsic deficit in dendritic spines, the primary site of excitatory inputs. That is, lower levels of PV and GAT1 in chandelier neuron axon terminals, and increased post-synaptic α2-containing GABA-A receptors, would all serve to increase GABA-mediated depolarization at pyramidal neuron AIS. In addition, reduced GAD67 expression in PV-containing basket neurons would lead to lower levels of somatic hyperpolarization. Thus, the observed changes in both chandelier and basket neurons in schizophrenia could reflect homeostatic responses to preserve activity in the neural networks formed by DLPFC layer 3 pyramidal neurons in the face of a disease-related deficiency in the capacity of these neurons to receive excitatory drive from other pyramidal cells.
Acknowledgments
Work by the author cited in this review was supported by NIH grants MH043784, MH051234, and MH084053. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Disclosure/Conflicts of Interest: David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2007–2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck and Neurogen.
REFERENCES
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Ango F, Di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119(2):257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Mirnics K. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. Eur J Neurosci. 2007;25(6):1843–1854. doi: 10.1111/j.1460-9568.2007.05396.x. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Comm. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Kanbara N, et al. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: Specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABA-A receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- Bourgeois J-P, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62(10):1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Castner SA, Arriza JL, Roberts JC, Mrzljak L, Christian EP, Williams GV. Reversal of ketamine-induced working memory impairments by the GABAAalpha2/3 agonist TPA023. Biol Psychiatry. 2010;67(10):998–1001. doi: 10.1016/j.biopsych.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Collin T, Chat M, Lucas MG, Moreno H, Racay P, Schwaller B, et al. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci. 2005;25(1):96–107. doi: 10.1523/JNEUROSCI.3748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k, or parvalbumin in monkey prefrontal cortex: Distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and post-synaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Lovallo EM, Stockton S, Rasband M, Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. J Comp Neurol. 2009a;514(4):353–367. doi: 10.1002/cne.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Weaver CL, Lovallo EM, Melchitzky DS, Lewis DA. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in subjects with schizophrenia. Neuropsychopharm. 2009b;34(9):2112–2124. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeLima AD, Morrison JH. Ultrastructural analysis of somatostatin-immunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J Comp Neurol. 1989;283:212–227. doi: 10.1002/cne.902830205. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy and biochemistry. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; London: 2002. pp. 466–503. [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon WC. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31(5):257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: An immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12(1):21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72(1):21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hanada S, Mita T, Nishino N, Tanaka C. [3H]Muscimol binding sites increased in autopsied brains of chronic schizophrenics. Life Sci. 1987;40:239–266. doi: 10.1016/0024-3205(87)90341-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25(2):372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65(12):1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183(4):635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: Implications for dendritic spine deficits. Biol Psychiatry. 2010;68(1):25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11(1):11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic action of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, et al. Gephyrin regulates the cell surface dynamics of synaptic GABA(A) receptors. J Neurosci. 2005;25(45):10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28(18):4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162(6):1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270(5):2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, et al. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol. 2005;94(5):3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, et al. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166(7):983–990. doi: 10.1083/jcb.200408007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophysical Journal. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24(1):207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165(12):1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: Natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119(4):706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Devel. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lund JS, Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26(19):5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Eggan SM, Lewis DA. Synaptic targets of calretinin-containing axon terminals in macaque monkey prefrontal cortex. Neuroscience. 2005;130(1):185–195. doi: 10.1016/j.neuroscience.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Lewis DA. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: Laminar, regional and target specificity of Type I and Type II synapses. J Comp Neurol. 1999;408:11–22. [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65(12):1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Molnar G, Olah S, Komlosi G, Fule M, Szabadics J, Varga C, et al. Complex events initiated by individual spikes in the human cerebral cortex. PLoS Biol. 2008;6(9):e222. doi: 10.1371/journal.pbio.0060222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Felmy F, Schwaller B, Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci. 2007;27(9):2261–2271. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: Focus on GABA content, GABAA receptor α-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23(7):2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo T-U, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156(11):1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Volk CLE, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T. Some aspects of the maturation of the human cerebral cortex. Mod Probl Paediat. 1974;13:44–56. [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: A postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167(2):160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MDC, Slater P, Deakin JFW, Royston MC, Skan WJ. Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neurosci Lett. 1989;107:211–215. doi: 10.1016/0304-3940(89)90819-7. [DOI] [PubMed] [Google Scholar]
- Stein V, Nicol RA. GABA generates Excitement. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12(9):854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Spectrin and ankyrin-based cytoskeletons at polarized domains in myelinated axons. Exp Biol Med (Maywood) 2008;233(4):394–400. doi: 10.3181/0709-MR-243. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharm. 2009;34(2):374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharm. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311(5758):233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. Am J Psychiatry. 2005;162(10):1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43(11):970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 Subunits to gephyrin. J Neurosci. 2008;28(6):1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20(5):497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: A preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: Decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy J-M, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89(3):1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997a;154(7):1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997b;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95(9):5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. beta IV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176(4):509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex. 2005;15(8):1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, et al. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex. 2009;19(7):1597–1615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou DX, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. Ankyrin(G) is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143(5):1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]