Abstract
During the lactation period mothers have a variety of adaptive changes in brain physiology and behaviour to allow them to properly raise their pups. The exact circuitries and mechanisms responsible for these changes are not fully understood. Recent evidence suggests that the neuropeptide tuberoinfundibular peptide of 39 residues (TIP39) and its receptor, the parathyroid-hormone 2 receptor (PTH2-R), contribute to these mechanisms. To further investigate this idea we evaluated the growth rate of pups from dams with a non-functional PTH2-R (PTH2-R-KO) as well as maternal behavioural and neuroendocrine parameters. We observed that PTH2-R-KO-reared pups had a slowed growth rate. This was associated with a reduced volume of milk yielded by PTH2-R-KO dams after 30-min suckling when compared with wild-type dams (WT) when pups were returned after five hours separation. Our data suggests a reduced sensitivity of PTH2-R-KO dams to pup stimulation. We also observed a significant reduction in suckling-induced c-Fos expression in the paraventricular nucleus of the hypothalamus and signs of lower prolactin levels in the PTH2-R-KO dams. Our data suggest that the reduced growth rate of PTH2-R-KO-reared pups was likely the result of alterations in the milk-production pathway rather than modifications in behaviour. Although PTH2-R-KO dams showed increased anxiety in the elevated zero-maze test, no differences from WT dams in maternal behaviour were observed. Altogether, our findings suggest involvement of the TIP39/PTH2-R system in the pathways involved in the successful development of the pups.
Keywords: lactation, TIP39/PTH2-R system, prolactin, pup development
Introduction
Successfully raising pups requires behavioural and physiological adaptations and interactions between these processes. Significant progress has been made toward identifying the mechanisms underlying maternal behaviour and the neuroendocrine reflex responsible for milk production (1, 2, 3). However, the critical circuitries are not fully elucidated. Neuroendocrine and behavioural modifications that occur during the lactation period are interdependent. For instance, the pathways that regulate milk production and ejection are also involved in the modulation of maternal behaviour expression (4, 5, 6, 7, 8). It is thus important to consider changes at the neuroendocrine and behavioural levels to fully understand the pathways activated during the lactation period.
Anatomical evidence suggests that tuberoinfundibular peptide of 39 residues (TIP39) and its receptor (the parathyroid hormone 2 receptor – PTH2-R) may play a role in lactation-related processes (9). TIP39-containing nerve fibers and PTH2-R-expressing cells and their fibers are abundant in the hypothalamic para- and periventricular and arcuate nuclei (10, 11), which are involved in several aspects of lactation (12, 13, 14). In lactating dams there is a dramatic increase in TIP39 expression within TIP39 neurons that project to these regions (15). Furthermore, acute block of the PTH2-R inhibits suckling-induced prolactin release in rat dams (15). Thus, neuroanatomical and neuroendocrinological studies suggest a role of TIP39 in stimulating prolactin release and in transferring sensory information into neurohormonal inputs that influence milk production. However, its potential role in other lactation-related events has not been addressed. The role played by prolactin in the expression of maternal behaviour (16) and on the proper development and growth of the offspring suggests that TIP39 signalling could be involved directly or indirectly in these processes.
To date, the role of TIP39 signalling in lactation has only been investigated using acute suppression of TIP39 signalling by intracerebroventricular administration of a PTH2-R antagonist in rats. This approach does not permit assessment of the role of TIP39 signalling in maternal behaviour or on offspring development over long periods of time. In the present study, we used a mouse line with an inactivating mutation in the PTH2-R to further study the role of the TIP39/PTH2-R system throughout the lactation period. Our goal was to assess the involvement of the TIP39/PTH2-R system in the expression of maternal behaviour and in the development of the offspring during the lactation period and in the pathways regulating milk production.
Material and methods
Animals
Housing conditions
Animals were housed under a reverse light-dark cycle (lights off at 0800h) in polycarbonate cages (35.5 × 14 × 12.5cm) starting at least two weeks before the beginning of the experiments. Wild-type (WT) females and PTH2-R mutant (PTH2-R-KO) females were paired with PTH2-R-KO and WT males, respectively, to obtain litters of heterozygous pups. When pregnant, females were housed individually until giving birth. The day of birth was noted as post-natal day 0 (PND0). Litters were culled randomly to 6 pups at PND1 and weaned at PND21. Animals had ad libitum access to food, water, bedding and nesting material. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and strictly followed NIH/ILAR guidelines.
Production and characterization of PTH2-R knockout mice
Adult WT and PTH2-R-KO female littermates (9 ± 1 weeks of age at the beginning of the experiment) generated from HET x HET matings were tested. Development of this mouse line, general characterization of males, and genotyping have been previously described (17). Briefly, mice with a null mutation of the PTH2-R were generated by first introducing loxP sites into intronic sequence flanking exon 5 of the receptor in 129S6 X C57BL6/N F1 ES cells. The “floxed exon 5” mice were then bred with a “Cre deleter” line (that express Cre recombinase in germ cells; Tg(Prm-cre)58Og). Mice with permanent deletion of exon 5 were identified by PCR and then bred with C57Bl/6J mice, producing heterozygous exon 5 deleted mice. Animals used in this study were backcrossed to C57Bl/6J for 4 generations. For this study an observational battery measuring general health and neurological functions was performed (modified from 18) on 10 WT and 10 PTH2-R-KO females. The general physical condition of the mice was noted (body weight, missing whiskers, bald patches and piloerection). Gross neurological function and reflexes were assessed as a percent of animals within each genotype approaching an object placed in their cage, eye blinking, ear and whisker twitching and presenting normal postural reflex when their cage is moved up and down or left to right. Motor co-ordination was assessed using the accelerating rotarod. Each mouse was placed on a 3-cm diameter dowel that was accelerated at a constant rate of 8 r.p.m./min up to 40 r.p.m. The latency to fall to the floor was automatically recorded by the apparatus with a maximum cutoff latency of 5 minutes. General response to novelty was assessed by placing the animals in new empty cages for 5 minutes. Wild-running, freezing and tremor were recorded as a percent of animals within each genotype demonstrating these behaviours. The occurrence of grooming and the number of feces dropped was used as an indicator of anxiety. Finally, the total distance traveled (mm) and the velocity (mm/s) were analyzed from video records (Topscan 2.0, Clever Sys, Inc., Reston, VA, USA). Data were analyzed using t-tests or Chi-square tests. No gross abnormality or defect was found in the PTH2-R-KO females. Although, 30% of the PTH2-R-KO mice were found with missing whiskers while no WT mice were (p=0.066 – Table 1).
Table 1.
General health and neurological functions in WT and PTH2-R-KO females
| WT | PTH2-R-KO | P value | |
|---|---|---|---|
| Physical Characteristics | |||
| Body Weight (g) | 18.5 ± .4 | 18.2 ± .5 | .650 |
| Missing Whiskers (%) | 0 | 30 | .066 |
| Bald Patches (%) | 0 | 0 | 1.000 |
| Piloerection (%) | 0 | 0 | 1.000 |
| Empty Cage Observations | |||
| Wild Running (%) | 0 | 0 | 1.000 |
| Spontaneous Freezing (%) | 0 | 0 | 1.000 |
| Tremor (%) | 0 | 0 | 1.000 |
| Grooming | 3.6 ± .7 | 4.8 ± 1.0 | .331 |
| Feces | .6 ± .2 | .8 ± .2 | .511 |
| Distance Traveled (mm) | 15838.2 ± 977.9 | 13174.7± 1326.2 | .123 |
| Velocity (m/s) | 26.4 ± 1.6 | 22.0 ± 2.2 | .123 |
| Neurological Functions and Reflexes | |||
| Approaching Object (%) | 90 | 70 | .264 |
| Postural Reflex (%) | 100 | 100 | 1.000 |
| Eye Blink (%) | 100 | 100 | 1.000 |
| Ear Twitch (%) | 100 | 100 | 1.000 |
| Whisker Twitch (%) | 100 | 70 | .066 |
| Motor Function | |||
| Rotarod latency (cs) | 58.23 ± 10.91 | 70.89 ± 12.82 | .462 |
Assessment of pups weight and mortality
A total of 12 WT and 12 PTH2-R-KO female mice were used. Dams were weighed a first time before pairing with the males. At PND0, the number of born-dead pups was noted and thereafter, the number of dead pups throughout lactation was scored. Starting at PND1 and until weaning (PND21), entire litters and dams were weighed daily. The average pup weight was calculated by dividing the entire litter weight by the number of pups weighed (six).
Assessment of maternal behaviour
Seven WT and 6 PTH2-R-KO females were used. Until PND5 the dams were left undisturbed. At PND2, 3 and 4 infra-red lights (Clover IR045, Clover Electronics, CA, USA) and infra-red sensitive cameras (KPC-Ex20H, KT&C co., LTD, Korea) were placed in front of the cages to record maternal behaviour. Videos were analyzed off-line using a scan sampling method (1 scan every 6 minutes for each hour considered), starting at 0000h, 0400h, 0800h, 1200h, 1600h and 2000h (total of 60 scans/PND/dam). At each scan, the location of the dam was noted as in or out of the nest and the behaviour was noted as active nursing (the dam is over the pups with a dorsal arch posture), passive nursing (the dam lies flat on top of the pups), licking/grooming the pups, self-grooming or non-nursing behaviour (see 19 for a detailed description of these behaviours). Data were expressed as the percent of scan spent performing each behaviour relative to the total of visible scans (in some case, the behaviour could not be scored because of reduced visibility).
A pup retrieval test was performed at PND6 on 9 WT and 6 PTH2-R-KO females. Pups were removed from the home cage for 5 minutes. They were then returned scattered about the dam’s cage and retrieval of the pups to the nest site was recorded for 2 minutes. Pup retrieval was quantified by measurement of the latency for the 1st and 3rd pup retrieved.
Assessment of anxiety in virgin and lactating females
Virgin (WT n=7, PTH2-R-KO n=9) and lactating dams (PND7; WT n=8, PTH2-R-KO n=7) were tested in the elevated-zero maze (EZM) test to assess their anxiety level. Dams were removed from their home-cage and individually carried to the testing room. The apparatus consisted of a circular platform (outer diameter 46 cm, width 5.5 cm) that was elevated 40 cm above the floor and made of grey plastic. The EZM featured two open and two enclosed (closed) segments. The closed segments were enclosed by walls extending 20 cm above the surface of the maze, while there were no walls in the open segments. Each test session started by placing the mouse in one of the two open sectors facing a close sector. The test session lasted for 5 minutes. Performance was recorded using a video-camera placed above the EZM and analyzed with TopScan video tracking software (CleverSys, Inc., VA, USA). Measurements of total path moved, percent of time spent in open and closed segments, latency to enter the closed segment and number of open segment entries were used for further analysis. In addition, the number of feces was counted at the end of the 5-min test session. The mouse was then returned to its home cage and the EZM was cleaned with a 70% alcohol solution.
Assessment of milk volume production
The milk volume was assessed in 2 groups of lactating dams at PND8. At 0800h, mothers were separated from their pups by being placed in a new cage. Pups were weighed and carried in their home-cage to an adjacent room to avoid ultrasonic communication between the dam and the litter during the separation. After a separation period of 5 hours, pups were weighed and reunited with their mothers by placing the dam back in its home-cage. For the first group (WT n=7; PTH2-R-KO n=11), pups were weighed again after a suckling period of 30-min. For the second group (WT n=7; PTH2-R-KO n=9), pups were weighed again after a suckling period of 90-min. The difference in the litters’ body weight before and after the reunion was measured as an indicator of the milk yield obtained (20).
Assessment of basal prolactin levels in serum
To measure the basal prolactin level in the serum of lactating dams, blood samples were taken from lactating dams not previously separated/reunited from their pups, but present in the nest with the pups. Blood was taken by tail incision at PND 5 (WT n=4; PTH2-R-KO n=6), PND 10 (WT n=5; PTH2-R-KO n=5) and PND19 (WT n=5; PTH2-R-KO n=6) at 1400h. For each sample, about 40 μl of blood was collected into prechilled ethylenediamine tetraacetic acid (EDTA)-coated tubes (Microvette CB300, Sarstedt, Germany). Sampling was finished within 3 min after the cage was first touched by the experimenter. All samples were centrifuged (4 °C) and the plasma was stored at −20 °C until assayed. Prolactin in serum was estimated using a commercial ELISA kit according to manufacturer’s instruction (Catalog number: DY1445; R&D Systems, Inc., MN, USA).
Assessment of suckling-induced c-Fos activation
WT (n=4) and PTH2-R-KO (n=4) lactating dams were removed from their pups at PND9 for 24 hours. During this time, pups were placed in a new cage with nesting material and sawdust and the cages were placed on a heating pad maintained at 30–33°C. After the separation (at PND10), pups were placed back with their mothers and cages were left undisturbed for 90 minutes. Dams were then anesthetized with isoflurane and perfused transcardially with 50ml of 0.1M PBS (pH 7.4) followed by 50ml of fresh 4% paraformaldehyde (PFA). Brains were removed and post-fixed overnight in PFA at 4°C, placed in 30% sucrose until they sank, and then frozen on dry ice and sectioned at 35 μm using a cryostat. Tissues were stored in cryoprotectant solution (21) at −20°C until c-Fos staining was performed.
For immunohistochemistry, free floating sections were labeled with a rabbit anti-c-Fos primary antibody (1:20000, Ab-5, Calbiochem/EMD, San Diegeo CA), detected using Vectastain Elite ABC reagents (Vector Laboratories, Burlingame CA) with nickel intensified diaminobenzidine, essentially as previously described (22). Images were captured with a CoolSnap FX camera (Photometrics, Tucson AZ) using a 10X objective on an Olympus IX-70 light microscope. Images were analyzed using ImageJ 1.42q software (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA. http://rsb.info.nih.gov/ij/, 1997–2009). We used one section per animal for each area of consideration and averaged the left and right side to obtain a single count. We selected the section most similar to the area of interest based on the mouse brain atlas from Franklin and Paxinos (23). The following regions were considered: the hypothalamic supraoptic nuclues (SON – Bregma −0.70mm), the paraventricular hypothalamic nucleus (PVN – Bregma −0.70mm), the medial preoptic area (MPA – Bregma −0.10mm), the ventral bed nucleus of the stria terminalis (vBNST – Bregma −0.46mm) and the arcuate nucleus (ArcN – Bregma −1.94mm). The selected brain region was outlined and the “binary/find maxima” command of the software was used, which allows the determination of local maxima and reports the count of black objects, which corresponded to c-Fos immunoreactive cells. Figures for illustration were assembled in Adobe Photoshop, cropped, and contrast adjusted. No manipulation of individual image elements was performed.
Data analysis
Data were analyzed using Prism 4 software (GraphPad Software Inc., SanDiego, CA, USA). Maternal behaviour was analyzed using a mixed-model ANOVA, with genotype being the between-subject factor and PND and time-window the within-subjects factors (PND and time-window being factors with repeated measures). The general model to analyze maternal behaviour was 3 PND × 6 time-windows × 2 genotypes. The data from the EZM were analyzed using a 2-way ANOVA with the genotype and the stage (virgin vs. lactating) as independent variables. The mortality rate (as a percentage of pups dead) was analyzed using a χ2 test and the litters and dams weight using an ANOVA with repeated measure for the PND. The data from the pup retrieval test, the milk volume, the prolactin level and the c-Fos studies were analyzed using t-tests. Statistical significance was set as p≤0.05 and trend as p≤0.1. All data are presented as mean ± standard error of the mean.
Results
Females lacking the PTH2 receptor have smaller pups
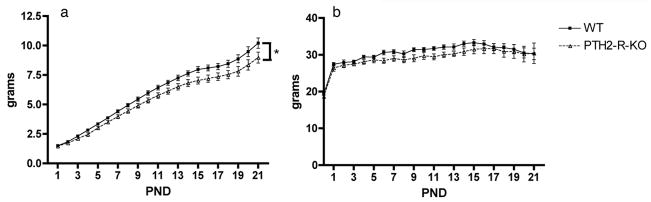
The 12 WT females gave birth to a total of 98 pups while the 12 PTH2-R-KO females gave birth to a total of 100 pups. The number of pups born dead (at PND0) was not different between the WT and the PTH2-R-KO females (2.04% vs. 4%; p=0.428). The number of pups dead after PND1 was higher in PTH2-R-KO females when compared to WT females but without statistical significance (16.67% vs. 6.94%; p=0.089). The litter weight during the lactation period was significantly affected by the genotype (F1=4.70, p=0.043), PTH2-R-KO females raising smaller pups than WT (Figure 1a). We also observed a significant PND effect (F20=707.0, p<0.001) and a significant PND x genotype interaction (F20= 3.12, p<0.001 – Figure 1a). We noticed a similar genotype effect in the pups’ growth rate in two additional cohorts of mice (data not shown). The weight of the dams was significantly affected by the PND (F21=42.36, p<0.001) but no effects of the genotype or of the interaction between the PND and genotype were found (F1=1.37, p=0.259; F21=0.72, p=0.811, respectively – Figure 1b).
Figure 1.
(a) Average pup body weight during the lactation period in litters reared by WT and PTH2-R-KO dams. * main genotype effect; p= 0.043. (b) Average body weight of WT and PTH2-R-KO dams before pregnancy and throughout the lactation period. PND: postnatal day.
Maternal behaviour is not affected by the absence of the PTH2 receptor
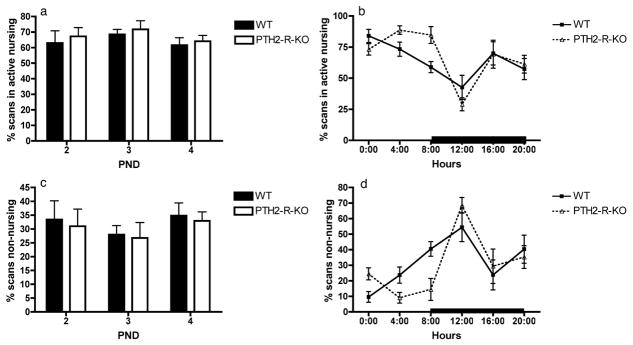
No difference was observed between the maternal behaviour of WT and the PTH2-R-KO females. The time spent in the nest (data not shown) was not affected by the PND (F2=1.25, p=0.305) or the genotype (F1=0.45, p=0.514), but was significantly affected by the time-window, dams spending more time in the nest during the light phase (F5=8.98, p<0.001). The levels of active nursing and passive nursing were not affected by the PND (F2=0.89, p=0.426; F2=1.15, p=0.337, respectively) or the genotype (F1=0.67, p=0.432; F1=0.02, p=0.096, respectively), but were affected by the time window (F5=10.71, p<0.001; F5=2.47, p=0.047, respectively). A non-significant trend for an interaction between the genotype and the time-window was observed for the level of active nursing (F5=2.23, p =0.066 – Figure 2a and 2b). However, post-hoc analyses did not reveal any significant difference between the WT and the PTH-2-R-KO dams at any particular time. The level of licking/grooming of the pups was not affected by the PND (F2=0.32, p=0.733), the genotype (F1=2.23, p=0.166) or the time window (F5=0.86, p=0.512). Finally, the time spent performing non-nursing behaviour was affected by the time window (F5=10.42, p<0.001) but not by the genotype (F1=2.23, p=0.166) or the PND (F2=0.26, p=0.619) and no significant interactions were found (Figure 2c and 2d).
Figure 2.
Representation of the maternal behavior observed in WT and PTH2-R-KO dams as a percentage of scans spent in active nursing (a and b) or spent in non-nursing behavior (c and d). (a) and (c) the percentage of scans spent in each behavior over the 6 time-windows was averaged to obtain one value per PND (post-natal day); (b) and (d) the percentage of scans spent in each behavior over the 3 PND was averaged to obtain one value per hour. The black bar represents the dark phase of the light/dark cycle.
In the pup retrieval test, WT and PTH2-R-KO dams showed a similar latency to retrieve the 1st pup (24.12 ± 3.24 sec vs. 30.66 ± 13.39 sec, respectively; p=0.578) and the 3rd pup (61.19 ± 7.10 sec vs. 69.02 ± 17.16 sec, respectively; p=0.639).
Females lacking the PTH2 receptor showed increased anxiety
The data from the EZM are presented in the table 2. We observed that PTH2-R-KO females spent more time in the closed segment of the EZM (main genotype effect: F1=10.05, p=0.004), entered the closed segment sooner (F1=5.94, p=0.022) and tended to make fewer entries into the open segment (F1=3.55, p=0.071) compared with WT females. The stage (virgin vs. lactating) significantly affected most variables of the EZM: lactating females travelled a shorter distance in the EZM (F1=55.16, p<0.001), dropped more feces (F1=18.51, p<0.001), made fewer entries in the open segment (F1=30.99, p<0.001) and had a greater latency to enter the closed sector (F1=7.84, p=0.009) compared with virgin females. No significant genotype x stage interaction was found.
Table 2.
Data from the elevated-zero maze test in WT and PTH2-R-KO females tested while virgin or while lactating at PND7.
| Virgin | Lactating | Virgin | Lactating | |
|---|---|---|---|---|
| WT | PTH2-R-KO | |||
| Time in closed segment (%) | 80.40 ± 3.16a | 81.14 ± 4.79a | 87.83 ± 2.05b | 94.00 ± 1.67b |
| Latency to closed segment (sec) | 15.27 ± 4.75c, e | 71.71 ± 21.55c, f | 8.00 ± 3.70d, e | 19.68 ± 8.00d, f |
| Entries in open segments | 17.71 ± 2.77g | 5.88 ± 0.97h | 12.44 ± 1.85g | 4.43 ± 0.95h |
| Total path (mm) | 13855.06 ± 1334.23i | 8039.60 ± 722.53j | 15233.78 ± 940.48i | 6887.46 ± 697.39j |
| Feces | 0.57 ± 0.37k | 4.25 ± 0.80l | 1.33 ± 0.47k | 3.29 ± 0.87l |
main genotype effect p= 0.004;
main genotype effect p=0.022;
main stage effect p=0.009;
main stage effect p<0.001;
main stage effect p<0.001;
main stage effect p<0.001.
PTH2-R-KO females yield less milk and tend to have lower levels of prolactin
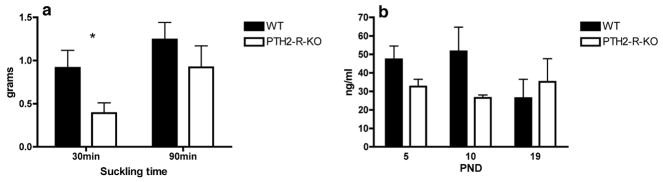
We observed that after a 30-min suckling period PTH2-R-KO dams yielded less milk than WT females (t16=2.37, p=0.031 – Figure 3a). When evaluated after a suckling period of 90-min, the milk yield by PTH2-R-KO dams was not significantly different from the yield of WT dams (t14=0.97, p=0.350 – Figure 3a). The basal prolactin concentration in serum was reduced in PTH2-R-KO dams when compared to WT dams but without reaching statistical significance. This was especially true at PND 5 and 10 (t8=1.94, p=0.088; t8=1.91, p=0.093, respectively) but not at PND19 (t9=0.53, p=0.607) suggesting that there may be a lower level of prolactin in mice lacking the PTH2 receptor during the early and mid-lactation but not late lactation (Figure 3b).
Figure 3.
Milk yield (in grams) by WT and PTH2-R-KO dams after a 30-min or a 90-min suckling period (a) and basal serum prolactin level (ng/ml) of WT and PTH2-R-KO dams at postnatal day (PND) 5, 10 and 19 (b). * p=0.031.
PTH2-R-KO mice show a different pattern of c-Fos activation in the PVN following a suckling stimulus
We observed that the number of c-Fos positive cells in the MPA, the vBNST, the ArcN and the SON of PTH2-R-KO and WT mice were similar after a 90-min suckling stimulus (Figure 4 – t6=1.58, p=0.166; t6=0.98, p=0.365; t6=1.72, p=0.137; t6=0.31, p=0.773, respectively). However we noted lower c-Fos activation in the PVN of PTH2-R-KO females compared with WT (t6=2.48, p=0.047 – Figure 4 and 5).
Figure 4.
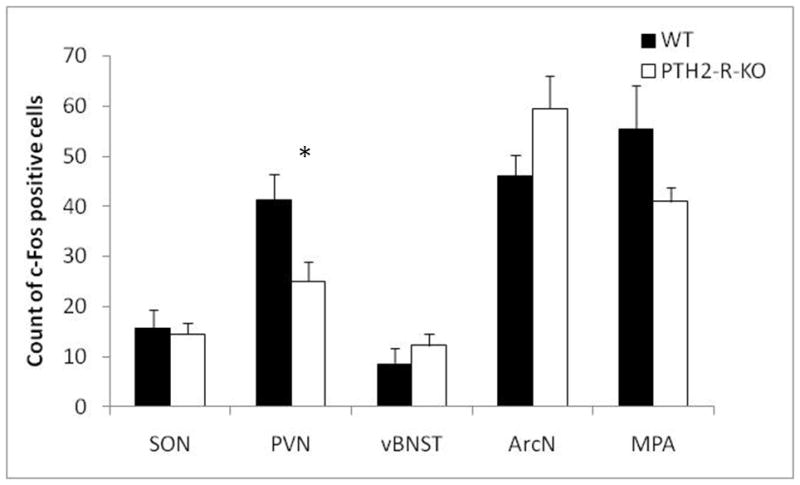
Number of c-Fos positive cells counted in the paraventricular nucleus of the supraoptic nucleus (SON), the hypothalamus (PVN), the ventral bed nucleus of the stria terminalis (vBNST), the arcuate nucleus (ArcN), and the medial pre-optic area (MPA) in WT and PTH2-R-KO dams after a 90-min suckling stimulus. * p<0.05.
Figure 5.
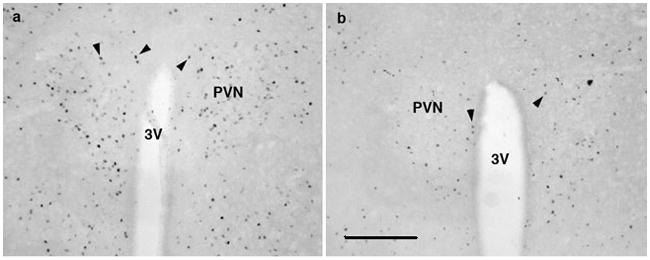
Representative micrographs showing the expression of c-Fos protein within the paraventricular nucleus of the hypothalamus (PVN) of WT and PTH2-R-KO dams (a and b, respectively) after a 90-min suckling stimulus. 3V: 3rd ventricule. The bar scale represents 100μm.
Discussion
In the present study we examined the potential involvement of the TIP39/PTH2-R system in lactation and maternal behaviour. We found that the pups of lactating females lacking a functional PTH2 receptor gain weight at a reduced rate. The slowed pup development is likely to result from neuroendocrine modifications leading to a reduced volume of milk-yield rather than from impairment in maternal behaviour. The lower c-Fos activation in the paraventricular nucleus of the hypothalamus following suckling and a trend toward a lower basal prolactin level in PTH2-R-KO dams suggest an effect in the milk production pathways.
We observed that pups of PTH2-R-KO dams have a slower growth rate than WT-reared litters. This difference is not likely linked to any prenatal factor: there was no difference in the mortality rate or in the body weight one day after birth between the PTH2-R-KO-and WT-reared pups. We produced heterozygote litters by crossing PTH2-R-KO and WT females with WT and PTH2-R-KO males, respectively, to limit the effects of the pup genotypes on the results. Thus, the slower growth throughout lactation in PTH2-R-KO-reared pups is likely due to postnatal factors and to maternal factors rather than offspring factors. However, since we did not use a cross-fostering design, we cannot fully rule out possible long-term in utero effects on the pups. Based on the neuroanatomical distribution of TIP39 fibers and of the PTH2-R, we hypothesised that TIP39 signalling could modulate the expression of maternal behaviour. However, we did not observe any impairment in maternal behaviour in PTH2-R-KO dams in comparison to the WT. This behavioural observation correlates well with the results from the c-Fos study. The medial preoptic area and the ventral bed nucleus of the stria terminalis together contribute to most maternal behaviours and pup retrieval (1, 24). No differences in the c-Fos activation in these two brain regions were observed between PTH2-R-KO and WT dams. This supports the idea that TIP39 signalling is not necessary for the development and the expression of maternal behaviour in mice and that variations in maternal behaviour between WT and PTH2-R-KO dams do not account for the slower growth rate observed in PTH-2-R-KO-reared pups.
Our data suggest that the slower growth rate of pups reared by dams lacking the PTH2-R may be the result of changes in the milk-production or release pathways. Indeed, PTH2-R-KO dams yield less milk than WT dams in a paradigm in which pup weight increase from nursing is measured following a five-hour separation. The PTH2-R-KO/WT difference was most obvious, and statistically significant, 30 minutes following pup return. Ninety minutes after the return of the pups, this difference was no longer statistically significant. The larger initial effect is consistent with the possibility of a lower sensitivity of the PTH2-R-KO dams to nipple stimulation or of alteration in the transfer of sensory information into neurohormonal inputs that are implicated in milk production. The significant activation during lactation of TIP39 neurons in the thalamic region (15) known to integrate and transfer sensory information (25) supports this idea. In rodents, the pathway by which the suckling stimulation induces the production and ejection of milk, involves the release of prolactin (26) by suppression of tuberoinfundibular dopamine neural activity in the hypothalamic arcuate nucleus (27, 28, 29) and the release of oxytocin by neurons from the supraoptic and paraventricular nuclei of the hypothalamus (30). In the present study, we did not observe difference in c-Fos activation in the region of the arcuate nucleus (however, c-Fos is thought not to report tuberoinfundibular dopamine neural activity (29)), but we noticed a lower level of c-Fos activation in the paraventricular nucleus of the hypothalamus of PTH2-R-KO dams after the suckling stimulus. The hypothalamic paraventricular nucleus plays an important role in the regulation of both prolactin and oxytocin secretion and milk production and ejection (31, 32, 33 and 34). Considering the abundance of PTH2 receptors-expressing neurons in the PVN of the hypothalamus (10), a lack of TIP39 signalling in this region during lactation might alter the pathways involved milk production and/or ejection. For instance, we noticed some signs of lower basal prolactin level in PTH2-R-KO dams, although the difference did not reach statistical significance. Since prolactin is the primary neuropeptide involved in milk production (see 31 for a review), a reduced level of prolactin in PTH2-R-KO dams would explain the reduced milk volume yield at the beginning of a suckling session. However, the assessment of suckling-induced prolactin levels would be necessary to confirm the potential role of TIP39 signalling in milk production pathways.
Another interesting observation in females lacking the PTH2-R was their increased anxiety in the elevated-zero maze test as shown by more time spent in the closed segment and by the shorter latency to enter the close segment of the maze. Studies in humans and animals show that psychosocial stress or anxiety in postpartum females reduces prolactin secretion, which leads to alterations in normal lactation mechanisms (35). Further studies demonstrated that stress induces a marked reduction in plasma prolactin when pre-stress prolactin levels are high (e.g. during lactation – 36). It is thus possible that the increased anxiety of the PTH2-R-KO dams contributes to the reduction of milk production and to the impairment in the pups’ development. Other studies have demonstrated that low prolactin level during pregnancy is associated with increased anxiety postpartum (37). However, with the current data, we are not able to demonstrate a direct correlation between the level of anxiety of dams lacking the PTH2-R and the alterations in the milk-production pathways.
Altogether, our findings strongly suggest that the TIP39/PTH2-R system modulates some of the pathways involved in the successful development of the pups. However, more work is necessary to elucidate the exact mechanisms by which the TIP39/PTH2-R system modulates the neuroendocrine changes that occur during the lactation period.
Acknowledgments
This research was supported by the Intramural Program of the NIH, National Institute of Mental Health.
Footnotes
The authors declare that there are no potential conflicts of interest.
References
- 1.Gammie SC. Current models and future directions of understanding the neural circuitries of maternal behaviours in rodents. Behav Cog Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- 2.Leong DA, Frawley LS, Neill JS. Neuroendocrine control of prolactin secretion. Ann Rev Physiol. 1983;45:109–127. doi: 10.1146/annurev.ph.45.030183.000545. [DOI] [PubMed] [Google Scholar]
- 3.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behaviour in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Factor EM, Mayer AD, Rosenblatt JS. Peripeduncular nucleus lesions in the rat: I. Effects on maternal aggression, lactation, and maternal behaviour during pre- and postpartum periods. Behav Neurosci. 1993;107:166–185. doi: 10.1037//0735-7044.107.1.166. [DOI] [PubMed] [Google Scholar]
- 5.Olazabal DE, Ferreira A. Maternal behaviour in rats with kainic acid-induced lesions of the hypothalamic paraventricular nucleus. Physiol Behav. 1997;61:779–784. doi: 10.1016/s0031-9384(96)00567-7. [DOI] [PubMed] [Google Scholar]
- 6.Grattan DR, Pi XJ, Andrews ZB, Augustine RA, Kokay IC, Summerfield MR, Todd B, Bunn SJ. Prolactin receptors in the brain during pregnancy and lactation: implications for behavior. Horm Behav. 2001;40:115–124. doi: 10.1006/hbeh.2001.1698. [DOI] [PubMed] [Google Scholar]
- 7.Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- 8.Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobolyi A, Palkovits M, Usdin TB. The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms. Prog Neurobiol. 2010;90:29–59. doi: 10.1016/j.pneurobio.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber CA, Dobolyi A, Sleeman M, Usdin TB. Distribution of tuberinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol. 2007;502:563–583. doi: 10.1002/cne.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Palkovits M, Usdin TB, Dobolyi A. Forebrain projections of tuberoinfundibular peptide of 39 residues (TIP39)-containing subparafascicular neurons. Neuroscience. 2006;138:1245–1263. doi: 10.1016/j.neuroscience.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocrine Rev. 1985;6:564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar I, Banky ZS, Toth BE, Nagy GM, Halasz B. Brain structures mediating the suckling stimulus-induced release of prolactin. J Neuroendocrinol. 2002;14:384–396. doi: 10.1046/j.0007-1331.2002.00789.x. [DOI] [PubMed] [Google Scholar]
- 14.Giordano AL, Siegel HI, Rosenblatt JS. Nuclear estrogen receptor binding in the preoptic area and hypothalamus of pregnancy-terminated rats: correlation with the onset of maternal behaviour. Neuroendocrinology. 1989;50:248–258. doi: 10.1159/000125230. [DOI] [PubMed] [Google Scholar]
- 15.Cservenak M, Bodnar I, Usdin TB, Palkovits M, Nagy GM, Dobolyi A. Tuberounfundibular peptide of 39 residues is activated during lactation and participates in the suckling-induced prolactin release in rat. Endocrinology. 2010;151:5830–5840. doi: 10.1210/en.2010-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behaviour in steroid-treated, nulliparous female rats. Proc Nat Acad Sci. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutellier L, Logemann A, Kuo J, Rusnak M, Usdin TB. TIP39 modulates effects of novelty-induced arousal on memory. Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00643.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacol. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 19.Coutellier L, Friedrich AC, Failing K, Wurbel H. Variations in the postnatal environment in mice: effects on maternal behaviour and behavioural and endocrine responses in the adult offspring. Physiol Behav. 2008;93:395–407. doi: 10.1016/j.physbeh.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Palmer CA, Neville MC, Anderson SM, McManaman JL. Analysis of lactation defects in transgenic mice. J Mammary Gland Biol Neoplasia. 2006;11:269–282. doi: 10.1007/s10911-006-9023-3. [DOI] [PubMed] [Google Scholar]
- 21.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 22.Dobolyi A, Palkovits M, Bodnar I, Usdin TB. Neurons containing tuberoinfundibular peptide of 39 residues project to limbic, endocrine, auditory and spinal areas in rat. Neuroscience. 2003;122:1093–1105. doi: 10.1016/j.neuroscience.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; 2008. [Google Scholar]
- 24.Neuman M. Maternal behaviour. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. Raven Press; New York: 1994. p. 221. [Google Scholar]
- 25.Arboglast LA. Tuberoinfundibular peptide of 39 residues (TIP39): a new player in the translation of sensory input to prolactin release during lactation. Endocrinology. 2010;151:5568–5569. doi: 10.1210/en.2010-1107. [DOI] [PubMed] [Google Scholar]
- 26.Tucker HA. Lactation and its hormonal control. In: Knobil E, Neill JD, editors. The physiology of Reproduction. Raven Press; New York: 1994. pp. 1065–1098. [Google Scholar]
- 27.Demarest KT, McKay DW, Riegel GD, Moore KE. Biochemical indices of tuberoinfundibular dopaminergic neuronal activity during lactation: a lack of response to prolactin. Neuroendocrinology. 1983;36:130–137. doi: 10.1159/000123449. [DOI] [PubMed] [Google Scholar]
- 28.Demarest KT, Moore KE, Riegel GD. Acute restraint stress decreases tuberoinfundibular dopaminergic neuronalactivity: evidence for a differential response in male versus female rats. Neuroendocrinology. 1985;41:504–510. doi: 10.1159/000124227. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman GE, Le WW, Abbud R, Lee WS, Smith MS. Use of Fos-related antigens (FRAs) as markers of neuronal activity: FRA changes in dopamine neurons during proestrus, pregnancy and lactation. Brain Res. 1994;654:207–215. doi: 10.1016/0006-8993(94)90481-2. [DOI] [PubMed] [Google Scholar]
- 30.Belin V, Moos F. Paired recording from surpaoptic paraventricular of oxytocin cells in suckled rats: recruitment and synchronisation. J Physiol. 1986;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 32.Lyons DJ, Horjales-Araujo E, Broberger C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: switch to tonic discharge by thyrotropin-releasing hormone. Neuron. 2010;65:217–29. doi: 10.1016/j.neuron.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Kádár A, Sánchez E, Wittmann G, Singru PS, Füzesi T, Marsili A, Larsen PR, Liposits Z, Lechan RM, Fekete C. Distribution of hypophysiotropic thyrotropin-releasing hormone (TRH)-synthesizing neurons in the hypothalamic paraventricular nucleus of the mouse. J Comp Neurol. 2010;518:3948–61. doi: 10.1002/cne.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakerley JB, Juss TS, Farrington R, Ingram CD. Role of the paraventricular nucleus in controlling the frequency of milk ejection and the facilitatory effect of centrally administered oxytocin in the suckled rat. J Endocrino. 1990;125:467–75. doi: 10.1677/joe.0.1250467. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs M, Meinschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- 36.Donadio MV, Sagae SC, Franci CR, Anselmo-Franci JA, Lucion AB, Sanvitto GL. Angiotensin II receptors in the arcuate nucleus mediate stress-induced reduction of prolactin secretion in steroid-primed ovariectomized and lactating rats. Brain Res. 2004;1006:59–65. doi: 10.1016/j.brainres.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 37.Larsen CM, Gratten DR. Prolactin-Induced Mitogenesis in the Subventricular Zone of the Maternal Brain during Early Pregnancy Is Essential for Normal Postpartum Behavioral Responses in the Mother. Endocrinoogyl. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]